Laponite Composites: In Situ Films Forming as a Possible Healing Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
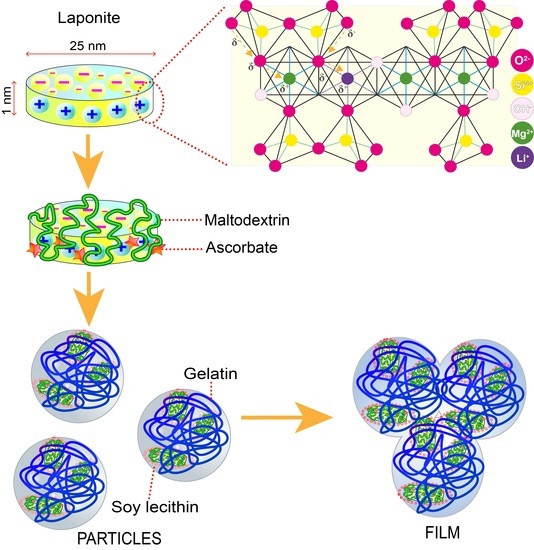
2.2. Preparation of Nanoparticles and Films
2.3. Characterization of Nanoparticles
2.3.1. Particle Size, Polidispersity Index (PDI), and Zeta Potential (ξ)
2.3.2. Entrapment Efficiency
2.3.3. Rheology Study
2.3.4. Morphology
2.4. Characterization of Films
2.4.1. Film Thickness
2.4.2. Mechanical Properties
2.4.3. Swelling Behavior and Mass Loss by Solubilization
2.4.4. Occlusive Effects
2.4.5. Clarity
2.4.6. Bioadhesion and Postwetting Bioadhesion
2.4.7. ATR-FT-IR
2.4.8. Differential Scanning Calorimetry (DSC)
2.4.9. Surface Morphology—Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM)
2.4.10. Uniformity of Content
2.4.11. Drug Release
3. Results and Discussion
3.1. Preparation of Nanoparticles
3.2. Characterization of Nanoparticles
3.2.1. Particle Size, Polydispersity Index (PDI), and Zeta Potential (ξ)
3.2.2. Entrapment Efficiency
3.2.3. Rheological Analysis
3.2.4. Morphology
3.3. Characterization of Films
3.3.1. Film Thickness
3.3.2. Mechanical Properties
3.3.3. Swelling Behavior and Mass Loss by Solubilization
3.3.4. Occlusive Effects
3.3.5. Clarity
3.3.6. Bioadhesion and Post-Wetting Bioadhesion
3.3.7. ATR-FT-IR
3.3.8. Differential Scanning Calorimetry (DSC)
3.3.9. Surface Morphology, Scanning Electron Microscopy (SEM), and Atomic Force Microscopy (AFM)
3.3.10. Uniformity of Content
3.3.11. Drug Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vowden, K.; Vowden, P. Wound Dressings: Principles and Practice. Surgery 2017, 35, 489–494. [Google Scholar] [CrossRef]
- Madrid Martínez, A.; Santillan Reyes, E.R. Caracterización Fisicoquímica y Biológica de Un Biopolímero Obtenido Por Irradiación Gamma a Base de Quitosano y Poloxámero. Bachelor’s Tesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2015. [Google Scholar]
- Rouf, T.B.; Schmidt, G.; Kokini, J.L. Zein–Laponite Nanocomposites with Improved Mechanical, Thermal and Barrier Properties. J. Mater. Sci. 2018, 53, 7387–7402. [Google Scholar] [CrossRef]
- Gonzaga, V.D.A.M.; Poli, A.L.; Gabriel, J.S.; Tezuka, D.Y.; Valdes, T.A.; Leitão, A.; Rodero, C.F.; Bauab, T.M.; Chorilli, M.; Schmitt, C.C. Chitosan-Laponite Nanocomposite Scaffolds for Wound Dressing Application. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1388–1397. [Google Scholar] [CrossRef]
- Olivera, N.; Rouf, T.B.; Bonilla, J.C.; Carriazo, J.G.; Dianda, N.; Kokini, J.L. Effect of LAPONITE® Addition on the Mechanical, Barrier and Surface Properties of Novel Biodegradable Kafirin Nanocomposite Films. J. Food Eng. 2019, 245, 24–32. [Google Scholar] [CrossRef]
- Das, S.S.; Neelam; Hussain, K.; Singh, S.; Hussain, A.; Faruk, A.; Tebyetekerwa, M. Laponite-Based Nanomaterials for Biomedical Applications: A Review. Curr. Pharm. Des. 2019, 25, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Viseras, C.; Carazo, E.; Borrego-Sánchez, A.; García-Villén, F.; Sánchez-Espejo, R.; Cerezo, P.; Aguzzi, C. Clay Minerals in Skin Drug Delivery. Clays Clay Miner. 2019, 67, 59–71. [Google Scholar] [CrossRef]
- Tomás, H.; Alves, C.S.; Rodrigues, J. Laponite®: A Key Nanoplatform for Biomedical Applications? Nanomedicine 2018, 14, 2407–2420. [Google Scholar] [CrossRef]
- Ghadiri, M.; Chrzanowski, W.; Lee, W.H.; Rohanizadeh, R. Layered Silicate Clay Functionalized with Amino Acids: Wound Healing Application. RSC Adv. 2014, 4, 35332–35343. [Google Scholar] [CrossRef]
- Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Antibiotic Eluting Clay Mineral (Laponite®) for Wound Healing Application: An in Vitro Study. J. Mater. Sci. Mater. Med. 2014, 25, 2513–2526. [Google Scholar] [CrossRef]
- Golafshan, N.; Rezahasani, R.; Tarkesh Esfahani, M.; Kharaziha, M.; Khorasani, S.N. Nanohybrid Hydrogels of Laponite: PVA-Alginate as a Potential Wound Healing Material. Carbohydr. Polym. 2017, 176, 392–401. [Google Scholar] [CrossRef]
- Teng, L.; Xia, K.; Qian, T.; Hu, Z.; Hong, L.; Liao, Y.; Peng, G.; Yuan, Z.; Chen, Y.; Zeng, Z. Shape-Recoverable Macroporous Nanocomposite Hydrogels Created via Ice Templating Polymerization for Noncompressible Wound Hemorrhage. ACS BioMater. Sci. Eng. 2022, 8, 2076–2087. [Google Scholar] [CrossRef]
- Rajabi, N.; Kharaziha, M.; Emadi, R.; Zarrabi, A.; Mokhtari, H.; Salehi, S. An Adhesive and Injectable Nanocomposite Hydrogel of Thiolated Gelatin/Gelatin Methacrylate/Laponite® as a Potential Surgical Sealant. J. Colloid Interface Sci. 2020, 564, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Castañeda, O.; Cano-Colín, S.; Pat, L.; Salgado, R.M.; Krötzsch, E.; Elizondo-Vázquez, F.; de la Garza, A.; Baena-Ocampo, L. Maltodextrin/Ascorbic Acid Stimulates Wound Closure by Increasing Collagen Turnover and TGF-Β1 Expression in Vitro and Changing the Stage of Inflammation from Chronic to Acute in Vivo. J. Tissue Viability 2017, 26, 131–137. [Google Scholar] [CrossRef]
- Pineda-Álvarez, R.A.; Bernad-Bernad, M.J.; Rodríguez-Cruz, I.M.; Escobar-Chávez, J.J. Development and Characterization of Starch/Gelatin Microneedle Arrays Loaded with Lecithin–Gelatin Nanoparticles of Losartan for Transdermal Delivery. J. Pharm. Innov. 2020, 17, 71–84. [Google Scholar] [CrossRef]
- Rasouli, M. Characterization and Improvement of Phenol-Sulfuric Acid Microassay for Glucose-Based Glycogen. Eur. Rev. Med Pharmacol. Sci. 2014, 18, 2020–2024. [Google Scholar] [PubMed]
- Ludwig, N.; Formenti, D.; Gargano, M.; Alberti, G. Skin Temperature Evaluation by Infrared Thermography: Comparison of Image Analysis Methods. Infrared Phys. Technol. 2014, 62, 1–6. [Google Scholar] [CrossRef]
- Lenhardt, R.; Sessler, D.I. Estimation of Mean Body Temperature from Mean Skin and Core Temperature. Anesthesiology 2006, 105, 1117–1121. [Google Scholar] [CrossRef]
- López Garcia, M.d.R. Preparación y Caracterización de Nanopartículas Lipídicas y Evaluación de Su Efecto Sobre Las Propiedades de Barrera de La Piel. Master’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2014. [Google Scholar]
- Valencia, G.A.; Luciano, C.G.; Lourenço, R.V.; Do Amaral Sobral, P.J. Microstructure and Physical Properties of Nano-Biocomposite Films Based on Cassava Starch and Laponite. Int. J. Biol. Macromol. 2018, 107, 1576–1583. [Google Scholar] [CrossRef]
- Xue, J.; Zhong, Q. Blending Lecithin and Gelatin Improves the Formation of Thymol Nanodispersions. J. Agric. Food Chem. 2014, 62, 2956–2962. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of PH on Wound-Healing: A New Perspective for Wound-Therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Brown, G.L. Formation of Films from Polymer Dispersions. J. Polym. Sci. 1956, 22, 423–434. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Lei, X.; Miao, S.; Zhang, S.; Cheng, P.; Song, Y.; Wu, H.; Gao, Y.; Bi, L.; et al. Cell-Loaded Injectable Gelatin/Alginate/LAPONITE® Nanocomposite Hydrogel Promotes Bone Healing in a Critical-Size Rat Calvarial Defect Model. R. Soc. Chem. 2020, 10, 25652–25661. [Google Scholar] [CrossRef] [PubMed]
- Berthet, M.; Gauthier, Y.; Lacroix, C.; Verrier, B.; Monge, C. Nanoparticle-Based Dressing: The Future of Wound Treatment? Trends Biotechnol. 2017, 35, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Karponis, D.; Mosahebi, A.; Seifalian, A.M. Nanoparticles in Wound Healing; from Hope to Promise, from Promise to Routine. Front. Biosci.-Landmark 2018, 23, 1038–1059. [Google Scholar] [CrossRef]
- Ding, J.; Venkatesan, R.; Zhai, Z.; Muhammad, W.; Nakkala, J.R.; Gao, C. Micro- and Nanoparticles-Based Immunoregulation of Macrophages for Tissue Repair and Regeneration. Colloids Surf. B Biointerfaces 2020, 192, 111075. [Google Scholar] [CrossRef]
- Li, C.; Mu, C.; Lin, W.; Ngai, T. Gelatin Effects on the Physicochemical and Hemocompatible Properties of Gelatin/PAAm/Laponite Nanocomposite Hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 18732–18741. [Google Scholar] [CrossRef]
- Nasab, M.E.; Takzaree, N.; Saffaria, P.M.; Partoazar, A. In Vitro Antioxidant Activity and in Vivo Wound-Healing Effect of Lecithin Liposomes: A Comparative Study. J. Comp. Eff. Res. 2019, 8, 633–643. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Becher, T.B.; Mendonça, M.C.P.; De Farias, M.A.; Portugal, R.v.; De Jesus, M.B.; Ornelas, C. Soft Nanohydrogels Based on Laponite Nanodiscs: A Versatile Drug Delivery Platform for Theranostics and Drug Cocktails. ACS Appl. Mater. Interfaces 2018, 10, 21891–21900. [Google Scholar] [CrossRef]
- García-Guzmán, P.; Medina-Torres, L.; Calderas, F.; Bernad-Bernad, M.J.; Gracia-Mora, J.; Marcos, X.; Correa-Basurto, J.; Núñez-Ramírez, D.M.; Manero, O. Rheological Mucoadhesion and Cytotoxicity of Montmorillonite Clay Mineral/Hybrid Microparticles Biocomposite. Appl. Clay Sci. 2019, 180, 105202. [Google Scholar] [CrossRef]
- López-Angulo, D.; Bittante, A.M.Q.B.; Luciano, C.G.; Ayala-Valencia, G.; Flaker, C.H.C.; Djabourov, M.; José do Amaral Sobral, P. Effect of Laponite® on the Structure, Thermal Stability and Barrier Properties of Nanocomposite Gelatin Films. Food Biosci. 2020, 35, 100596. [Google Scholar] [CrossRef]
- García-Guzmán, P.; Medina-Torres, L.; Calderas, F.; Bernad-Bernad, M.J.; Gracia-Mora, J.; Mena, B.; Manero, O. Characterization of Hybrid Microparticles/Montmorillonite Composite with Raspberry-like Morphology for Atorvastatin Controlled Release. Colloids Surf. B Biointerfaces 2018, 167, 397–406. [Google Scholar] [CrossRef]
- Finotelli, P.V. Microencapsulation of Ascorbic Acid in Maltodextrin and Capsul Using Spray-Drying. In Proceedings of ENPROMER; E-Papers Serviços Editoriais: Rio de Janeiro, Brasil, 2016. [Google Scholar]
- Yang, J.H.; Lee, S.Y.; Han, Y.S.; Park, K.C.; Choy, J.H. Efficient Transdermal Penetration and Improved Stability of L-Ascorbic Acid Encapsulated in an Inorganic Nanocapsule. Bull. Korean Chem. Soc. 2003, 24, 499–503. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Medina-Torres, L.; González-Laredo, R.F.; Rodríguez-González, V.M.; Eim, V.; Femenia, A. Influence of Water Deficit on the Main Polysaccharides and the Rheological Properties of Aloe Vera (Aloe Barbadensis Miller) Mucilage. Ind. Crop. Prod. 2017, 109, 644–653. [Google Scholar] [CrossRef]
- Blanco-López, M.; González-Garcinuño, Á.; Tabernero, A.; Martín Del Valle, E.M. Steady and Oscillatory Shear Flow Behavior of Different Polysaccharides with Laponite. Polymers 2021, 13, 2520. [Google Scholar] [CrossRef]
- Liu, P.; Du, M.; Leong, Y.K.; Clode, P.; Liu, J. Spherical Metal Oxides-LAPONITE® Sheets Interactions: Microstructure, Rheology and Thixotropy of Composite Gels. Appl. Clay Sci. 2021, 208, 106113. [Google Scholar] [CrossRef]
- Vanin, F.M.; Sobral, P.J.A.; Menegalli, F.C.; Carvalho, R.A.; Habitante, A.M.Q.B. Effects of Plasticizers and Their Concentrations on Thermal and Functional Properties of Gelatin-Based Films. Food Hydrocoll. 2005, 19, 899–907. [Google Scholar] [CrossRef]
- Negrete-Herrera, N.; Putaux, J.L.; David, L.; de Haas, F.; Bourgeat-Lami, E. Polymer/Laponite Composite Latexes: Particle Morphology, Film Microstructure, and Properties. Macromol. Rapid Commun. 2007, 28, 1567–1573. [Google Scholar] [CrossRef]
- Al-Hassan, A.A.; Norziah, M.H. Starch-Gelatin Edible Films: Water Vapor Permeability and Mechanical Properties as Affected by Plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Physical and Chemical Methods Used to Enhance the Structure and Mechanical Properties of Protein Films: A Review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of Advances in Polymeric Wound Dressing Films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Castro, N.; Durrieu, V.; Raynaud, C.; Rouilly, A. Influence of DE-Value on the Physicochemical Properties of Maltodextrin for Melt Extrusion Processes. Carbohydr. Polym. 2016, 144, 464–473. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Benko, A.; Palka, K.; Wojcik, M.; Przekora, A. Elastic and Biodegradable Chitosan/Agarose Film Revealing Slightly Acidic PH for Potential Applications in Regenerative Medicine as Artificial Skin Graft. Int. J. Biol. Macromol. 2020, 164, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and Thermal Properties of Gelatin Films at Different Degree of Crosslinking. Clin. Gastroenterol. Hepatol. 2001, 22, 3–8. [Google Scholar] [CrossRef]
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. (Eds.) Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780444533548. [Google Scholar]
- Teeranachaideekul, V.; Boonme, P.; Souto, E.B.; Müller, R.H.; Junyaprasert, V.B. Influence of Oil Content on Physicochemical Properties and Skin Distribution of Nile Red-Loaded NLC. J. Control. Release 2008, 128, 134–141. [Google Scholar] [CrossRef]
- Urbán-Morlán, Z.; Ganem-Rondero, A.; Melgoza-Contreras, L.M.; Escobar-Chávez, J.J.; Nava-Arzaluz, M.G.; Quintanar-Guerrero, D. Preparation and Characterization of Solid Lipid Nanoparticles Containing Cyclosporine by the Emulsification-Diffusion Method. Int. J. Nanomed. 2010, 5, 611–620. [Google Scholar] [CrossRef]
- Wissing, S.A.; Mü, R.H. The Influence of the Crystallinity of Lipid Nanoparticles on Their Occlusive Properties. Int. J. Pharm. 2002, 242, 377–379. [Google Scholar] [CrossRef]
- Li, X.; Ma, M.; Ahn, D.U.; Huang, X. Preparation and Characterization of Novel Eggshell Membrane-Chitosan Blend Films for Potential Wound-Care Dressing: From Waste to Medicinal Products. Int. J. Biol. Macromol. 2019, 123, 477–484. [Google Scholar] [CrossRef]
- Pan, N.; Qin, J.; Feng, P.; Song, B. Window Screen Inspired Fibrous Materials with Anisotropic Thickness Gradients for Improving Light Transmittance. Nanoscale 2019, 11, 13521–13531. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; von Klitzing, R.; Erim, F.B. Antimicrobial Cerium Ion-Chitosan Crosslinked Alginate Biopolymer Films: A Novel and Potential Wound Dressing. Int. J. Biol. Macromol. 2017, 105, 1161–1165. [Google Scholar] [CrossRef]
- Khanlari, S.; Dubé, M.A. Bioadhesives: A Review. Macromol. React. Eng. 2013, 7, 573–587. [Google Scholar] [CrossRef]
- Waring, M.; Rippon, M.; Bielfeldt, S.; Brandt, M. Cell Attachment to Adhesive Dressings: Qualitative and Quantitative Analysis. Wounds 2008, 4, 35–47. [Google Scholar]
- Blacklow, S.O.; Li, J.; Freedman, B.R.; Zeidi, M.; Chen, C.; Mooney, D.J. Bioinspired Mechanically Active Adhesive Dressings to Accelerate Wound Closure. Synth Biol. 2019, 5, eaaw3963. [Google Scholar] [CrossRef]
- Arilla, E.; Igual, M.; Martínez Monzó, J.; Codoñer Franch, P.; García Segovia, P. Impact of Resistant Maltodextrin Addition on the Orange Juice. Foods 2020, 1832, 1832. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Pálková, H.; Madejová, J.; Zimowska, M.; Serwicka, E.M. Laponite-Derived Porous Clay Heterostructures: II. FTIR Study of the Structure Evolution. Microporous Mesoporous Mater. 2010, 127, 237–244. [Google Scholar] [CrossRef]
- Hamid-Akash, M.S.; Rehman, K. Drug Stability and Chemical Kinetics; Springer: Singapore, 2020; Volume 1, ISBN 9789811564260. [Google Scholar]
- Varnik, F.; Baschnagel, J.; Binder, K. Static and Dynamic Properties of Supercooled Thin Polymer Films. Eur. Phys. J. E 2002, 8, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Sobral, P.J.A.; Habitante, A.M.Q.B. Phase Transitions of Pigskin Gelatin. Food Hydrocoll. 2001, 15, 377–382. [Google Scholar] [CrossRef]
- Abrusci, C.; Martín-González, A.; Del Amo, A.; Catalina, F.; Bosch, P.; Corrales, T. Chemiluminescence Study of Commercial Type-B Gelatines. J. Photochem. Photobiol. A Chem. 2004, 163, 537–546. [Google Scholar] [CrossRef]
- Ismail, Y.; Mauer, L.J. Phase Transitions of Ascorbic Acid and Sodium Ascorbate in a Polymer Matrix and Effects on Vitamin Degradation. J. Food Process. Eng. 2020, 43, e13073. [Google Scholar] [CrossRef]
- Nurhadi, B.; Roos, Y.H.; Maidannyk, V. Physical Properties of Maltodextrin de 10: Water Sorption, Water Plasticization and Enthalpy Relaxation. J. Food Eng. 2016, 174, 68–74. [Google Scholar] [CrossRef]
- Ziderman, I.I.; Gregorski, K.S.; Lopez, S.V.; Friedman, M. Thermal Interaction of Ascorbic Acid and Sodium Ascorbate with Proteins in Relation to Nonenzymatic Browning and Maillard Reactions of Foods. Food Chem. 1989, 37, 1480–1486. [Google Scholar] [CrossRef]
- Hellio-Serughetti, D.; Djabourov, M. Gelatin Hydrogels Cross-Linked with Bisvinyl Sulfonemethyl. 2. The Physical and Chemical Networks. Langmuir 2006, 22, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Comisión Permanente de la Farmacopea de los Estados Unidos Mexicanos. Farmacopea de Los Estados Unidos Mexicanos, Tomo 1; Unidécima; Secretaria de Salud: Mexico City, Mexico, 2020.
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Kalam, M.; Humayun, M.; Parvez, N.; Yadav, S.; Garg, A.; Amin, S.; Sultana, Y.; Ali, A. Release Kinetics of Modified Pharmaceutical Dosage Forms: A Review. Cont. J. Pharm. Sci. 2007, 1, 30–35. [Google Scholar]
- Mulye, N.V.; Turco, S.J. A Simple Model Based on First Order Kinetics to Explain Release of Highly Water Soluble Drugs from Porous Dicalcium Phosphate Dlhy Drate Matrices. Drug Dev. Ind. Pharm. 1995, 21, 943–953. [Google Scholar] [CrossRef]
- Sáez, V.; Hernáez, E.; Sanz Angulo, L. Mecanismos de Liberación de Fármaco Desde Materiales Polímericos. Rev. Iberoam. Polímeros 2004, 5, 55–70. [Google Scholar]










| Sample | L:G mg:mg | Laponite mg | M:AS mg:mg | Size nm | PDI | Z Potential mV | % Entrapment | |
|---|---|---|---|---|---|---|---|---|
| M | AS | |||||||
| LG | 125:100 | - | - | 311.5 ± 36.4 | 0.179 ± 0.034 | −43.2 ± 2.8 | - | - |
| LG MAS | 10:10 | 1801.5 ± 171.3 | 0.578 ± 0.183 | −27.3 ± 0.8 | 50.03 ± 0.99 | 92.98 ± 0.90 | ||
| LGL 4.4 | 4.4 | - | 371.8 ± 34.0 | 0.464 ± 0.046 | −27.1 ± 1.0 | - | - | |
| LGL 4.4 MAS | 10:10 | 1488.7 ± 118.5 | 0.513 ± 0.108 | −15.8 ± 1.6 | 53.85 ± 0.98 | 96.59 ± 0.33 | ||
| LGL 6.6 | 6.6 | - | 336.7 ± 18.9 | 0.625 ± 0.031 | −28.2 ± 1.1 | - | - | |
| LGL 6.6 MAS | 10:10 | 403.4 ± 65.1 | 0.433 ± 0.130 | −16.4 ± 1.9 | 55.41 ± 0.25 | 97.68 ± 0.19 | ||
| LGL | 8.8 | - | 286.4 ± 28.7 | 0.222 ± 0.008 | −34.5 ± 1.7 | - | - | |
| LGL MAS | 10:10 | 391.8 ± 20.7 | 0.145 ± 0.043 | −24.2 ± 2.2 | 57.12 ± 0.50 | 97.96 ± 0.06 | ||
| 150:100 | - | - | 217.6 ± 12.3 | 0.295 ± 0.071 | −38.2 ± 1.2 | - | - | |
| 10:10 | 1363.8 ± 24.2 | 0.508 ± 0.112 | −24.3 ± 1.2 | 50.12 ± 0.28 | 93.28 ± 0.25 | |||
| 4.4 | - | 266.6 ± 30.2 | 0.346 ± 0.030 | −9.3 ± 0.3 | - | - | ||
| 10:10 | 1574.3 ± 160.6 | 0.926 ± 0.092 | −1.8 ± 0.8 | 54.26 ± 1.10 | 97.92 ± 0.50 | |||
| 6.6 | - | 259.8 ± 4.4 | 0.400 ± 0.020 | −12.2 ± 0.6 | - | - | ||
| 10:10 | 772.6 ± 54.4 | 0.699 ± 0.044 | −1.9 ± 0.3 | 54.81 ± 0.48 | 98.47 ± 0.50 | |||
| 8.8 | - | 259.7 ± 26.2 | 0.408 ± 0.019 | −15.1 ± 1.5 | - | - | ||
| 10:10 | 372.6 ± 22.3 | 0.493 ± 0.050 | −2.4 ± 1.4 | 57.08 ± 0.11 | 98.00 ± 0.39 | |||
| Sample | Thickness mm | Young’s Modulus MPa | Tensile Strength MPa | Swelling % | Loss by Solubilization % | Occlusive Effects % |
|---|---|---|---|---|---|---|
| LG | 0.096 ± 0.007 | 23.9 ± 7.2 | 1.8 ± 0.3 | - | - | 88.58 ± 1.27 |
| LG MAS | 0.101 ± 0.005 | 164.3 ± 26.6 | 2.4 ± 0.2 | - | - | 86.43 ± 0.48 |
| LGL 4.4 | 0.097 ± 0.005 | 27.6 ± 6.6 | 0.4 ± 0.2 | 442.87 ± 30.65 | 14.55 ± 2.57 | 77.23 ± 3.32 |
| LGL 4.4 MAS | 0.102 ± 0.012 | 101.7 ± 20.3 | 1.6 ± 0.8 | 171.66 ± 21.20 | 19.17 ± 0.39 | 80.73 ± 1.12 |
| LGL 6.6 | 0.102 ± 0.007 | 18.7 ± 3.8 | 0.9 ± 0.1 | 523.74 ± 43.61 | 8.28 ± 1.53 | 70.33 ± 1.98 |
| LGL 6.6 MAS | 0.103 ± 0.010 | 72.7 ± 9.8 | 2.9 ± 0.5 | 277.17 ± 40.29 | 9.39 ± 0.64 | 81.11 ± 0.68 |
| LGL | 0.111 ± 0.009 | 4.7 ± 0.5 | 4.7 ± 0.8 | 672.46 ± 24.26 | 6.84 ± 0.87 | 69.56 ± 3.40 |
| LGL MAS | 0.113 ± 0.007 | 12.5 ± 2.7 | 7.1 ± 0.6 | 289.06 ± 7.73 | 6.85 ± 0.92 | 79.37 ± 1.00 |
| Drug (%) | ||||
|---|---|---|---|---|
| LG MAS | LGL MAS | |||
| Assay | M | AS | M | AS |
| 1 | 94.07 | 54.23 | 98.53 | 87.19 |
| 2 | 98.01 | 45.12 | 97.27 | 91.27 |
| 3 | 101.28 | 42.73 | 98.83 | 90.01 |
| 4 | 95.33 | 55.04 | 97.97 | 85.55 |
| 5 | 95.58 | 51.09 | 93.74 | 90.88 |
| 6 | 94.67 | 55.37 | 98.35 | 86.85 |
| 7 | 95.99 | 51.56 | 100.43 | 86.91 |
| 8 | 92.53 | 51.09 | 96.09 | 86.76 |
| 9 | 97.19 | 45.31 | 92.70 | 90.25 |
| 10 | 101.25 | 47.24 | 100.90 | 85.74 |
| Mean | 96.59 | 49.88 | 97.48 | 88.14 |
| SD | 2.90 | 4.51 | 2.65 | 2.20 |
| CV | 3.00 | 9.04 | 2.72 | 2.50 |
| Drug | Parameters | Zero-Order F = kt | First-Order F = 100[1-ekt] | Higuchi F = kt0.5 | Korsmeyer–Peppas F = ktn |
|---|---|---|---|---|---|
| M | r2 | 0.6930 | 0.9464 | 0.371 | 0.8612 |
| k | 0.897 ± 0.070 | 0.376 ± 0.026 | 10.100 ± 0.472 | 41.139 ± 1.925 | |
| n | - | - | - | 0.183 ± 0.011 | |
| AS | r2 | 0.9219 | 0.8848 | 0.9501 | 0.9680 |
| k | 0.256 ± 0.007 | 0.013 ± 0.001 | 3.575 ± 0.042 | 6.896 ± 0.402 | |
| n | - | - | - | 0.370 ± 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda-Álvarez, R.A.; Flores-Avila, C.; Medina-Torres, L.; Gracia-Mora, J.; Escobar-Chávez, J.J.; Leyva-Gómez, G.; Shahbazi, M.-A.; Bernad-Bernad, M.J. Laponite Composites: In Situ Films Forming as a Possible Healing Agent. Pharmaceutics 2023, 15, 1634. https://doi.org/10.3390/pharmaceutics15061634
Pineda-Álvarez RA, Flores-Avila C, Medina-Torres L, Gracia-Mora J, Escobar-Chávez JJ, Leyva-Gómez G, Shahbazi M-A, Bernad-Bernad MJ. Laponite Composites: In Situ Films Forming as a Possible Healing Agent. Pharmaceutics. 2023; 15(6):1634. https://doi.org/10.3390/pharmaceutics15061634
Chicago/Turabian StylePineda-Álvarez, Ramón Andrés, Carolina Flores-Avila, Luis Medina-Torres, Jesús Gracia-Mora, José Juan Escobar-Chávez, Gerardo Leyva-Gómez, Mohammad-Ali Shahbazi, and María Josefa Bernad-Bernad. 2023. "Laponite Composites: In Situ Films Forming as a Possible Healing Agent" Pharmaceutics 15, no. 6: 1634. https://doi.org/10.3390/pharmaceutics15061634
APA StylePineda-Álvarez, R. A., Flores-Avila, C., Medina-Torres, L., Gracia-Mora, J., Escobar-Chávez, J. J., Leyva-Gómez, G., Shahbazi, M. -A., & Bernad-Bernad, M. J. (2023). Laponite Composites: In Situ Films Forming as a Possible Healing Agent. Pharmaceutics, 15(6), 1634. https://doi.org/10.3390/pharmaceutics15061634