Advance and Challenges in the Treatment of Skin Diseases with the Transdermal Drug Delivery System
Abstract
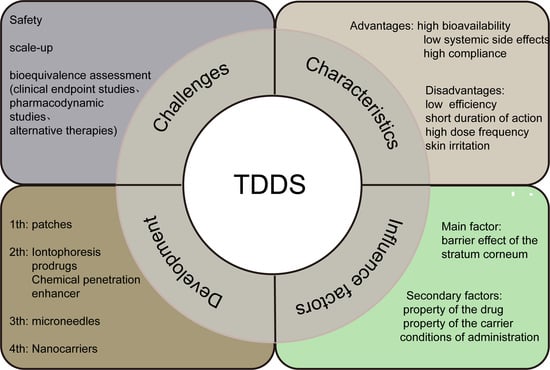
:1. Introduction
2. Absorption Process of TDDS
2.1. The Process of Absorption
2.2. Factors Affecting TDDS Penetration
2.2.1. Physiological Factors
2.2.2. Drug Physicochemical Qualities
2.2.3. Delivery Settings
3. Development of the TDDS
3.1. Frist-Generation TDDS
3.2. Second-Generation TDDS
3.2.1. Iontophoresis
3.2.2. Prodrugs
3.2.3. Chemical Penetration Enhancer
3.2.4. Ultrasound
3.3. Third-Generation TDDS
3.3.1. Electroporation
3.3.2. Microneedles
3.4. Fourth-Generation TDDS
3.4.1. Lipid Vesicular Carriers
3.4.2. Lipid Nanoparticles
3.4.3. Emulsion Based Carriers
3.4.4. Polymeric Nanoparticles
3.4.5. Nanocrystals
4. Challenges and Outlook
4.1. Safety
4.2. Difficulties in Large-Scale Production
4.3. Lack of Standards for Bioequivalence Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TDDS | Transdermal drug delivery system |
| FDA | The Food and Drug Administration |
| ER | Electro-rejection |
| EO | Electro-osmosis |
| KCC | Ketoprofen choline chloride |
| DIPG | Dipropylene glycol |
| PG | Propylene glycol |
| BG | Butanediol |
| SLNs | Solid lipid nanoparticles |
| NLCs | Nanostructured lipid carriers |
| CYC | Cyclosporine |
| MF | Mometasone furoate |
| 8-MOP | 8-methoxypsoralen |
| CLOT | Clotrimazole |
| CSA | Ciclosporin A |
| BPO | Benzoyl peroxide |
| EMA | European Medicines Agency |
| HPLC | High-performance liquid chromatography |
References
- Qu, F.; Geng, R.; Liu, Y.; Zhu, J. Advanced nanocarrier- and microneedle-based transdermal drug delivery strategies. Theranostics 2022, 12, 3372–3406. [Google Scholar] [CrossRef]
- Jee, M.H.; Mraz, V.; Geisler, C.; Bonefeld, C.M. γδ T cells and inflammatory skin diseases. Immunol. Rev. 2020, 298, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Goodyear, B.; Dholaria, N.; Puri, V.; Michniak-Kohn, B. Nanostructured Non-Ionic Surfactant Carrier-Based Gel for Topical Delivery of desoximetasone. Int. J. Mol. Sci. 2021, 22, 1535. [Google Scholar] [CrossRef] [PubMed]
- Mahant, S.; Kumar, S.; Nanda, S.; Rao, R. Microsponges for dermatological applications: Perspectives and challenges. Asian J. Pharm. Sci. 2020, 15, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.H. Transdermal drug delivery. Handb. Exp. Pharmacol. 2010, 197, 399–410. [Google Scholar]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.-C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef]
- Kurmi, B.D.; Tekchandani, P.; Paliwal, R.; Paliwal, S.R. Transdermal Drug Delivery: Opportunities and Challenges for Controlled Delivery. Curr. Drug Metab. 2017, 18, 481–495. [Google Scholar] [CrossRef]
- Pereira, M.N.; Ushirobira, C.Y.; Cunha-Filho, M.S.; Gelfuso, G.M.; Gratieri, T. Nanotechnology advances for hair loss. Ther. Deliv. 2018, 9, 593–603. [Google Scholar] [CrossRef]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert. Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef]
- Proksch, E.; Fölster-Holst, R.; Jensen, J.M. Skin barrier function, epidermal proliferation and differentiation in eczema. J. Dermatol. Sci. 2006, 43, 159–169. [Google Scholar] [CrossRef]
- Malik, D.S.; Mital, N.; Kaur, G. Topical drug delivery systems: A patent review. Expert. Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, S.R.; Andonova, V.Y. Lipid Nanoparticulate Drug Delivery Systems: Recent Advances in the Treatment of Skin Disorders. Pharmaceuticals 2021, 14, 1083. [Google Scholar] [CrossRef]
- Uchida, N.; Yanagi, M.; Hamada, H. Physical Enhancement? Nanocarrier? Current Progress in Transdermal Drug Delivery. Nanomaterials 2021, 11, 335. [Google Scholar] [CrossRef]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef]
- Choy, Y.B.; Prausnitz, M.R. The rule of five for non-oral routes of drug delivery: Ophthalmic, inhalation and transdermal. Pharm. Res. 2011, 28, 943–948. [Google Scholar] [CrossRef]
- Santos, L.F.; Correia, I.J.; Silva, A.S.; Mano, J.F. Biomaterials for drug delivery patches. Eur. J. Pharm. Sci. 2018, 118, 49–66. [Google Scholar] [CrossRef]
- Ünlü, B.; Türsen, Ü. Transdermal patches in dermatology. Dermatol. Ther. 2019, 32, 25. [Google Scholar] [CrossRef]
- Boyaci, A.; Tutoglu, A.; Boyaci, N.; Aridici, R.; Koca, I. Comparison of the efficacy of ketoprofen phonophoresis, ultrasound, and short-wave diathermy in knee osteoarthritis. Rheumatol. Int. 2013, 33, 2811–2818. [Google Scholar] [CrossRef]
- Bozorg, B.D.; Bhattaccharjee, S.A.; Somayaji, M.R.; Banga, A.K. Topical and transdermal delivery with diseased human skin: Passive and iontophoretic delivery of hydrocortisone into psoriatic and eczematous skin. Drug Deliv. Transl. Res. 2022, 12, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Srichakravarthy, N.; Bal, T.R. Synthesis of stavudine amino acid ester prodrugs with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Bioorganic Med. Chem. Lett. 2004, 14, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Maibach, H. Combined use of nanocarriers and physical methods for percutaneous penetration. Adv. Drug Deliv. Rev. 2018, 127, 58–84. [Google Scholar] [CrossRef]
- Burnette, R.R.; Ongpipattanakul, B. Characterization of the permselective properties of excised human skin during. J. Pharm. Sci. 1987, 76, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Cordery, S.F.; Husbands, S.M.; Bailey, C.P.; Guy, R.H.; Delgado-Charro, M.B. Simultaneous Transdermal Delivery of Buprenorphine Hydrochloride and Naltrexone. Mol. Pharm. 2019, 16, 2808–2816. [Google Scholar] [CrossRef]
- Bakshi, P.; Vora, D.; Hemmady, K.; Banga, A.K. Iontophoretic skin delivery systems: Success and failures. Int. J. Pharm. 2020, 586, 119584. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Takeshita, T.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for. Colloids Surf. B Biointerfaces 2017, 160, 520–526. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Ye, R.; Zheng, Y.; Li, X.; Chen, Y.; Xie, X.; Jiang, L. Smartphone-powered iontophoresis-microneedle array patch for controlled. Microsyst. Nanoeng. 2020, 6, 112. [Google Scholar] [CrossRef]
- Patel, D.; Wairkar, S. Recent advances in cyclosporine drug delivery: Challenges and opportunities. Drug Deliv. Transl. Res. 2019, 9, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, T.; Oshima, Y.; Michiue, K.; Tanaka, D.; Kogure, K. Non-invasive delivery of biological macromolecular drugs into the skin by. J. Control Release 2020, 323, 323–332. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, L.; Song, W.; Liu, J. Influencing factors and drug application of iontophoresis in transdermal drug. Drug Deliv. Transl. Res. 2022, 12, 15–26. [Google Scholar] [CrossRef]
- Ita, K. Transdermal iontophoretic drug delivery: Advances and challenges. J. Drug Target. 2016, 24, 386–391. [Google Scholar] [CrossRef]
- N’Da, D.D. Prodrug strategies for enhancing the percutaneous absorption of drugs. Molecules 2014, 19, 20780–20807. [Google Scholar] [CrossRef]
- Ackaert, O.W.; De Graan, J.; Capancioni, R.; Della Pasqua, O.E.; Dijkstra, D.; Westerink, B.H.; Danhof, M.; Bouwstra, J.A. The in vitro and in vivo evaluation of new synthesized prodrugs of 5-OH-DPAT for. J. Control Release 2010, 144, 296–305. [Google Scholar] [CrossRef]
- Ita, K.B. Prodrugs for transdermal drug delivery—Trends and challenges. J. Drug Target. 2016, 24, 671–678. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Meng, S. Site-specific drug delivery in the skin for the localized treatment of skin. Expert. Opin. Drug Deliv. 2019, 16, 847–867. [Google Scholar] [CrossRef]
- Clas, S.D.; Sanchez, R.I.; Nofsinger, R. Chemistry-enabled drug delivery (prodrugs): Recent progress and challenges. Drug Discov. Today 2014, 19, 79–87. [Google Scholar] [CrossRef]
- Lobo, S.; Yan, G. Improving the direct penetration into tissues underneath the skin with. Int. J. Pharm. 2018, 535, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zahui, T.; Alberti, I.; Kalia, Y.N. Cutaneous biodistribution of ionizable, biolabile aciclovir prodrugs after short. Eur. J. Pharm. Biopharm. 2016, 99, 94–102. [Google Scholar] [CrossRef]
- Kasting, G.B.; Miller, M.A.; LaCount, T.D.; Jaworska, J. A Composite Model for the Transport of Hydrophilic and Lipophilic Compounds. J. Pharm. Sci. 2019, 108, 337–349. [Google Scholar] [CrossRef]
- Vasyuchenko, E.P.; Orekhov, P.S.; Armeev, G.A.; Bozdaganyan, M.E. CPE-DB: An Open Database of Chemical Penetration Enhancers. Pharmaceutics 2021, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert. Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Kis, N.; Gunnarsson, M.; Berkó, S.; Sparr, E. The effects of glycols on molecular mobility, structure, and permeability in. J. Control Release 2022, 343, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qin, Y.; Dai, D.; Wang, P.; Shi, M.; Gao, J.; Yang, J.; Xiao, W.; Song, P.; Xu, R. Transdermal Delivery of Therapeutic Compounds With Nanotechnological Approaches in Psoriasis. Front. Bioeng. Biotechnol. 2021, 9, 804415. [Google Scholar] [CrossRef]
- Yadav, P.R.; Munni, M.N.; Campbell, L.; Mostofa, G.; Dobson, L.; Shittu, M.; Pattanayek, S.K.; Uddin, J.; Das, D.B. Translation of Polymeric Microneedles for Treatment of Human Diseases: Recent Trends, Progress, and Challenges. Pharmaceutics 2021, 13, 1132. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; Gunasegaran, T.A.P.; Nathan, S.S.; Md, S.; Gorain, B.; Tripathy, M.; Hussain, Z. Hyaluronic acid-modified betamethasone encapsulated polymeric nanoparticles. Drug Deliv. Transl. Res. 2019, 9, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gill, H.S.; Andrews, S.N.; Prausnitz, M.R. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Control Release 2011, 154, 148–155. [Google Scholar] [CrossRef]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar]
- Lau, S.; Fei, J.; Liu, H.; Chen, W.; Liu, R. Multilayered pyramidal dissolving microneedle patches with flexible pedestals for. J. Control Release 2017, 265, 113–119. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McAlister, E.; McCrudden, M.T.C.; Vora, L.; Steiner, L.; Levin, G.; Levy-Nissenbaum, E.; Shterman, N.; Kearney, M.C.; McCarthy, H.O.; et al. Hydrogel-forming microneedle arrays as a therapeutic option for transdermal. J. Control Release 2020, 322, 177–186. [Google Scholar] [CrossRef]
- Arya, J.; Henry, S.; Kalluri, H.; McAllister, D.V.; Pewin, W.P.; Prausnitz, M.R. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials. Biomaterials 2017, 128, 1–7. [Google Scholar] [CrossRef]
- Lee, J.W.; Prausnitz, M.R. Drug delivery using microneedle patches: Not just for skin. Expert. Opin. Drug Deliv. 2018, 15, 541–543. [Google Scholar] [CrossRef]
- Wang, R.; Zhong, T.; Bian, Q.; Zhang, S.; Ma, X.; Li, L.; Xu, Y.; Gu, Y.; Yuan, A.; Hu, W.; et al. PROTAC Degraders of Androgen Receptor-Integrated Dissolving Microneedles for androgenetic alopecia and recrudescence treatment via single topical administration. Small Methods 2023, 7, 2201293. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Warner, K.S.; Zhang, J.; Sharma, S.; Gale, B.K. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int. J. Pharm. 2010, 391, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Al-Naggar, M.R.; Al-Adl, A.S.; Rabie, A.R.; Abdelkhalk, M.R.; Elsaie, M.L. Intralesional bleomycin injection vs. microneedling-assisted topical bleomycin. J. Cosmet. Dermatol. 2019, 18, 124–128. [Google Scholar] [CrossRef]
- Borowska, K.; Wołowiec, S.; Rubaj, A.; Głowniak, K.; Sieniawska, E.; Radej, S. Effect of polyamidoamine dendrimer G3 and G4 on skin permeation of 8-methoxypsoralene—In Vivo study. Int. J. Pharm. 2012, 426, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.; Pilz, M.; Steinle, H.; Kochba, E.; Levin, Y.; Lunter, D.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Intradermal Delivery of Synthetic mRNA Using Hollow Microneedles for Efficient and rapid production of exogenous proteins in skin. Mol. Ther.-Nucleic Acids 2018, 11, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Ogai, N.; Nonaka, I.; Toda, Y.; Ono, T.; Minegishi, S.; Inou, A.; Hachiya, M.; Fukamizu, H. Enhanced immunity in intradermal vaccination by novel hollow microneedles. Skin. Res. Technol. 2018, 24, 630–635. [Google Scholar] [CrossRef]
- Cárcamo-Martínez, Á.; Mallon, B.; Anjani, Q.K.; Domínguez-Robles, J.; Utomo, E.; Vora, L.K.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Enhancing intradermal delivery of tofacitinib citrate: Comparison between powder-loaded hollow microneedle arrays and dissolving microneedle arrays. Int. J. Pharm. 2021, 593, 120152. [Google Scholar] [CrossRef]
- Du, H.; Liu, P.; Zhu, J.; Lan, J.; Li, Y.; Zhang, L.; Zhu, J.; Tao, J. Hyaluronic Acid-Based Dissolving Microneedle Patch Loaded with Methotrexate for Improved Treatment of Psoriasis. ACS Appl. Mater. Interfaces 2019, 11, 43588–43598. [Google Scholar] [CrossRef]
- Lan, X.; She, J.; Lin, D.A.; Xu, Y.; Li, X.; Yang, W.F.; Lui, V.W.Y.; Jin, L.; Xie, X.; Su, Y.X. Microneedle-Mediated Delivery of Lipid-Coated Cisplatin Nanoparticles for Efficient and Safe Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 33060–33069. [Google Scholar] [CrossRef]
- Sachan, R.; Jaipan, P.; Zhang, J.Y.; Degan, S.; Erdmann, D.; Tedesco, J.; Vanderwal, L.; Stafslien, S.J.; Negut, I.; Visan, A.; et al. Printing amphotericin B on microneedles using matrix-assisted pulsed laser evaporation. Int. J. Bioprinting 2017, 3, 147–157. [Google Scholar] [CrossRef]
- Nasiri, M.I.; Vora, L.K.; Ershaid, J.A.; Peng, K.; Tekko, I.A.; Donnelly, R.F. Nanoemulsion-based dissolving microneedle arrays for enhanced intradermal and. Drug Deliv. Transl. Res. 2022, 12, 881–896. [Google Scholar] [CrossRef]
- Jeong, H.R.; Kim, J.Y.; Kim, S.N.; Park, J.H. Local dermal delivery of cyclosporin A, a hydrophobic and high molecular weight. Eur. J. Pharm. Biopharm. 2018, 127, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Kang, B.M.; Yang, H.; Ohn, J.; Kwon, O.; Jung, H. High-Dose Steroid Dissolving Microneedle for Relieving Atopic Dermatitis. Adv. Healthc. Mater. 2021, 10, e2001691. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.H.B.; Anjani, Q.K.; Donnelly, R.F. Synthesis and characterization of sorbitol laced hydrogel-forming microneedles. Int. J. Pharm. 2021, 607, 121049. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, H.; Shi, Z.; Lin, L.; Li, Y.; Wang, M.; Pan, G.; Lei, Y.; Xue, L. Responsive hydrogel-based microneedle dressing for diabetic wound healing. J. Mater. Chem. B 2022, 10, 3501–3511. [Google Scholar] [CrossRef]
- Jain, S.; Kale, D.P.; Swami, R.; Katiyar, S.S. Codelivery of benzoyl peroxide & adapalene using modified liposomal gel for improved acne therapy. Nanomedicine 2018, 13, 1481–1493. [Google Scholar]
- Cevc, G.; Blume, G. Hydrocortisone and dexamethasone in very deformable drug carriers have increased biological potency, prolonged effect, and reduced therapeutic dosage. Biochim. Et Biophys. Acta 2004, 1663, 61–73. [Google Scholar] [CrossRef]
- Fesq, H.; Lehmann, J.; Kontny, A.; Erdmann, I.; Theiling, K.; Rother, M.; Ring, J.; Cevc, G.; Abeck, D. Improved risk-benefit ratio for topical triamcinolone acetonide in Transfersome in comparison with equipotent cream and ointment: A randomized controlled trial. Br. J. Dermatol. 2003, 149, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Celia, C.; Trapasso, E.; Cilurzo, F.; Fresta, M. Paclitaxel-loaded ethosomes®: Potential treatment of squamous cell carcinoma, a malignant transformation of actinic keratoses. Eur. J. Pharm. Biopharm. 2012, 81, 102–112. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Fan, C.; Li, X.; Wang, X.; Li, M.; Liu, Y. Tacrolimus-loaded ethosomes: Physicochemical characterization and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 82, 49–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, X.; Li, W.; Chen, W.; Wang, X.; Ma, Z.; Lin, N. Tripterygium wilfordii: An inspiring resource for rheumatoid arthritis treatment. Med. Res. Rev. 2021, 41, 1337–1374. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Wang, X.; Tian, C.; Liu, L.; Xia, M.; Jiang, J.; Gui, S. Dual drug-loaded cubic liquid crystal gels for transdermal delivery: Inner structure and percutaneous mechanism evaluations. Drug Dev. Ind. Pharm. 2019, 45, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Gamal, F.A.; Sayed, O.M.; El-Ela, F.I.A.; Kharshoum, R.M.; Salem, H.F. Treatment of Basal Cell Carcinoma Via Binary Ethosomes of Vismodegib: In Vitro and In Vivo Studies. AAPS PharmSciTech 2020, 21, 51. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, N.; Tikoo, K.; Sinha, V.R. Development of mirtazapine loaded solid lipid nanoparticles for topical delivery: Optimization, characterization and cytotoxicity evaluation. Int. J. Pharm. 2020, 586, 119439. [Google Scholar] [CrossRef]
- El-Housiny, S.; Shams Eldeen, M.A.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.R.; El-Nabarawi, M.A. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of. Drug Deliv. 2018, 25, 78–90. [Google Scholar] [CrossRef]
- Jain, S.; Addan, R.; Kushwah, V.; Harde, H.; Mahajan, R.R. Comparative assessment of efficacy and safety potential of multifarious lipid. Int. J. Pharm. 2019, 562, 96–104. [Google Scholar] [CrossRef]
- Gupta, S.; Wairkar, S.; Bhatt, L.K. Isotretinoin and α-tocopherol acetate-loaded solid lipid nanoparticle topical gel. J. Microencapsul. 2020, 37, 557–565. [Google Scholar] [CrossRef]
- Passos, J.S.; Martino, L.C.; Dartora, V.F.C.; Araujo, G.L.B.; Ishida, K.; Lopes, L.B. Development, skin targeting and antifungal efficacy of topical lipid. Eur. J. Pharm. Sci. 2020, 149, 105296. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, K.; Bedi, N. Topical Nanostructured Lipid Carrier Based Hydrogel of Mometasone Furoate for the. Pharm. Nanotechnol. 2018, 6, 133–143. [Google Scholar] [CrossRef]
- Vasanth, S.; Dubey, A.; Ravi, G.S.; Lewis, S.A.; Ghate, V.M.; El-Zahaby, S.A.; Hebbar, S. Development and Investigation of Vitamin C-Enriched Adapalene-Loaded Transfersome Gel: A Collegial Approach for the Treatment of Acne Vulgaris. AAPS PharmSciTech 2020, 21, 61. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Kaul, V.; Waghule, T.; Gorantla, S.; Sharma, S.; Roy, A.; Dubey, S.K.; Singhvi, G. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: Optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur. J. Pharm. Sci. 2020, 152, 105438. [Google Scholar] [CrossRef]
- Argenta, D.F.; Bidone, J.; Koester, L.S.; Bassani, V.L.; Simões, C.M.O.; Teixeira, H.F. Topical Delivery of Coumestrol from Lipid Nanoemulsions Thickened with Hydroxyethylcellulose for Antiherpes Treatment. AAPS PharmSciTech 2018, 19, 192–200. [Google Scholar] [CrossRef]
- Rashid, S.A.; Bashir, S.; Naseem, F.; Farid, A.; Rather, I.A.; Hakeem, K.R. Olive Oil Based Methotrexate Loaded Topical Nanoemulsion Gel for the Treatment of imiquimod induced psoriasis-like skin inflammation in an animal model. Biology 2021, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.S.; Maulvi, F.A.; Patel, P.S.; Shukla, M.R.; Shah, K.M.; Gupta, A.R.; Joshi, S.V.; Shah, D.O. Cyclosporine laden tailored microemulsion-gel depot for effective treatment of psoriasis: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2020, 186, 110681. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lee, S.H.; Chia, V.D.; Chow, P.S.; Macbeath, C.; Liu, Y.; Shlieout, G. Development of microemulsion based topical ivermectin formulations: Pre-formulation and formulation studies. Colloids Surf. B Biointerfaces 2020, 189, 110823. [Google Scholar] [CrossRef]
- Yu, K.; Wang, Y.; Wan, T.; Zhai, Y.; Cao, S.; Ruan, W.; Wu, C.; Xu, Y. Tacrolimus nanoparticles based on chitosan combined with nicotinamide: Enhancing percutaneous delivery and treatment efficacy for atopic dermatitis and reducing dose. Int. J. Nanomedicine 2017, 13, 129–142. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Development of methotrexate loaded fucoidan/chitosan nanoparticles with. Int. J. Biol. Macromol. 2019, 124, 1115–1122. [Google Scholar] [CrossRef]
- Gao, J.; Chen, F.; Fang, H.; Mi, J.; Qi, Q.; Yang, M. Daphnetin inhibits proliferation and inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced psoriasis-like skin lesion in mice. Biol. Res. 2020, 53, 48. [Google Scholar] [CrossRef]
- Kandekar, S.G.; Del Río-Sancho, S.; Lapteva, M.; Kalia, Y.N. Selective delivery of adapalene to the human hair follicle under finite dose. Nanoscale 2018, 10, 1099–1110. [Google Scholar] [CrossRef]
- Yotsumoto, K.; Ishii, K.; Kokubo, M.; Yasuoka, S. Improvement of the skin penetration of hydrophobic drugs by polymeric micelles. Int. J. Pharm. 2018, 553, 132–140. [Google Scholar] [CrossRef]
- Pelikh, O.; Eckert, R.W.; Pinnapireddy, S.R.; Keck, C.M. Hair follicle targeting with curcumin nanocrystals: Influence of the formulation. J. Control Release 2021, 329, 598–613. [Google Scholar] [CrossRef]
- Pyo, S.M.; Hespeler, D.; Keck, C.M.; Müller, R.H. Dermal miconazole nitrate nanocrystals—Formulation development, increased. Int. J. Pharm. 2017, 531, 350–359. [Google Scholar] [CrossRef]
- Walunj, M.; Doppalapudi, S.; Bulbake, U.; Khan, W. Preparation, characterization, and in vivo evaluation of cyclosporine cationic liposomes for the treatment of psoriasis. J. Liposome Res. 2020, 30, 68–79. [Google Scholar] [CrossRef]
- Kesharwani, S.S.; Kaur, S.; Tummala, H.; Sangamwar, A.T. Multifunctional approaches utilizing polymeric micelles to circumvent multidrug. Colloids Surf. B Biointerfaces 2019, 173, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, A.; Mishra, N.; Narang, R.K. Development and Characterization of a Clobetasol Propionate Nanostructured Lipid. Curr. Mol. Pharmacol. 2021, 14, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bahreman, A.; Daudey, G.; Bussmann, J.; Olsthoorn, R.C.; Kros, A. Drug Delivery via Cell Membrane Fusion Using Lipopeptide Modified Liposomes. ACS Cent. Sci. 2016, 2, 621–630. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim. Et Biophys. Acta 1992, 1104, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, T.; Zhu, S.; Pi, J.; Guo, P.; Qi, D.; Liu, Z.; Li, N. Natural medicine combined with nanobased topical delivery systems: A new strategy. Drug Deliv. Transl. Res. 2022, 12, 1326–1338. [Google Scholar] [CrossRef]
- Kilian, D.; Shahzad, Y.; Fox, L.; Gerber, M.; Du Plessis, J. Vesicular carriers for skin drug delivery: The Pheroid™ technology. Curr. Pharm. Des. 2015, 21, 2758–2770. [Google Scholar] [CrossRef]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. Bmj 2020, 28, 369. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Sun, L.; Wang, L.; Lin, Z.; Liu, Z.; Xi, L.; Wang, Z.; Zheng, Y. Loading of water-insoluble celastrol into niosome hydrogels for improved topical. Colloids Surf. B Biointerfaces 2019, 182, 110352. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Rostamkalaei, S.S.; Asadi, M.; Asare-Addo, K.; Nokhodchi, A. The design of naproxen solid lipid nanoparticles to target skin layers. Colloids Surf. B Biointerfaces 2016, 145, 626–633. [Google Scholar] [CrossRef]
- Madan, J.R.; Khude, P.A.; Dua, K. Development and evaluation of solid lipid nanoparticles of mometasone furoate for. Int J Pharm. Investig. 2014, 4, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Lambe, U.P.; Brar, B.; Shah, I.; Manimegalai, J.; Ranjan, K.; Rao, R.; Kumar, S.; Mahant, S.; Khurana, S.K.; et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 2018, 97, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Jacob, S.; Patel, S.S.; Venugopala, K.N.; Morsy, M.A.; Gupta, S.; Attimarad, M.; Sreeharsha, N.; et al. Clarithromycin Solid Lipid Nanoparticles for Topical Ocular Therapy: Optimization, evaluation and in vivo studies. Pharmaceutics 2021, 13, 523. [Google Scholar] [CrossRef]
- Mahant, S.; Rao, R.; Souto, E.B.; Nanda, S. Analytical tools and evaluation strategies for nanostructured lipid carrier-based. Expert. Opin. Drug Deliv. 2020, 17, 963–992. [Google Scholar] [CrossRef]
- Mahmood, A.; Rapalli, V.K.; Gorantla, S.; Waghule, T.; Singhvi, G. Dermatokinetic assessment of luliconazole-loaded nanostructured lipid carriers. Drug Deliv. Transl. Res. 2022, 12, 1118–1135. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Gorain, B.; Choudhury, H.; Nair, A.B.; Dubey, S.K.; Kesharwani, P. Theranostic application of nanoemulsions in chemotherapy. Drug Discov. Today 2020, 25, 1174–1188. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef] [PubMed]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; de Holanda ESilva, K.G.; Elias Mansur, C.R. Formulation characterization and in vitro drug release of hydrogel-thickened. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 245–253. [Google Scholar] [CrossRef]
- Zhengguang, L.; Jie, H.; Yong, Z.; Jiaojiao, C.; Xingqi, W.; Xiaoqin, C. Study on the transdermal penetration mechanism of ibuprofen nanoemulsions. Drug Dev. Ind. Pharm. 2019, 45, 465–473. [Google Scholar] [CrossRef]
- Zhang, J.; Michniak-Kohn, B.B. Investigation of microemulsion and microemulsion gel formulations for dermal. Int. J. Pharm. 2018, 536, 345–352. [Google Scholar] [CrossRef]
- Erdal, M.S.; Gürbüz, A.; Birteksöz Tan, S.; Güngör, S.; Özsoy, Y. In Vitro Skin Permeation and Antifungal Activity of Naftifine Microemulsions. Turk. J. Pharm. Sci. 2020, 17, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Pandey, M.; Thu, H.E.; Kaur, T.; Jia, G.W.; Ying, P.C.; Xian, T.M.; Abourehab, M.A.S. Hyaluronic acid functionalization improves dermal targeting of polymeric nanoparticles for management of burn wounds: In vitro, ex vivo and in vivo evaluations. Biomed. Pharmacother. 2022, 150, 112992. [Google Scholar] [CrossRef]
- Balzus, B.; Sahle, F.F.; Hönzke, S.; Gerecke, C.; Schumacher, F.; Hedtrich, S.; Kleuser, B.; Bodmeier, R. Formulation and ex vivo evaluation of polymeric nanoparticles for controlled. Eur. J. Pharm. Biopharm. 2017, 115, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Caon, T.; Mazzarino, L.; Simões, C.M.; Senna, E.L.; Silva, M.A. Lipid- and Polymer-Based Nanostructures for Cutaneous Delivery of Curcumin. AAPS PharmSciTech 2017, 18, 920–925. [Google Scholar] [CrossRef]
- Campbell, C.S.; Contreras-Rojas, L.R.; Delgado-Charro, M.B.; Guy, R.H. Objective assessment of nanoparticle disposition in mammalian skin after topical. J. Control Release 2012, 162, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.; Sanchez-Fernandez, A.; Leung, A.E.; Schweins, R.; Wu, B.; Nylander, T.; Ulvenlund, S.; Wahlgren, M. Molecular structure of maltoside surfactants controls micelle formation and. J. Colloid Interface Sci. 2021, 581, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Makhmalzade, B.S.; Chavoshy, F. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J. Adv. Pharm. Technol. Res. 2018, 9, 2–8. [Google Scholar] [CrossRef]
- Abolmaali, S.S.; Tamaddon, A.M.; Salmanpour, M.; Mohammadi, S.; Dinarvand, R. Block ionomer micellar nanoparticles from double hydrophilic copolymers. Eur. J. Pharm. Sci. 2017, 104, 393–405. [Google Scholar] [CrossRef]
- Lapteva, M.; Santer, V.; Mondon, K.; Patmanidis, I.; Chiriano, G.; Scapozza, L.; Gurny, R.; Möller, M.; Kalia, Y.N. Targeted cutaneous delivery of ciclosporin A using micellar nanocarriers and the. J. Control Release 2014, 196, 9–18. [Google Scholar] [CrossRef]
- Kahraman, E.; Özhan, G.; Özsoy, Y.; Güngör, S. Polymeric micellar nanocarriers of benzoyl peroxide as potential follicular. Colloids Surf. B Biointerfaces 2016, 146, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Raszewska-Famielec, M.; Flieger, J. Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions—An Overview of Dermo-Cosmetic and Dermatological Products. Int. J. Mol. Sci. 2022, 23, 15980. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Pireddu, R.; Sinico, C.; Ennas, G.; Schlich, M.; Valenti, D.; Murgia, S.; Marongiu, F.; Fadda, A.M.; Lai, F. The effect of diethylene glycol monoethyl ether on skin penetration ability of. Colloids Surf. B Biointerfaces 2018, 162, 8–15. [Google Scholar] [CrossRef]
- Parmar, P.K.; Wadhawan, J.; Bansal, A.K. Pharmaceutical nanocrystals: A promising approach for improved topical drug. Drug Discov. Today 2021, 26, 2329–2349. [Google Scholar] [CrossRef]
- Pelikh, O.; Stahr, P.L.; Huang, J.; Gerst, M.; Scholz, P.; Dietrich, H.; Geisel, N.; Keck, C.M. Nanocrystals for improved dermal drug delivery. Eur. J. Pharm. Biopharm. 2018, 128, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Alalaiwe, A.; Lin, C.F.; Hsiao, C.Y.; Chen, E.L.; Lin, C.Y.; Lien, W.C.; Fang, J.Y. Development of flavanone and its derivatives as topical agents against psoriasis. Int. J. Pharm. 2020, 581, 119256. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert. Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, L.; Wu, D.; Shi, D.; Wang, T.; Zhu, X. Mechanism of transdermal permeation promotion of lipophilic drugs by ethosomes. Int. J. Nanomed. 2017, 12, 3357–3364. [Google Scholar] [CrossRef]
- Ji, T.; Kohane, D.S. Nanoscale systems for local drug delivery. Nano Today 2019, 28, 100765. [Google Scholar] [CrossRef]
- Takahashi, H.; Tsuji, H.; Minami-Hori, M.; Miyauchi, Y.; Iizuka, H. Defective barrier function accompanied by structural changes of psoriatic stratum. J. Dermatol. 2014, 41, 144–148. [Google Scholar] [CrossRef]
- Liu, R.; Zuo, R.; Hudalla, G.A. Harnessing molecular recognition for localized drug delivery. Adv. Drug Deliv. Rev. 2021, 170, 238–260. [Google Scholar] [CrossRef]
- Quinn, H.L.; Hughes, C.M.; Donnelly, R.F. In vivo and qualitative studies investigating the translational potential of microneedles for use in the older population. Drug Deliv. Transl. Res. 2018, 8, 307–316. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, N.; Feng, X. The role of internal and external stimuli in the rational design of skin-specific. Int. J. Pharm. 2021, 592, 120081. [Google Scholar] [CrossRef]
- Thakur, S.; Riyaz, B.; Patil, A.; Kaur, A.; Kapoor, B.; Mishra, V. Novel drug delivery systems for NSAIDs in management of rheumatoid arthritis: An overview. Biomed. Pharmacother. 2018, 106, 1011–1023. [Google Scholar] [CrossRef]
- Schlich, M.; Musazzi, U.M.; Campani, V.; Biondi, M.; Franzé, S.; Lai, F.; De Rosa, G.; Sinico, C.; Cilurzo, F. Design and development of topical liposomal formulations in a regulatory. Drug Deliv. Transl. Res. 2022, 12, 1811–1828. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Shah, V.P.; Crommelin, D.J.; Shargel, L.; Bashaw, D.; Bhatti, M.; Blume, H.; Dressman, J.; Ducharme, M.; Fackler, P.; et al. Harmonization of regulatory approaches for evaluating therapeutic equivalence and interchangeability of multisource drug products: Workshop summary report. Eur. J. Pharm. Sci. 2011, 44, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Wiedersberg, S.; Leopold, C.S.; Guy, R.H. Bioavailability and bioequivalence of topical glucocorticoids. 2008, 68, 453–466. Eur. J. Pharm. Biopharm. 2008, 68, 453–466. [Google Scholar] [CrossRef]
- Patel, P.; Schmieder, S.; Krishnamurthy, K. Research Techniques Made Simple: Drug Delivery Techniques, Part 2: Commonly Used. J. Investig. Dermatol. 2016, 136, e43–e49. [Google Scholar] [CrossRef] [PubMed]
- Braddy, A.C.; Davit, B.M.; Stier, E.M.; Conner, D.P. Survey of international regulatory bioequivalence recommendations for approval of Generic Topical Dermatological Drug Products. AAPS J. 2015, 17, 121–133. [Google Scholar] [CrossRef]




| Transdermal Technique | Mechanism | Advantage | Disadvantage | Drug |
|---|---|---|---|---|
| Ultrasound | Heat effect and cavitation effect | Delivering many different types of drugs | Higher precision instrument requirements; impacted by ultrasonic frequency, intensity, and mode | Ketoprofen [18] |
| Iontophoresis | Electro-rejection and electro-osmosis | Realization of macromolecule transdermal penetration | Higher demands on instrumentation; complex to use | Hydrocortisone [19] |
| Prodrugs | By attaching the inactive ingredient to the medicine, the parent drug becomes more hydrophobic than the active form | Targeted | Designed specifically for a particular drug | Stavudine [20] |
| Chemical penetration enhancer | Direct interaction with the stratum corneum or the drug to improve drug penetration efficiency | Small-molecule drug transdermal penetration | Toxic; may cause skin irritation when used in high concentrations |
| Category | Materials of the Substrate | Mechanism | Preparation Methods | Drug | Treatable Diseases |
|---|---|---|---|---|---|
| Solid microneedles | Silicon, titanium, stainless steel, and other polymer materials insoluble in water | Does not contain drugs and leaves micropores in the skin during use. The active drug components penetrate the skin through these micropores, belonging to passive transport | Etching process, mechanical cutting | Acyclovir [52] | Herpes |
| Coated microneedles | Metal or polymer materials | After insertion into the skin, the drug coating dissolves from microneedles and quickly enters the tissue for one-step administration | Dip coating method, gas jet drying method, and spraying method | Bleomycin [53] | Plantar wart |
| Hollow microneedles | Polymer materials that are insoluble in water, including silicon, glass, stainless steel, etc. | The drug penetrates into the skin under pressure, acting like a microsyringe | Lithography technology | Synthetic [54] mRNA [55] | Skin diseases |
| Vaccinum [56] | |||||
| and tofacitinib citrate [57] | Psoriasis, alopecia areata, and vitiligo | ||||
| Soluble microneedles | Polymer materials with degradability and biocompatibility (e.g., maltose, carboxymethyl cellulose, etc.) | After insertion into the skin, the needle tip matrix remains in the skin while the drug is released, requiring only a one-step application | Hollow method, centrifugal method, fusion method, and casting method | Methotrexate [58] | Psoriasis |
| Cisplatin [59] | Superficial tumors | ||||
| Amphotericin B [60] | Mycosis | ||||
| Nanoemulsion [61] | |||||
| Cyclosporin A [62] | Psoriasis | ||||
| Tofacitinib citrate [57] | Psoriasis | ||||
| Triamcinolone Acetonide [63] | Psoriasis | ||||
| Hydrogel microneedles | Expandable hyperlinked polymer | By absorbing tissue fluid and expanding in the skin, porous microducts are formed through which drugs can be diffused into the skin microcirculation | Vacuum method, centrifugal method after crosslinking, and freeze-drying method | Sorbitol [64] | |
| Insulin [65] | Diabetic wound |
| Category | Penetration Method | Advantages | Limiations | Medication | Treatable Diseases |
|---|---|---|---|---|---|
| Liposomes | Intercellular pathway | low toxicity; biocompatibility and biodegradability; simple production process | Low stability; large volume and lack of elasticity | Adapalene and benzoyl peroxide combination [66] | Acne |
| Transfersomes | Intercellular pathway | Highly elastic; deformable | Hydrophobic drug loading is challenging; loading hydrophobic drugs poses challenges | Dexamethasone [67] | Skin disease |
| Triamcinolone [68] | Skin disease | ||||
| Ethosomes | Intercellular pathway | Suitable for hydrophilic and lipophilic drugs; can be used under both blocked and non-blocked conditions | Long-term impact still needs to be evaluated | Paclitaxel [69] | Skin cancer |
| Tacrolimus [70] | Atopic dermatitis | ||||
| Niosomes | Intercellular pathway | High stability | Tripterygium wilfordii [71] | Psoriasis | |
| Cubosomes | Intercellular pathway | Good adhesion performance; thermodynamic stability | Insufficient carrier materials; research on the lack of in vitro transdermal performance | Cinnamaldehyde [72] | |
| Binary ethosomes | Intercellular pathway | Vismodegib [73] | Skin cancer | ||
| SLNs | Accessory pathway | High stability; low toxicity; good flexibility | High moisture content; low drug loading; tends to gel | Mirtazapine [74] | Itch |
| Fluconazole [75] | Pityriasis rosea | ||||
| Tacrolimus [76] | Atopic dermatitis | ||||
| Combination of isotretinoin and α-tocopherol [77] | Acne | ||||
| NLCs | Accessory pathway | High drug-loading capacity; high stability; high biodegradability and biocompatibility; suitable for large-scale production | Tend to gel; lack of long-term stability data | Itraconazole [78] | Fungal infection |
| Mometasone furoate [79] | Psoriasis | ||||
| Adapalene combined with vitamin C [80] | Acne | ||||
| Curcumin [81] | Chronic inflammatory diseases, psoriasis, acne | ||||
| Nanoemulsions | Accessory pathway | Improve solubility; enhanced permeability | Irritability; low stability; low viscosity | Coumestrol [82] | Herpes |
| Methotrexate [83] | Psoriasis | ||||
| Microemulsions | Accessory pathway | Mass production; thermodynamic stability | Toxicity | Cyclosporin [84] | Psoriasis |
| Retinol palmitate [85] | Acne, skin aging, psoriasis | ||||
| Ivermectin [86] | Parasite infestation | ||||
| Polymer nanoparticles | Accessory pathway | High stability; targeting | Difficulties in large-scale production | Betamethasone Valerate [44] | Atopic dermatitis |
| Tacrolimus [87] | Atopic dermatitis | ||||
| Methotrexate [88] | Inflammatory diseases | ||||
| Polymer micelles | Intercellular pathway | Accurate release | Limited to lipophilic drugs; low drug-loading capacity | Imiquimod [89] | Basal cell carcinoma |
| Adapalene [90] | Acne | ||||
| Benzoyl Peroxide | Acne | ||||
| combination of Indomethacin and Resveratrol [91] | Skin cancer | ||||
| Dendrimers | Intercellular pathway | Increase the solubility of high lipophilic drugs; Targeting | Not suitable for hydrophilic drugs; cytotoxicity; high cost | 8-methoxypsoralene [54] | |
| Nanocrystals | Accessory pathway | High solubility; high drug-loading capacity; scalable production | Difficulty in optimizing size and dosage | Curcumin [92] | |
| Miconazole nitrate [93] | Fungal skin disease |
| Trade Name | Formulations | Indication | Time to Market |
|---|---|---|---|
| Ztlido | Patch | Relieve neuropathic pain associated with herpes zoster | February 2018 |
| Naftin | Gel | Foot moss | June 2013 |
| Aczone | Gel | Acne | July 2015 |
| Impoyz | Cream | plaque psoriasis | November 2017 |
| Vectical | Ointment | plaque psoriasis | January 2009 |
| Finacea | Foam agent | Lupus erythematosus pustule | July 2015 |
| Altreno | lotion | Acne vulgaris | November 2018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, T.; Tai, Z.; Shen, M.; Li, Y.; Yu, J.; Wang, J.; Zhu, Q.; Chen, Z. Advance and Challenges in the Treatment of Skin Diseases with the Transdermal Drug Delivery System. Pharmaceutics 2023, 15, 2165. https://doi.org/10.3390/pharmaceutics15082165
Cheng T, Tai Z, Shen M, Li Y, Yu J, Wang J, Zhu Q, Chen Z. Advance and Challenges in the Treatment of Skin Diseases with the Transdermal Drug Delivery System. Pharmaceutics. 2023; 15(8):2165. https://doi.org/10.3390/pharmaceutics15082165
Chicago/Turabian StyleCheng, Tingting, Zongguang Tai, Min Shen, Ying Li, Junxia Yu, Jiandong Wang, Quangang Zhu, and Zhongjian Chen. 2023. "Advance and Challenges in the Treatment of Skin Diseases with the Transdermal Drug Delivery System" Pharmaceutics 15, no. 8: 2165. https://doi.org/10.3390/pharmaceutics15082165
APA StyleCheng, T., Tai, Z., Shen, M., Li, Y., Yu, J., Wang, J., Zhu, Q., & Chen, Z. (2023). Advance and Challenges in the Treatment of Skin Diseases with the Transdermal Drug Delivery System. Pharmaceutics, 15(8), 2165. https://doi.org/10.3390/pharmaceutics15082165