In Vitro and Biological Evaluation of Oral Fast-Disintegrating Films Containing Ranitidine HCl and Syloid® 244FP-Based Ternary Solid Dispersion of Flurbiprofen
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Methods
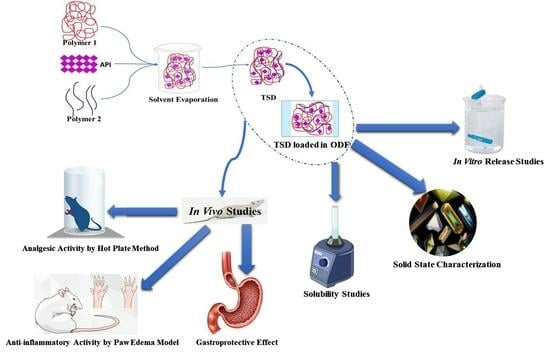
2.2.1. Development of Solid Dispersion
2.2.2. Development of Composite ODFs
2.3. Characterization of TSD and Composite ODFs
2.3.1. Micromeritic Properties of TSD
Powder Density
Compressibility Index (Ci)
Hausner’s Ratio
Angle of Repose
2.3.2. Solubility Study of TSD
2.3.3. Drug Content in TSD
2.3.4. Physical Parameters of Composite ODFs
Thickness
In Vitro Disintegration Time
2.3.5. Mechanical Parameters of Composite ODFs
Folding Endurance
Tensile Strength
2.3.6. Drug Content of Composite ODFs
2.3.7. Solid State Characterization of TSD and Composite ODFs
Scanning Electron Microscopy (SEM)
Fourier Transform Infrared Spectroscopy (FTIR)
X-ray Diffractometry (XRD)
2.4. In Vitro Drug Release Profile of TSD and Composite ODFs
2.5. In Vivo Study Protocols
2.5.1. In Vivo Analgesic Activity
2.5.2. In Vivo Anti-Inflammatory Activity
Detection of Pro-Inflammatory Cytokines
2.5.3. Assessment of Gastroprotective Effect
Gastric Lesion Index (GLI)
Histopathology
3. Results and Discussion
3.1. Evaluation of Binary and Ternary Solid Dispersions
3.2. Composite ODF Properties
3.3. Physical Parameters of Composite ODFs
3.3.1. Thickness
3.3.2. In Vitro Disintegration Time (DT)
3.4. Mechanical Parameters of Composite ODFs
3.4.1. Folding Endurance
3.4.2. Tensile Strength
3.5. Solid State Characterization of TSD and Composite ODF
3.5.1. SEM
3.5.2. FTIR
3.5.3. XRD
3.6. In Vitro Drug Release of TSD and Composite ODFs
3.7. In Vivo Study
3.7.1. Assay of Analgesic Activity
3.7.2. In Vivo Anti-Inflammatory Activity
Detection of Pro-Inflammatory Cytokines
3.7.3. Evaluation of Gastroprotective Activity
Gastric Lesion Index (GLI)
Histopathological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Abbasi, M.; Mousavi, M.J.; Jamalzehi, S.; Alimohammadi, R.; Bezvan, M.H.; Mohammadi, H.; Aslani, S. Strategies toward rheumatoid arthritis therapy; the old and the new. J. Cell. Physiol. 2019, 234, 10018–10031. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; John, P.; Fan, K.-H.; Bhatti, A.; Aziz, W.; Ahmed, B.; Feingold, E.; Demirci, F.Y.; Kamboh, M.I. Investigating the GWAS-Implicated Loci for Rheumatoid Arthritis in the Pakistani Population. Dis. Markers 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Akhter, E.; Bilal, S.; Haque, U. Prevalence of arthritis in India and Pakistan: A review. Rheumatol. Int. 2011, 31, 849–855. [Google Scholar] [CrossRef]
- Allesø, M.; Chieng, N.; Rehder, S.; Rantanen, J.; Rades, T.; Aaltonen, J. Enhanced dissolution rate and synchronized release of drugs in binary systems through formulation: Amorphous naproxen–cimetidine mixtures prepared by mechanical activation. J. Control. Release 2009, 136, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Tran, T.H.; Kim, S.Y.; Woo, K.B.; Choi, Y.J.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Preparation and characterization of fast dissolving flurbiprofen and esomeprazole solid dispersion using spray drying technique. Int. J. Pharm. 2016, 502, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G. Ranitidine Hydrochloride. Saunders Handbook of Veterinary Drugs, 4th ed.; Small and Large Animal; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Basit, A.W.; Lacey, L.F. Colonic metabolism of ranitidine: Implications for its delivery and absorption. Int. J. Pharm. 2001, 227, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Alhayali, A.; Vuddanda, P.R.; Velaga, S. Silodosin oral films: Development, physico-mechanical properties and in vitro dissolution studies in simulated saliva. J. Drug Deliv. Sci. Technol. 2019, 53, 101122. [Google Scholar] [CrossRef]
- Visser, J.C.; Dohmen, W.M.; Hinrichs, W.L.; Breitkreutz, J.; Frijlink, H.W.; Woerdenbag, H.J. Quality by design approach for optimizing the formulation and physical properties of extemporaneously prepared orodispersible films. Int. J. Pharm. 2015, 485, 70–76. [Google Scholar] [CrossRef]
- Łyszczarz, E.; Hofmanova, J.; Szafraniec-Szczęsny, J.; Jachowicz, R. Orodispersible films containing ball milled aripiprazole-poloxamer® 407 solid dispersions. Int. J. Pharm. 2020, 575, 118955. [Google Scholar] [CrossRef]
- Senta-Loys, Z.; Bourgeois, S.; Valour, J.-P.; Briançon, S.; Fessi, H. Orodispersible films based on amorphous solid dispersions of tetrabenazine. Int. J. Pharm. 2017, 518, 242–252. [Google Scholar] [CrossRef]
- Sohn, J.S.; Choi, J.-S. Febuxostat solubilization and stabilization approach using solid dispersion method: Synergistic effect of dicalcium phosphate dehydrate and chitosan. Int. J. Biol. Macromol. 2023, 253, 127266. [Google Scholar] [CrossRef] [PubMed]
- Tambe, S.; Jain, D.; Meruva, S.K.; Rongala, G.; Juluri, A.; Nihalani, G.; Mamidi, H.K.; Nukala, P.K.; Bolla, P.K. Recent advances in amorphous solid dispersions: Preformulation, formulation strategies, technological advancements and characterization. Pharmaceutics 2022, 14, 2203. [Google Scholar] [CrossRef] [PubMed]
- De Caro, V.; Ajovalasit, A.; Sutera, F.M.; Murgia, D.; Sabatino, M.A.; Dispenza, C. Development and characterization of an amorphous solid dispersion of furosemide in the form of a sublingual bioadhesive film to enhance bioavailability. Pharmaceutics 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.; An, J.; Park, C.; Kim, D.; Lee, J. Design and characterization of phosphatidylcholine-based solid dispersions of aprepitant for enhanced solubility and dissolution. Pharmaceutics 2020, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Waters, L.J.; Parkes, G.M.; Shahzad, Y. Microwave processed solid dispersions for enhanced dissolution of gemfibrozil using non-ordered mesoporous silica. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 428–435. [Google Scholar] [CrossRef]
- Madan, J.R.; Patil, S.; Mathure, D.; Bahirat, S.P.; Awasthi, R.; Dua, K. Improving dissolution profile of poorly water-soluble drug using non-ordered mesoporous silica. Marmara Pharm. J. 2018, 22, 249–258. [Google Scholar] [CrossRef]
- Özdoğan, A.I.; Akca, G.; Şenel, S. Development and in vitro evaluation of gel formulation of atorvastatin solid dispersions. J. Drug Deliv. Sci. Technol. 2021, 61, 102199. [Google Scholar] [CrossRef]
- Eedara, B.B.; Nyavanandi, D.; Narala, S.; Veerareddy, P.R.; Bandari, S. Improved Dissolution Rate and Intestinal Absorption of Fexofenadine Hydrochloride by the Preparation of Solid Dispersions: In Vitro and In Situ Evaluation. Pharmaceutics 2021, 13, 310. [Google Scholar] [CrossRef]
- Shi, X.; Ding, Z.; Bao, J.; Wang, C. Preparation and In Vitro/Vivo Evaluation of New Celecoxib Solid Dispersions with Co-Carrier Containing Aerosil and Poloxamer 188. Pharm. Chem. J. 2021, 54, 1033–1039. [Google Scholar] [CrossRef]
- Alruwaili, N.K.; Zafar, A.; Imam, S.S.; Alharbi, K.S.; Alshehri, S.; Elsaman, T.; Alomar, F.A.; Akhtar, S.; Fahmy, U.A.; Alhakamy, N.A.; et al. Formulation of amorphous ternary solid dispersions of dapagliflozin using PEG 6000 and Poloxamer 188: Solid-state characterization, ex vivo study, and molecular simulation assessment. Drug Dev. Ind. Pharm. 2020, 46, 1458–1467. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Ikeda, N.; Tahara, K.; Takeuchi, H. Mechanical characteristics of orally disintegrating films: Comparison of folding endurance and tensile properties. Int. J. Pharm. 2020, 589, 119876. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-L.; Zheng, S.-D.; Liu, Y.-Y.; Cui, W.-Q.; Hao, M.-Q.; God’spower, B.-O.; Chen, X.-Y.; Li, Y.-H. Albendazole solid dispersions prepared using PEG6000 and Poloxamer188: Formulation, characterization and in vivo evaluation. Pharm. Dev. Technol. 2020, 25, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, Y.; Zhou, L.; Jiang, Z.; Yang, X.; Feng, Y. Osmotic pump tablets with solid dispersions synergized by hydrophilic polymers and mesoporous silica improve in vitro/in vivo performance of cilostazol. Int. J. Pharm. 2020, 588, 119759. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Lee, S.-E.; Jang, W.S.; Byeon, J.C.; Park, J.-S. Solid dispersion of dutasteride using the solvent evaporation method: Approaches to improve dissolution rate and oral bioavailability in rats. Mater. Sci. Eng. C 2018, 90, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yan, J.; Ren, L.; Xue, M.; Yuan, Z.; Wang, T.; Yan, Z.; Yin, L.; Yang, L.; Qin, C. Preparation and evaluation of orally disintegrating film containing donepezil for Alzheimer disease. J. Drug Deliv. Sci. Technol. 2019, 54, 101321. [Google Scholar] [CrossRef]
- Chandra, A.; Chondkar, A.D.; Shirodkar, R.; Lewis, S.A. Rapidly dissolving lacidipine nanoparticle strips for transbuccal administration. J. Drug Deliv. Sci. Technol. 2018, 47, 259–267. [Google Scholar] [CrossRef]
- Sharma, R.; Kamboj, S.; Singh, G.; Rana, V. Development of aprepitant loaded orally disintegrating films for enhanced pharmacokinetic performance. Eur. J. Pharm. Sci. 2016, 84, 55–69. [Google Scholar] [CrossRef]
- Islam, N.; Irfan, M.; Khan, S.-U.; Syed, H.K.; Iqbal, M.S.; Khan, I.U.; Mahdy, A.; Raafat, M.; Hossain, M.A.; Inam, S.; et al. Poloxamer-188 and d-α-Tocopheryl Polyethylene Glycol Succinate (TPGS-1000) Mixed Micelles Integrated Orodispersible Sublingual Films to Improve Oral Bioavailability of Ebastine; In Vitro and In Vivo Characterization. Pharmaceutics 2021, 13, 54. [Google Scholar] [CrossRef]
- Fasihi, H.; Fazilati, M.; Hashemi, M.; Noshirvani, N. Novel carboxymethyl cellulose-polyvinyl alcohol blend films stabilized by Pickering emulsion incorporation method. Carbohydr. Polym. 2017, 167, 79–89. [Google Scholar] [CrossRef]
- Mehmood, Y.; Khan, I.U.; Shahzad, Y.; Khan, R.U.; Iqbal, M.S.; Khan, H.A.; Khalid, I.; Yousaf, A.M.; Khalid, S.H.; Asghar, S. In-vitro and in-vivo evaluation of velpatasvir-loaded mesoporous silica scaffolds. A prospective carrier for drug bioavailability enhancement. Pharmaceutics 2020, 12, 307. [Google Scholar] [CrossRef]
- Gajra, B.; Dalwadi, C.; Patel, R. Formulation and optimization of itraconazole polymeric lipid hybrid nanoparticles (Lipomer) using box behnken design. DARU J. Pharm. Sci. 2015, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Oktay, A.N.; Ilbasmis-Tamer, S.; Han, S.; Uludag, O.; Celebi, N. Preparation and in vitro/in vivo evaluation of flurbiprofen nanosuspension-based gel for dermal application. Eur. J. Pharm. Sci. 2020, 155, 105548. [Google Scholar] [CrossRef]
- Macdonald, A.D.; Woolfe, G.; Bergel, F.; Morrison, A.L.; Rinderknecht, H. Analgesic action of pethidine derivatives and related compounds. Br. J. Pharmacol. Chemother. 1946, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.; Zia-Ur-Rehman, M.; Munir, R.; Jamil, N.; Ishtiaq, S.; Saleem, R.S.Z.; Elsegood, M.R.J. (Benzylideneamino) triazole–Thione Derivatives of Flurbiprofen: An Efficient Microwave-Assisted Synthesis and In Vivo Analgesic Potential. ACS Omega 2021, 6, 31348–31357. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-X.; Qian, P.; Guo, Y.-T.; Gu, L.; Jurat, J.; Bai, Y.; Zhang, D.-F. Myrtenal and β-caryophyllene oxide screened from Liquidambaris Fructus suppress NLRP3 inflammasome components in rheumatoid arthritis. BMC Complement. Med. Ther. 2021, 21, 242. [Google Scholar] [CrossRef] [PubMed]
- Al-Thamarani, S.; Gardouh, A. Enhanced oral bioavailability and gastroprotective effect of ibuprofen through mixed polymer–lipid nanoparticles. Ther. Deliv. 2021, 12, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, E.S.; Steidel, A.; Rosenblatt, K.M.; Gessner, D.; Liepold, B. Assessment of the amorphous solid dispersion erosion behavior following a novel small-scale predictive approach. Eur. J. Pharm. Sci. 2021, 158, 105682. [Google Scholar] [CrossRef]
- Triboandas, H.; Pitt, K.; Bezerra, M.; Ach-Hubert, D.; Schlindwein, W. Itraconazole Amorphous Solid Dispersion Tablets: Formulation and Compaction Process Optimization Using Quality by Design Principles and Tools. Pharmaceutics 2022, 14, 2398. [Google Scholar] [CrossRef]
- Rashid, A.; Khan, I.U.; Khalid, S.H.; Asghar, S.; Munir, M.U. Development and evaluation of oral fast disintegrating film of ranitidine HCl by solvent casting method. Pak. J. Pharm. Sci. 2021, 34, 1527–1534. [Google Scholar]
- Lai, K.L.; Fang, Y.; Han, H.; Li, Q.; Zhang, S.; Li, H.Y.; Chow, S.F.; Lam, T.N.; Lee, W.Y.T. Orally-dissolving film for sublingual and buccal delivery of ropinirole. Colloids Surf. B Biointerfaces 2018, 163, 9–18. [Google Scholar] [CrossRef]
- Banarjee, T.; Ansari, V.A.; Singh, S.; Mahmood, T.; Akhtar, J. A review on fast dissolving films for buccal delivery of low dose drugs. Int. J. Life Sci. Rev 2015, 1, 117–123. [Google Scholar]
- Dogan, N.; McHugh, T. Effects of microcrystalline cellulose on functional properties of hydroxy propyl methyl cellulose microcomposite films. J. Food Sci. 2007, 72, E016–E022. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Panraksa, P.; Udomsom, S.; Rachtanapun, P.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. Hydroxypropyl Methylcellulose E15: A Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer. Polymers 2020, 12, 2666. [Google Scholar] [CrossRef] [PubMed]
- Bodini, R.B.; Guimarães, J.d.G.L.; Monaco-Lourenço, C.A.; de Carvalho, R.A. Effect of starch and hydroxypropyl methylcellulose polymers on the properties of orally disintegrating films. J. Drug Deliv. Sci. Technol. 2019, 51, 403–410. [Google Scholar] [CrossRef]
- Chonkar, A.D.; Rao, J.V.; Managuli, R.S.; Mutalik, S.; Dengale, S.; Jain, P.; Udupa, N. Development of fast dissolving oral films containing lercanidipine HCl nanoparticles in semicrystalline polymeric matrix for enhanced dissolution and ex vivo permeation. Eur. J. Pharm. Biopharm. 2016, 103, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Brniak, W.; Maślak, E.; Jachowicz, R. Orodispersible films and tablets with prednisolone microparticles. Eur. J. Pharm. Sci. 2015, 75, 81–90. [Google Scholar] [CrossRef]
- Bala, R.; Khanna, S.; Pawar, P.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67. [Google Scholar] [CrossRef]
- Speer, I.; Lenhart, V.; Preis, M.; Breitkreutz, J. Prolonged release from orodispersible films by incorporation of diclofenac-loaded micropellets. Int. J. Pharm. 2019, 554, 149–160. [Google Scholar] [CrossRef]
- Kawadkar, J.; Chauhan, M.K. Intra-articular delivery of genipin cross-linked chitosan microspheres of flurbiprofen: Preparation, characterization, in vitro and in vivo studies. Eur. J. Pharm. Biopharm. 2012, 81, 563–572. [Google Scholar] [CrossRef]
- Liw, J.J.; Teoh, X.-Y.; Teoh, A.X.Y.; Chan, S.-Y. The Effect of Carrier-Drug Ratios on Dissolution Performances of Poorly Soluble Drug in Crystalline Solid Dispersion System. J. Pharm. Sci. 2022, 111, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gaitano, R.O.; Calvo, N.L.; Narda, G.E.; Kaufman, T.S.; Maggio, R.M.; Brusau, E.V. Preparation and physical characterization of a diclofenac-ranitidine co-precipitate for improving the dissolution of diclofenac. J. Pharm. Sci. 2016, 105, 1258–1268. [Google Scholar] [CrossRef]
- Chieng, N.; Aaltonen, J.; Saville, D.; Rades, T. Physical characterization and stability of amorphous indomethacin and ranitidine hydrochloride binary systems prepared by mechanical activation. Eur. J. Pharm. Biopharm. 2009, 71, 47–54. [Google Scholar] [CrossRef]
- Pardhi, V.; Jain, K. Impact of binary/ternary solid dispersion utilizing poloxamer 188 and TPGS to improve pharmaceutical attributes of bedaquiline fumarate. J. Drug Deliv. Sci. Technol. 2021, 62, 102349. [Google Scholar] [CrossRef]
- Akhlaq, M.; Maryam, F.; Elaissari, A.; Ullah, H.; Adeel, M.; Hussain, A.; Ramzan, M.; Ullah, O.; Danish, M.Z.; Iftikhar, S.; et al. Pharmacokinetic evaluation of quetiapine fumarate controlled release hybrid hydrogel: A healthier treatment of schizophrenia. Drug Deliv. 2018, 25, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, S.; Sethi, S.; Rana, V. A spray dried nelfinavir mesylate particles for enhanced oral bioavailability: Systematic formulation optimization and in-vivo performance. Colloids Surf. B Biointerfaces 2019, 176, 288–299. [Google Scholar] [CrossRef]
- Sievens-Figueroa, L.; Bhakay, A.; Jerez-Rozo, J.I.; Pandya, N.; Romañach, R.J.; Michniak-Kohn, B.; Iqbal, Z.; Bilgili, E.; Davé, R.N. Preparation and characterization of hydroxypropyl methyl cellulose films containing stable BCS Class II drug nanoparticles for pharmaceutical applications. Int. J. Pharm. 2012, 423, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.M.; Jang, D.-J.; Kim, Y.C.; Yoon, J.-H.; Min, K.A.; Maeng, H.-J.; Cho, K.H. Flurbiprofen-loaded solid SNEDDS preconcentrate for the enhanced solubility, in-vitro dissolution and bioavailability in rats. Pharmaceutics 2018, 10, 247. [Google Scholar] [CrossRef]
- Oktay, A.N.; Ilbasmis-Tamer, S.; Karakucuk, A.; Celebi, N. Screening of stabilizing agents to optimize flurbiprofen nanosuspensions using experimental design. J. Drug Deliv. Sci. Technol. 2020, 57, 101690. [Google Scholar] [CrossRef]
- Yamamoto, E.; Takeda, Y.; Ando, D.; Koide, T.; Amano, Y.; Miyazaki, S.; Miyazaki, T.; Izutsu, K.-I.; Kanazawa, H.; Goda, Y. Discrimination of ranitidine hydrochloride crystals using X-ray micro-computed tomography for the evaluation of three-dimensional spatial distribution in solid dosage forms. Int. J. Pharm. 2021, 605, 120834. [Google Scholar] [CrossRef]
- Lotfy, N.S.; Borg, T.M.; Mohamed, E.A. The Promising Role of Chitosan–Poloxamer 188 Nanocrystals in Improving Diosmin Dissolution and Therapeutic Efficacy against Ferrous Sulfate-Induced Hepatic Injury in Rats. Pharmaceutics 2021, 13, 2087. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Shiono, K.; Otsuka, Y.; Shimada, Y.; Terada, H.; Komatsu, K.; Goto, S. Molecular recognizable ion-paired complex formation between diclofenac/indomethacin and famotidine/cimetidine regulates their aqueous solubility. Int. J. Pharm. 2020, 590, 119841. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, D.A.; Keshavarao, K.P. Fast disintegrating films containing anastrozole as a dosage form for dysphagia patients. Arch. Pharmacal Res. 2012, 35, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Babu, N.P.; Pandikumar, P.; Ignacimuthu, S. Pandikumar, and S. Ignacimuthu, Anti-inflammatory activity of Albizia lebbeck Benth., an ethnomedicinal plant, in acute and chronic animal models of inflammation. J. Ethnopharmacol. 2009, 125, 356–360. [Google Scholar] [CrossRef]
- Jin, J.; He, H.; Zhang, X.; Wu, R.; Gan, L.; Li, D.; Lu, Y.; Wu, P.; Wong, W.-L.; Zhang, K. The in vitro and in vivo study of oleanolic acid indole derivatives as novel anti-inflammatory agents: Synthesis, biological evaluation, and mechanistic analysis. Bioorganic Chem. 2021, 113, 104981. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, H.; Niu, X.; Fan, T.; Mu, Q.; Li, H. Protective effect of tetrahydrocoptisine against ethanol-induced gastric ulcer in mice. Toxicol. Appl. Pharmacol. 2013, 272, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hu, Y.; Wu, T.; Feng, Y.; Jiang, C.; Du, H.; Lu, S. Apigenin-oxymatrine binary co-amorphous mixture: Enhanced solubility, bioavailability, and anti-inflammatory effect. Food Chem. 2022, 373, 131485. [Google Scholar] [CrossRef]













| Code | HPMC E5 | PG | Pearlitol Flash® | FBP TSD | RHCl | Dis. Water |
|---|---|---|---|---|---|---|
| S0 | 0.3 g | 0.03 g | 0.03 g | 10 mL | ||
| S1 | 0.3 g | 0.03 g | 0.03 g | 0.15 g | 10 mL | |
| S2 | 0.3 g | 0.03 g | 0.03 g | 0.15 g | 0.075 g | 10 mL |
| Code | Thickness (µm) | Disintegration Time (s) | Folding Endurance | Tensile Strength (N/mm2) |
|---|---|---|---|---|
| S0 | 35 ± 0.58 | 19.51 ± 0.21 | >300 | 8.3 ± 1.4 |
| S1 | 62 ± 0.61 | 15.78 ± 0.94 | >300 | 6.17 ± 0.31 |
| S2 | 66.4 ± 0.67 | 15.02 ± 0.9 | >300 | 5.3 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, A.; Irfan, M.; Kamal, Y.; Asghar, S.; Khalid, S.H.; Hussain, G.; Alshammari, A.; Albekairi, T.H.; Alharbi, M.; Khan, H.U.; et al. In Vitro and Biological Evaluation of Oral Fast-Disintegrating Films Containing Ranitidine HCl and Syloid® 244FP-Based Ternary Solid Dispersion of Flurbiprofen. Pharmaceutics 2024, 16, 164. https://doi.org/10.3390/pharmaceutics16020164
Rashid A, Irfan M, Kamal Y, Asghar S, Khalid SH, Hussain G, Alshammari A, Albekairi TH, Alharbi M, Khan HU, et al. In Vitro and Biological Evaluation of Oral Fast-Disintegrating Films Containing Ranitidine HCl and Syloid® 244FP-Based Ternary Solid Dispersion of Flurbiprofen. Pharmaceutics. 2024; 16(2):164. https://doi.org/10.3390/pharmaceutics16020164
Chicago/Turabian StyleRashid, Aisha, Muhammad Irfan, Yousaf Kamal, Sajid Asghar, Syed Haroon Khalid, Ghulam Hussain, Abdulrahman Alshammari, Thamer H. Albekairi, Metab Alharbi, Hafeez Ullah Khan, and et al. 2024. "In Vitro and Biological Evaluation of Oral Fast-Disintegrating Films Containing Ranitidine HCl and Syloid® 244FP-Based Ternary Solid Dispersion of Flurbiprofen" Pharmaceutics 16, no. 2: 164. https://doi.org/10.3390/pharmaceutics16020164
APA StyleRashid, A., Irfan, M., Kamal, Y., Asghar, S., Khalid, S. H., Hussain, G., Alshammari, A., Albekairi, T. H., Alharbi, M., Khan, H. U., Chauhdary, Z., Vandamme, T. F., & Khan, I. U. (2024). In Vitro and Biological Evaluation of Oral Fast-Disintegrating Films Containing Ranitidine HCl and Syloid® 244FP-Based Ternary Solid Dispersion of Flurbiprofen. Pharmaceutics, 16(2), 164. https://doi.org/10.3390/pharmaceutics16020164