Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects
Abstract
:1. Introduction
2. Materials and Methods
3. 3D Printing in Pharmaceutical Areas
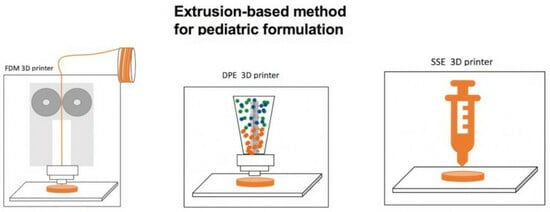
3.1. Comparison of the Representative 3DP Technologies in Pharmaceutical Applications
3.1.1. Fused Deposition Modeling (FDM)
3.1.2. Direct Powder Extrusion Technology (DPE)
3.1.3. Semi-Solid Extrusion (SSE)
3.1.4. Inkjet Printing
3.1.5. Stereolithography (SLA)
3.1.6. Selective Laser Sintering (SLS)
3.2. Availability of Pharmaceutical Materials
3.3. Upcoming 3DP Systems for Personalized Medicine
3.3.1. FabRx Ltd.
3.3.2. Triastek Inc.
3.4. State of the Art of the Clinical Development of 3D-Printed Drugs
| 3D Printing Systems | GMP-Manufactured Pharmaceutical Printer | FDA IND Clearance 3DP Drug | FDA Approval and Commercially Available 3DP Drug | Ref. | |
|---|---|---|---|---|---|
| Extrusion-based | |||||
| Fused deposition modeling | FabRx Ltd. (London, UK) ° | ||||
| DiHeSys (Schwäbisch Gmünd, Germany) ° | |||||
| Semi-solid extrusion | FabRx Ltd. (London, UK) ° | Isoleucine chewable tablets, 2019 | [38] | ||
| Craft Health (Tulsa, USA) ° | |||||
| Direct extrusion printing | FabRx Ltd. (London, UK) ° | ||||
| Melt extrusion deposition (MED) | Triastek Inc. (Nanjing, China) * | T19, 2021 T20, 2022 T21, 2022 | [42] | ||
| Powder bed fusion | |||||
| Binder jetting | Aprecia Pharmaceuticals (Blue Ash, USA) * | Spritam®, 2015 | [43] | ||
| Selective laser sintering | FabRx Ltd. (London, UK) ° | ||||
| Vat polymerization | |||||
| Stereolithography | FabRx Ltd. (London, UK) ° | ||||
| Direct light processing | |||||
| Inkjet printing | DiHeSys (Schwäbisch Gmünd, Germany) ° | ||||
| Drop-on-solid | |||||
| Drop-on-drop | |||||
| Lamination-based | |||||
| Laminated object manufacturing | |||||
4. Extrusion-Based Discoveries in Pediatric 3D Printing Formulations
4.1. Palatable Personalized Pediatric Forms
4.2. Pediatric Formulation Release and Size
4.3. Taste Masking
4.4. Challenge of Poorly Water-Soluble Drugs
4.5. HME and FDM Coupling Potential
| Technique | Dosage Form | API | Polymer (s) and Other Excipients | T °C and Extruder Model | T °C and 3D Printer Model | Patient Acceptability | Target Release Profile | Ref. |
|---|---|---|---|---|---|---|---|---|
| FDM | Chewable tablets | Indomethacin BCS class II | PEG 6000, HPMCAS | 40–120 °C Eurolab16, Thermo Fisher Scientific (Waltham, MA, USA) | 165 °C HD2xR Airwolf (Costa Mesa, CA, USA) | “Candy-like” with various shapes and taste masking | Immediate release | [46] |
| Minitablets | Baclofen BCS class III | PVA, sorbitol | 160 °C Process 11, Thermo Scientific | 170 °C Makerbot Industries (New York, NY, USA) | Size suitable for children | Sustained release | [56] | |
| Tablets | Rufinamide BCS class II | Kollidon® VA64, HPMC, Soluplus®, triacetin, Gelucire® (44/14 and 48/16) | 120–150 °C Filabot EX2, Barre, VT, USA | 215 °C CraftBot3, Budapest, Hungary | Improved dissolution | Enhanced drug release | [63] | |
| Tablets | Caffeine Citrate BCS class I | Eudragit® EPO, HPC (LF and HF), HPMC K4M | 155 °C Thermo Fischer | 200 °C Prusa i3 3D printer (Prague, Czech Republic) | Taste-masked formulation | Modified release | [48] | |
| Tablets | Amiodarone BCS class II | D-sorbitol, PEO, glycerol, SiO2 | 50–80 °C Thermo Fischer | 80 °C Prusa i3 Mk3S 3D printer | Reproducible filament batches for oral formulation | Immediate release | [65] | |
| Tablets | Lumefantrine BCS class II | Eudragit® EPO, Xylisorb 300, maltodextrin | >100 °C Pharma11, Thermo Scientific | 160 °C BoltPro 3D-printer, Alphen aan den Rijn, The Netherlands | Size suitable for children > 6 years | Immediate release | [63] | |
| Minitablets | Caffeine and propranolol hydrochloride BCS class II and I | HPMC, HPC, PEG 6000 | HPMC 120 °C//HPC 90 °C Turbula T2F, System Schatz, Lauterhofen, Germany | HPMC 200 °C//HPC 170 °C Ultimaker, Zaltbommel, The Netherlands | Size suitable for children | Dependent on tablet size | [55] | |
| Chewable tablets | Diphenhydramine Hydrochloride BCS class I | Hydroxypropyl cellulose, Gelucire® 48/16, sucralose, strawberry flavour | 120 °C Eurolab16, Thermo Fisher | 165 °C Ultimaker 3 extended, The Netherlands | Taste masking | Immediate release | [61] | |
| Tablets | Hydrocortisone BCS class I | Eudragit® EPO, Triethyl citrate, talc, sodium stearyl fumarate, TiO2 | 110 °C Thermo Fisher | 140 °C MarkerBot Industries (New York, NY, USA) | Size suitable for children and lower drug contents | Immediate release | [67] | |
| Tablets | Hydrocortisone BCS class I | Kollidon® VA64, Affinsol® 15LV, PEG 6000, Sorbitol, Triethyl citrate | 90–140 °C Thermo Fisher | 155–180–210 °C Prusa i3MK3 3D desktop printer (Czech Republic) | Pediatric-friendly printed shapes | Personalized sustained release | [49] | |
| FDM and DPE | Minitablets | Ritonavir and Lopinavir BCS class IV | PEG 4000, HPMCAS, magnesium stearate | 120 °C Noztek (Shoreham, UK) | 80 °C DPE: Hyrel 3D SR (Norcross, GA, USA) | HIV-infected children | Zero-order sustained release profile over 24h | [66] |
| DPE | Tablets | Praziquantel BCS class II | Kollidon® VA 64, Surfactants (Span™ 20 or Kolliphor® SLS) | 50–180 °C Thermo Fisher | 140–200 °C physical mixtures; 130–140 °C pellets and milled powder obtained from HME FabRx Ltd., London, UK | Masking of taste | Increased API release | [20] |
| Minitablets | Budesonide BCS class II | PEG6000, HPMC, HP-β-CD | ------- | 180 °C 3DForMe® printer (Farmalabor, Italy) | Size and dose suitable | Sustained colonic release | [58] | |
| Orodisperdible films | Clobetasol propionate BCS class II | PEO 100.000, HPMC, HP-β-CD, chitosan | ------- | 170 °C 3DForMe® Printer (Farmalabor, Italy) | Mucoadhesive films | Sustained release | [68] | |
| HMPE | Chewable tablets | Ibuprofen BCS class II | Soluplus®, Kollidon ® VA64, Eudragit® EPO | ------- | IBU/VA64: 120 °C, IBU/EPO: 90 °C IBU/Soluplus: 105 °C Bio-X (Celink, Sweden) | Taste masking | Specific release profile tailored to individual patient | [69] |
| SSE | Chewable printlets | Isoleucine BCS class II | Sucrose, pectin, maltodextrin, water, flavourings and colorants | ------- | 70 °C The magic candy factory | Patient acceptability and colour and flavour patient choice | Immediate release | [38] |
| Chewable dosage form | Paracetamol and Ibuprofen BCS class I and class II | Glycerol, gelatin, locust bean gum, water, food dyes | ------- | 75 °C Makerbot Industries | Lego™-like brick shapes | Dual drug release | [51] | |
| Chewable dosage form | Paracetamol and Ibuprofen BCS class I and class II | Bitter chocolate, corn syrup | ------- | 45 °C 3D Food Printer (Model 3C10A) | Cartoon character shapes and palate acceptability | Immediate release for paracetamol and pH- dependent for Ibuprofen | [52] | |
| Chewable dosage form | Ranatidine BCS class III | Xanthan gum, strawberry essence, gelatin, corn starch, carrageenan, food colouring | ------- | 37 °C A syringe-based extrusion 3D printer (bIDO-I, Idonial Technological Centre, Gijón, Spain) | To improve the organoleptic characteristics and palatability of the formula | Immediate- and sustained-release profiles | [54] | |
| Tablets | Furosemide and sildenafil citrate BCS class IV and class II | Gelucire® 48/16, Polysorbate 80 | ------- | 40–41 °C A modified Prusa i3 MK2 3DP | Smaller batch size suitable for children | Immediate release | [21] | |
| Oro-dispersible film | Diclofenac BCS class II | Maltodextrin, glycerol, TiO2, mint and licorice flavors, sucralose | ------- | 95 °C A modify FDM 3DP (Futura Group Srl, Gallarate, Italy) | Taste-masking | Immediate release | [59] | |
| Tablets | Isoniazid BCS class III | Corn starch | ------- | Cellink-ink- redible printer (Gothenburg, Sweden) | Pediatric-friendly formulations | Immediate release | [50] | |
| Chewable tablets | Propranolol hydrochloride BCS class I | Gelatin, carrageenan, sodium carboxymethyl starch | ------- | 80 °C FoodBot (Hangzhou, China) | Size and shape suitable | Immediate release | [60] | |
| Oro-dispersible tablets | Loratidine BCS class II | Cellulose, mannitol, carboxy methyl starch sodium, Kollidon® 30 | ------- | Suitable for elders and children with dysphagia | Immediate release | [70] | ||
| Oro-dispersible minitablet | Carbamazepine BCS class II | Ac-Di-Sol®, Kollidon® 30, sucralose, lactose monohydrate | ------- | Bioprinter Bio XTM (Cellink, Gothenburg, Sweden) | Taste masking | Sustained release | [71] | |
| Tablets | Caffeine BCS class I | Sodium alginate, HPMC, sodium croscarmellose | ------- | Body-weight-adjusted dosage, neonate targets | Rapid release | [72] | ||
| Chewable tablets | Amlodipine besylate BCS class I | Carboxymethyl cellulose sodium, sodium starch Glycolate, glycerin | ------- | Taste masking and cartoon shape | Immediate release | [73] | ||
| Printles | Hydrochlorothiazide BCS class III/IV | Sucrose, PVP, banana flavoring essence, croscarmellose sodium, lactose | ------- | FabRx M3dimaker (London, UK) | Personalized medicines with high drug load | Oro- dispersible printlets | [74,75] | |
| Tablets | Levetiracetam | Kollicoat® IR, | ------- | 27 °C 3D-Bioplotter (EnvisionTec, Gladbeck, Germany) | Dose suitable | Immediate release | [76] |
5. Benefits and Limitations of Various Aspects of 3D Printing Formulation
5.1. Benefits of 3D Printing of Pediatric Formulations
5.2. Regulatory Landscape
5.3. Market Landscape
5.4. Future Role of Pharmacy in 3D Pharmaceutical Formulation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hull, C.W.; UVP, Inc. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4575330, 1 March 1986. [Google Scholar]
- Hull, C.W. Method for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4929402, 29 May 1990. [Google Scholar]
- Hull, C.W.; Spence, S.T.; Albert, D.J.; Smalley, D.R.; Harlow, R.A.; Stinebaugh, P.; Tarnoff, H.L.; Nguyen, H.D.; Lewis, C.W.; Vorgitch, T.J.; et al. Method and Apparatus for Production of High Resolution Three-Dimensional Objects by Stereolithography. U.S. Patent 5184307, 2 February 1993. [Google Scholar]
- Crump, S.S.; Muir, A.E.P.D. Creating Three-Dimensional Objects. U.S. Patent 005121329A, 9 June 1992. [Google Scholar]
- Prasad, L.K.; Smyth, H. 3D Printing technologies for drug delivery: A review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Quodbach, J.; Bogdahn, M.; Breitkreutz, J.; Chamberlain, R.; Eggenreich, K.; Elia, A.G.; Gottschalk, N.; Gunkel-Grabole, G.; Hoffmann, L.; Kapote, D.; et al. Quality of FDM 3D Printed Medicines for Pediatrics: Considerations for Formulation Development, Filament Extrusion, Printing Process and Printer Design. Ther. Innov. Regul. Sci. 2022, 56, 910–928. [Google Scholar] [CrossRef]
- Karalia, D.; Siamidi, A.; Karalis, V.; Vlachou, M. 3D-Printed Oral Dosage Forms: Mechanical Properties, Computational Approaches and Applications. Pharmaceutics 2021, 13, 1401. [Google Scholar] [CrossRef]
- Kaval, B.; Kapkl, E.; Kaynak, M.S. Release kinetics of 3D printed oral solid dosage forms: An overview. Eur. J. Life Sci. 2022, 1, 70–88. [Google Scholar] [CrossRef]
- Spritam East Windsor, N.J. Aprecia Pharmaceuticals Company. 2015. Available online: https://www.aprecia.com/news/fda-approves-the-first-3d-printed-drug-product (accessed on 11 December 2023).
- Preis, M.; Öblom, H. 3D-Printed Drugs for Children—Are We Ready Yet? AAPS Pharm. Sci. Tech. 2017, 18, 303–308. [Google Scholar] [CrossRef]
- CAS Insight, American Chemistry Society. 2023. Available online: https://www.cas.org/sites/default/files/documents/CASGENENGWHP101008230110-3D-Printing-Insight-Report.pdf (accessed on 13 February 2024).
- Scopus®—Document Search Results. Available online: https://id.elsevier.com/as/authorization.oauth2?platSite=SC%2Fscopus&ui_locales=en-US&scope=openid+profile+email+els_auth_info+els_analytics_info+urn%3Acom%3Aelsevier%3Aidp%3Apolicy%3Aproduct%3Aindv_identity&els_policy=idp_policy_indv_identity_plus&respo (accessed on 13 February 2024).
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3D Printing Technology: Technological, Materials, and Applications. Proc. Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Varghese, R.; Sood, P.; Salvi, S.; Karsiya, J.; Kumar, D. 3D printing in the pharmaceutical sector: Advances and evidences. Sens. Int. 2022, 3, 100177. [Google Scholar] [CrossRef]
- Bhusnure, O.G.; Gholve, S.V.; Sugave, B.K.; Dongre, R.C.; Gore, S.A.; Giram, P.S. 3D Printing & Pharmaceutical Manufacturing: Opportunities and Challenges. Int. J. Bioassays 2016, 5, 4723–4738. [Google Scholar]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.S.; Kumar, S.; Zhou, Q.T. Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies. Acta Pharm. Sin. B. 2021, 11, 2505–2536. [Google Scholar] [CrossRef]
- Nashed, N.; Lam, M.; Nokhodchi, A. A comprehensive overview of extended release oral dosage forms manufactured through hot melt extrusion and its combination with 3D printing. Int. J. Pharm. 2021, 596, 120237. [Google Scholar] [CrossRef] [PubMed]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.-W.; Ahmed, W.; Arafat, B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Boniatti, J.; Januskaite, P.; Fonseca, L.B.D.; Viçosa, A.L.; Amendoeira, F.C.; Tuleu, C.; Basit, A.W.; Goyanes, A.; Ré, M.-I. Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations. Pharmaceutics 2021, 13, 1114. [Google Scholar] [CrossRef] [PubMed]
- Lafeber, I.; Tichem, J.M.; Ouwerkerk, N.; Van Unen, A.D.; Van Uitert, J.J.D.; Bijleveld-Olierook, H.C.M.; Kweekel, D.M.; Zaal, W.M.; Le Brun, P.P.H.; Guchelaar, H.J.; et al. 3D printed furosemide and sildenafil tablets: Innovative production and quality control. Int. J. Pharm. 2021, 603, 120694. [Google Scholar] [CrossRef] [PubMed]
- Sundarkumar, V.; Wang, W.; Nagy, Z.; Reklaitis, G. Manufacturing pharmaceutical mini-tablets for pediatric patients using drop-on-demand printing. Int. J. Pharm. 2023, 644, 123355. [Google Scholar] [CrossRef] [PubMed]
- Kyobula, M.; Adedeji, A.; Alexander, M.R.; Saleh, E.; Wildman, R.; Ashcroft, I.; Gellert, P.R.; Roberts, C.J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control. Release 2017, 261, 207–215. [Google Scholar] [CrossRef]
- Scoutaris, N.; Snowden, M.; Douroumis, D. Taste masked thin films printed by jet dispensing. Int. J. Pharm. 2015, 494, 619–622. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef]
- Yochana, S.; Yu, M.; Alvi, M.; Varenya, S.; Chatterjee, P. Pharmaceutical excipients and pediatric formulations. Chim. Oggi 2012, 30, 56–61. [Google Scholar]
- STEPDatabase—EuPFI. Available online: http://www.eupfi.org/step-database-info/ (accessed on 11 December 2023).
- Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer Selection for Hot-melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics 2020, 12, 795. [Google Scholar] [CrossRef]
- Tracy, T.; Wu, L.; Liu, X.; Cheng, S.; Li, X. 3D printing: Innovative solutions for patients pharmaceutical industry. Int. J. Pharm. 2023, 631, 122480. [Google Scholar] [CrossRef] [PubMed]
- FabRx’s Pharmaceutical 3D Printer for Personalised Medicines, M3dimakerTM, Is Now Available. Available online: https://www.fabrx.co.uk/2020/04/06/fabrxs-pharmaceutical-3d-printer-for-personalised-medicines-m3dimaker-isnowavailable#:~:text=April%2006,%202020,the%20manufacture%20of%20personalised%20medicines (accessed on 20 December 2023).
- Pharmaceutical 3D Printers for Personalized Medicine, FabrX LdT. Available online: https://www.fabrx.co.uk/products (accessed on 11 December 2023).
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef]
- Zheng, Y.; Deng, F.; Wang, B.; Wu, Y.; Luo, Q.; Zuo, X.; Liu, X.; Cao, L.; Li, M.; Lu, H.; et al. Melt extrusion deposition (MEDTM) 3D printing technology—A paradigm shift in design and development of modified release drug products. Int. J. Pharm. 2021, 602, 120639. [Google Scholar] [CrossRef]
- Personalisierte Medikamente aus dem Pharma-Drucker, DiHeSys. Available online: https://www.digital-health-systems.com/post/personalisierte-medikamente-aus-dem-pharma-drucker (accessed on 16 February 2024).
- Goyanes, A.; Madla, C.M.; Umerji, A.; Piñeiro, G.D.; Montero, J.M.G.; Diaz, M.J.L.; Barcia, M.G.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef] [PubMed]
- 3D Printing Could Give You Better Pill to Swallow. Available online: https://medium.com/mosaic-science/3d-printing-could-give-you-a-better-pill-to-swallow-d1114504f53f (accessed on 21 December 2023).
- FabRx and Gustave Roussy Enter Into An Agreement To Develop A Novel, Personalised, Multi-Drug Dosage form for the Treatment of Patients with Early-Stage Breast Cancer. Available online: https://www.fabrx.co.uk/2021/06/25/fabrx-and-%20gustave-roussy-enter-into-an-agreement-to-develop-a-novel-personalised-multi-drug-%20dosage-form-for-the-treatment-of-patients-with-early-stage-breast-cancer (accessed on 8 December 2023).
- Awad, A.; Yao, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; W Basit, A.W. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172. [Google Scholar] [CrossRef]
- Triastek Receives FDA IND Clearance for 3D Printed Medicine for the Treatment of Ulcerative Colitis. Available online: https://www.triastek.com/detail/36.html (accessed on 15 December 2023).
- Voelher, R. The printed pill. JAMA 2015, 314, 1108. [Google Scholar] [CrossRef]
- Bácskay, I.; Ujhelyi, Z.; Fehér, P.; Arany, P. The Evolution of the 3D-Printed Drug Delivery Systems: A Review. Pharmaceutics 2022, 14, 1312. [Google Scholar] [CrossRef]
- Lopez, F.L.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Formulation approaches to pediatric oral drug delivery: Benefits and limitations of current platforms. Expert Opin. Drug Deliv. 2015, 12, 1727–1740. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed Starmix” drug loaded dosage forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef] [PubMed]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dumpa, N.; Bandari, S.; Durig, T.; Repka, M.A. Fabrication of Taste-Masked Donut-Shaped Tablets Via Fused Filament Fabrication 3D Printing Paired with Hot-Melt Extrusion Techniques. AAPS Pharm. Sci. Tech. 2020, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Parulski, C.; Bya, L.A.; Goebel, J.; Servais, A.C.; Lechanteur, A.; Evrard, B. Development of 3D printed mini-waffle shapes containing hydrocortisone for children’s personalized medicine. Int. J. Pharm. 2023, 642, 123131. [Google Scholar] [CrossRef] [PubMed]
- Chatzitaki, A.T.; Mystiridou, E.; Bouropoulos, N.; Ritzoulis, C.; Karavasilil, C.; Fatouros, D.G. Semi-solid extrusion 3D printing of starch-based soft dosage forms for the treatment of paediatric latent tuberculosis infection. J. Pharm. Pharmacol. 2022, 74, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Rycerz, K.; Stepien, K.A.; Czapiewska, M.; Arafat, B.T.; Habashy, R.; Isreb, A.; Peak, M.; Alhnan, M.A. Embedded 3D Printing of Novel Bespoke Soft Dosage Form Concept for Pediatrics. Pharmaceutics 2019, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Karavasilia, C.; Gkaragkounisa, A.; Moschakisb, T.; Ritzoulisc, C.; Fatouros, D.G. Pediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3D printing. Eur. J. Pharm. Sci. 2020, 147, 105291. [Google Scholar] [CrossRef] [PubMed]
- Karavasilia, C.; Zgouro, P.; Manousi, N.; Lazaridou, A.; Zacharis, C.; Bouropoulod, N.; Moschakisb, T.; Fatouros, D.G. Cereal-Based 3D Printed Dosage Forms for Drug Administration During Breakfast in Pediatric Patients within a Hospital Setting. J. Pharm. Sci. 2022, 111, 2562–2570. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Suñé-Pou, M.; Pérez-Lozano, P.; García-Montoya, E.; Aguilar, E. 3D printed gummies: Personalized drug dosage in a safe and appealing way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef]
- Krause, J.; Müller, L.; Sarwinska, D.; Seidlitz, A.; Sznitowska, M.; Weitschies, W. 3D Printing of Mini Tablets for Pediatric Use. Pharmaceuticals 2021, 14, 143. [Google Scholar] [CrossRef]
- Palekar, S.; Nukala, P.K.; Mishra, S.M.; Kipping, T.; Patel, K. Application of 3D printing technology and quality by design approach for development of age-appropriate pediatric formulation of baclofen. Int. J. Pharm. 2019, 556, 106–116. [Google Scholar] [CrossRef]
- Thabet, Y.; Klingmann, V.; Breitkreutz, J. Drug Formulations: Standards and Novel Strategies for Drug Administration in Pediatrics. J. Clin. Pharmacol. 2018, 58, S26–S35. [Google Scholar] [CrossRef]
- Pistone, M.; Racaniello, G.F.; Rizzi, R.; Iacobazzi, R.M.; Arduino, I.; Lopalco, I.; Lopedota, A.A.; Denora, N. Direct cyclodextrin based powder extrusion 3D printing of budesonide loaded mini-tablets for the treatment of eosinophilic colitis in paediatric patients. Int. J. Pharm. 2023, 632, 122592. [Google Scholar] [CrossRef]
- Khalid, G.M.; Musazzi, U.M.; Selmin, F.; Franzr, S.; Minghetti, P.; Cilurzo, F. Extemporaneous printing of diclofenac orodispersible films for pediatrics. Drug Dev. Ind. Pharm. 2021, 47, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Tian, Y.; Zhang, E.; Gao, X.; Zhang, H.; Liu, N.; Han, X.; Sun, Y.; Wang, Z.; Zheng, A. Semisolid Extrusion 3D Printing of Propranolol Hydrochloride Gummy Chewable Tablets: An Innovative Approach to Prepare Personalized Medicine for Pediatrics. AAPS Pharm. Sci. Tech. 2022, 23, 166. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Fullbrook, D.H.G.; Vilain, L.; Derrar, Y.; Nandi, U.; Grau, C.; Morales, A.; Hooper, G.; Hiezl, Z.; Douroumis, D. Personalised Tasted Masked Chewable 3D Printed Fruit-Chews for Paediatric Patients. Pharmaceutics 2021, 13, 1301. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Saydam, M.; Takka, S. Improving the dissolution of a water-insoluble orphan drug through a fused deposition modelling 3-Dimensional printing technology approach. Eur. J. Pharm. Sci. 2020, 152, 105426. [Google Scholar] [CrossRef]
- Fanous, M.; Bitar, M.; Gold, S.; Sobczuk, A.; Hirsch, S.; Ogorka, J.; Imanidis, G. Development of immediate release 3D-printed dosage forms for a poorly water-soluble drug by fused deposition modeling: Study of morphology, solid state and dissolution. Int. J. Pharm. 2021, 599, 120417. [Google Scholar] [CrossRef] [PubMed]
- Roulon, S.; Soulairol, I.; Lavastre, V.; Payre, N.; Cazes, M.; Delbreilh, L.; Alié, J. Production of Reproducible Filament Batches for the Fabrication of 3D Printed Oral Forms. Pharmaceutics 2021, 13, 472. [Google Scholar] [CrossRef]
- Malebari, A.M.; Kara, A.; Khayyat, A.N.; Mohammad, K.A.; Serrano, D.R. Development of Advanced 3D-Printed Solid Dosage Pediatric Formulations for HIV Treatment. Pharmaceuticals 2022, 15, 435. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.L.; Stogiannari, M.; Janeczko, S.; Khoshan, M.; Lin, Y.; Isreb, A.; Habashy, R.; Giebułtowicz, J.; Peak, M.; Alhnan, M.A. Towards point-of-care manufacturing and analysis of immediate-release 3D printed hydrocortisone tablets for the treatment of congenital adrenal hyperplasia. Int. J. Pharm. 2023, 642, 123072. [Google Scholar] [CrossRef] [PubMed]
- Racaniello, G.F.; Pistone, M.; Meazzini, C.; Lopedota, A.; Arduino, I.; Rizzi, R.; Lopalco, A.; Musazzi, U.M.; Cilurzo, F.; Denora, N. 3D printed mucoadhesive orodispersible films manufactured by direct powder extrusion for personalized Clobetasol propionate based paediatric therapies. Int. J. Pharm. 2023, 643, 123214. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Nandi, U.; Scoutaris, N.; Sanfo, K.; Alexander, B.; Gong, Y.; Ho-Wah Hui, H.W.; Kumar, S.; Douroumis, D. Personalised paediatric chewable Ibuprofen tablets fabricated using 3D micro-extrusion printing technology. Int. J. Pharm. 2022, 626, 122135. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Xie, J.; Chen, L.; Xu, F. Preparation of Loratadine Orally Disintegrating Tablets by Semi-solid Extrusion 3D Printing. Curr. Drug Deliv. 2023, 20, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Fitaihi, R.; Abukhamees, S.; Abdelhakim, H.E. Formulation and Characterisation of Carbamazepine Orodispersible 3D-Printed Mini-Tablets for Paediatric Use. Pharmaceutics 2023, 15, 250. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.; Sanchez-Ballester, N.M.; Aubert, A.; Rossi, J.C.; Begu, S.; Soulairol, I. Preliminary Study on the Development of Caffeine Oral Solid Form 3D Printed by Semi-Solid Extrusion for Application in Neonates. AAPS Pharm. Sci. Tech. 2023, 24, 122. [Google Scholar] [CrossRef]
- Han, X.; Kang, D.; Liu, B.; Zhang, H.; Wang, Z.; Gao, X.; Zheng, A. Feasibility of developing hospital preparation by semisolid extrusion 3D printing: Personalized amlodipine besylate chewable tablets. Pharm. Dev. Technol. 2022, 27, 164–174. [Google Scholar] [CrossRef]
- Suárez-González, J.; Magarinos-Trivino, M.; Díaz-Torres, E.; Cáceres-Pérez, A.R.; Santovena-Estévez, A.; Farina, J.B. Individualized orodispersible pediatric dosage forms obtained by molding and semi-solid extrusion by 3D printing: A comparative study for hydrochlorothiazide. J. Drug Deliv. Sci. Technol. 2021, 66, 102884. [Google Scholar] [CrossRef]
- Díaz-Torres, E.; Santovena-Estévez, A.; Farina, J.B. A micro-extrusion 3D printing platform for fabrication of orodispersible printlets for pediatric use. Int. J. Pharm. 2021, 605, 120854. [Google Scholar]
- Aita, I.E.; Rahman, J.; Breitkreutz, J.; Quodbach, J. 3D-Printing with precise layer-wise dose adjustments for paediatric use via pressure-assisted microsyringe printing. Eur. J. Pharm. Biopharm. 2020, 157, 59–65. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Gerrard, S.E.; Walsh, J.; Bowers, N.; Salunke, S.; Hershenson, S. Innovations in Pediatric Drug Formulations and Administration Technologies for Low Resource Settings. Pharmaceutics 2019, 11, 518. [Google Scholar] [CrossRef]
- Zheng, Z.; Lv, J.; Yang, W.; Pi, X.; Lin, W.; Lin, Z.; Zhang, W.; Pang, J.; Zeng, Y.; Lv, Z.; et al. Preparation and application of subdivided tablets using 3D printing for precise hospital dispensing. Eur. J. Pharm. Sci. 2020, 149, 105293. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, E.; Brako, F.; Scarpa, M.; Bonifazi, D.; Pignataro, V.; Cavallo, M.; Cullufe, O.; Enache, C.; Nafria, B.; Claverol, J.; et al. Children’s Preferences for Oral Dosage Forms and Their Involvement in Formulation Research via EPTRI (European Paediatric Translational Research Infrastructure). Pharmaceutics 2021, 13, 730. [Google Scholar] [CrossRef] [PubMed]
- Cailleaux, S.; Sanchez-Ballester, N.M.; Gueche, Y.A.; Bataille, B.; Soulairol, I. 2021. Fused Deposition Modeling (FDM), the new asset for the production of tailored medicines. J. Control. Release 2021, 330, 821–841. [Google Scholar] [CrossRef]
- Lewis, S.; Fang, L.; Laxmi, K.V.; Tochukwu, C.O. Buccal films: A review of therapeutic opportunities, formulations & relevant evaluation approaches. J. Control. Release 2022, 352, 1071–1092. [Google Scholar]
- Technical Considerations for Additive Manufactured Medical Devices Guidance for Industry and Food and Drug Administration Staff. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/technical-considerations-additive-manufactured-medical-devices (accessed on 11 December 2023).
- Pediatric Regulation. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/paediatric-medicines-overview/paediatric-regulation (accessed on 20 December 2023).
- Zisowsky, J.; Krause, A.; Dingemanse, J. Drug Development for Pediatric Populations: Regulatory Aspects. Pharmaceutics 2010, 2, 364–388. [Google Scholar] [CrossRef] [PubMed]
- Nahata, M.C. Lack of Pediatric Drug Formulations. Pediatrics 1999, 104, 607–609. [Google Scholar] [CrossRef]
- 3D Printed Drugs Market to Reach US$ 2064.8 Million by 2027 Globally | CAGR: 15.2% | UnivDatos Market Insights. Available online: https://www.prnewswire.com/in/news-releases/3d-printed-drugs-market-to-reach-us-2-064-8-million-by-2027-globally-cagr-15-2-univdatos-market-insights-866286870.html (accessed on 13 February 2024).
- 3D Printed Drugs Market: Current Analysis and Forecast (2020–2027). Available online: https://univdatos.com/it/report/3d-printed-drugs-market/ (accessed on 11 December 2023).
- Annereau, M.; Toussaint, B.; Wojcicki, A.D.; Dufay, S.; Salmeron, R.D.; Boudy, V. 2D-3D printing in hospital pharmacies, what roles and challeges? Ann. Pharm. Françaises 2021, 79, 361–374. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianno, V.; Vurpillot, S.; Prillieux, S.; Espeau, P. Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects. Pharmaceutics 2024, 16, 441. https://doi.org/10.3390/pharmaceutics16040441
Ianno V, Vurpillot S, Prillieux S, Espeau P. Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects. Pharmaceutics. 2024; 16(4):441. https://doi.org/10.3390/pharmaceutics16040441
Chicago/Turabian StyleIanno, Veronica, Sarah Vurpillot, Sylvain Prillieux, and Philippe Espeau. 2024. "Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects" Pharmaceutics 16, no. 4: 441. https://doi.org/10.3390/pharmaceutics16040441
APA StyleIanno, V., Vurpillot, S., Prillieux, S., & Espeau, P. (2024). Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects. Pharmaceutics, 16(4), 441. https://doi.org/10.3390/pharmaceutics16040441