An Evaluation of the Binding Strength of Okra Gum and the Drug Release Characteristics of Tablets Prepared from It
Abstract
:1. Introduction
2. Materials and Methods
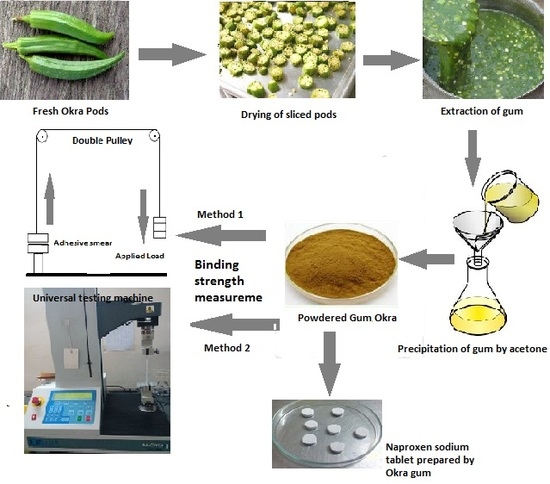
2.1. Preparation of the Aqueous Extract of Okra
2.2. Isolation of Okra Gum
2.3. The Determination of Binding/Adhesive Strength
2.3.1. Testing Using the Double Pulley Assembly/Modified Balance Apparatus
2.3.2. Universal Materials Testing Apparatus
2.4. Preparation of Tablets
2.5. Bulk Properties of Granules
2.6. Testing of Prepared Tablets
2.7. Drug Release Studies
3. Results
3.1. Binding Strength of Okra Gum and Pre-Gelatinized Starch
3.2. Bulk Properties of the Granules
3.3. Characteristics of Prepared Tablets
3.4. Dissolution Studies
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Symecko, C.; Rhodes, C. Binder functionality in tabletted systems. Drug Dev. Ind. Pharm. 1995, 21, 1091–1114. [Google Scholar] [CrossRef]
- Barbosa-Canovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005. [Google Scholar]
- Larry, L.; Augsburger, S.W.H. Pharmaceutical Dosage Forms: Tablets, 3rd ed.; CRC Press: New York, NY, USA, 2008. [Google Scholar]
- Miller, R.; Parikh, D. Handbook of Pharmaceutical Granulation Technology; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Okoye, E.I.; Onyekweli, A.O.; Kunle, O.O. Okra gum-an economic choice for the amelioration of capping and lamination in tablets. Ann. Biol. Res. 2011, 2, 30–42. [Google Scholar]
- Odeku, O.; Itiola, O. Evaluation of khaya gum as a binder in a paracetamol tablet formulation. Pharm. Pharmacol. Commun. 1998, 4, 183–188. [Google Scholar]
- Parikh, D.M. Handbook of Pharmaceutical Granulation Technology, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Ghori, M.U.; Alba, K.; Smith, A.M.; Conway, B.R.; Kontogiorgos, V. Okra extracts in pharmaceutical and food applications. Food Hydrocoll. 2014, 42, 342–347. [Google Scholar] [CrossRef]
- Tavakoli, N.; Ghasemi, N.; Hamishehkar, H. Evaluation of okra gum as a binder in tablet dosage forms. Iran. J. Pharm. Res. 2010, 2, 47. [Google Scholar]
- Emeje, M.; Isimi, C.; Kunle, O. Evaluation of Okra gum as a dry binder in Paracetamol tablet formulations. Cont. J. Pharm. Sci. 2007, 1, 15–22. [Google Scholar]
- Ogaji, I.; Nnoli, O. Film coating potential of okra gum using paracetamol tablets as a model drug. Asian J. Pharm. 2010, 4, 130. [Google Scholar] [CrossRef]
- Alba, K.; Ritzoulis, C.; Georgiadis, N.; Kontogiorgos, V. Okra extracts as emulsifiers for acidic emulsions. Food Res. Int. 2013, 54, 1730–1737. [Google Scholar] [CrossRef]
- Ikoni, O. Some physicochemical properties of acetaminophen pediatric suspensions formulated with okra gums obtained from different extraction processes as suspending agent. Asian J. Pharm. 2011, 5, 15. [Google Scholar] [CrossRef]
- Sharma, N.; Kulkarni, G.T.; Sharma, A. Development of Abelmoschus esculentus (Okra)-Based Mucoadhesive Gel for Nasal Delivery of Rizatriptan Benzoate. Trop. J. Pharm. Res. 2013, 12, 149–153. [Google Scholar] [CrossRef]
- Tavakoli, N.; Teimouri, R.; Hamishehkar, H. Characterization and evaluation of okra gum as a tablet binder. Jundishapur J. Nat. Pharm. Prod. 2007, 3, 33–38. [Google Scholar]
- Farooq, U.; Malviya, R.; Sharma, P.K. Extraction and Characterization of Okra Mucilage as Pharmaceutical Excipient. Acad. J. Plant Sci. 2013, 6, 168–172. [Google Scholar]
- Saxena, A.; Tewari, G.; Saraf, S.A. Formulation and evaluation of mucoadhesive buccal patch of acyclovir utilizing inclusion phenomenon. Braz. J. Pharm. Sci. 2011, 47, 887–897. [Google Scholar] [CrossRef]
- Sudarshan, S.; Sunil, B. In vivo mucoadhesive strength appraisal of gum Manilkara zapota. Braz. J. Pharm. Sci. 2015, 51, 689–698. [Google Scholar] [CrossRef]
- Shinde, G.; Sudharshini, S.; Stephenrathinaraj, B.; Rajveer, H.; Kumaraswamy, D.; GANESH, S. Formulation and evaluation of mucoadhesive tablets of niacin using different bioadhesive polymers. Int. J. Pharm. Biol. Sci. 2010, 1, 1–14. [Google Scholar] [CrossRef]
- Dharmalingam, S.R.; Ramamurthy, S.; Chidambaram, K.; Nadaraju, S. Development and validation of UV spectrophotometric method for the estimation of naproxen in bulk and semi-solid formulation. Int. J. Anal. Pharm. Biomed. Sci. 2013, 2, 49–55. [Google Scholar]
- Shah, R.B.; Tawakkul, M.A.; Khan, M.A. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech. 2008, 9, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Zaharuddin, N.D.; Noordin, M.I.; Kadivar, A. The Use of Hibiscus esculentus (Okra) Gum in Sustaining the Release of Propranolol Hydrochloride in a Solid Oral Dosage Form. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]


| Formulation Name | Naproxen Sodium (mg) | Okra Gum (% w/w) | Starch Slurry (% w/w) | Disintegrant Starch (% w/w) | Magnesium Stearate (% w/w) | Talc (% w/w) | Lactose (mg) | Total Weight of Tablet (mg) |
|---|---|---|---|---|---|---|---|---|
| F1 | 275 | 1 | - | - | 1 | 0.5 | 168 | 450 |
| F2 | 275 | 3 | - | - | 1 | 0.5 | 163 | 450 |
| F3 | 275 | 5 | - | - | 1 | 0.5 | 157 | 450 |
| F4 | 275 | 1 | - | 7 | 1 | 0.5 | 149 | 450 |
| F5 | 275 | 3 | - | 7 | 1 | 0.5 | 143 | 450 |
| F6 | 275 | 5 | - | 7 | 1 | 0.5 | 138 | 450 |
| F7 | 275 | - | 1 | - | 1 | 0.5 | 168 | 450 |
| F8 | 275 | - | 3 | - | 1 | 0.5 | 163 | 450 |
| F9 | 275 | - | 5 | - | 1 | 0.5 | 157 | 450 |
| F10 | 275 | - | 1 | 7 | 1 | 0.5 | 149 | 450 |
| F11 | 275 | - | 3 | 7 | 1 | 0.5 | 143 | 450 |
| F12 | 275 | - | 5 | 7 | 1 | 0.5 | 138 | 450 |
| Dispersion Sample Containing | Binding Strength (N) | |
|---|---|---|
| Double Pulley Method | Universal Testing Method | |
| Okra gum | ||
| 1% | 1.44 ± 0.21 | 2.51 ± 0.18 |
| 3% | 2.26 ± 1.52 | 3.63 ± 0.31 |
| 5% | 2.42 ± 0.95 | 4.51 ± 0.49 |
| Pre-gelatinized starch | ||
| 1% | 1.00 ± 0.32 | 1.34 ± 0.45 |
| 3% | 1.14 ± 0.25 | 1.48 ± 0.24 |
| 5% | 1.73 ± 0.34 | 1.81 ± 0.53 |
| Formulation | Bulk Density (g/cm2) | Tapped Density (g/cm2) | Hausner Ratio | Compressibility Index |
|---|---|---|---|---|
| F1 | 0.42 | 0.46 | 1.10 | 13 |
| F2 | 0.43 | 0.48 | 1.12 | 10 |
| F3 | 0.43 | 0.48 | 1.12 | 10 |
| F4 | 0.43 | 0.48 | 1.12 | 10 |
| F5 | 0.42 | 0.45 | 1.07 | 7 |
| F6 | 0.43 | 0.45 | 1.05 | 4 |
| F7 | 0.48 | 0.56 | 1.17 | 14 |
| F8 | 0.45 | 0.53 | 1.18 | 15 |
| F9 | 0.5 | 0.55 | 1.10 | 9 |
| F10 | 0.5 | 0.56 | 1.12 | 5 |
| F11 | 0.47 | 0.52 | 1.11 | 10 |
| F12 | 0.48 | 0.53 | 1.10 | 9 |
| Tablets Prepared with Okra Gum | Tablets Prepared with Pre-Gelatinized Starch | ||||||
|---|---|---|---|---|---|---|---|
| Formulation Name | Friability (%) | Hardness (Kg/cm2) | Disintegration Time (min) | Formulation Name | Friability (%) | Hardness (Kg/cm2) | Disintegration Time (min) |
| F1 | 0.50 | 7.0 ± 0.5 | 8.10 ± 0.8 | F7 | 0.62 | 6.5 ± 0.25 | 6.41 ± 0.1 |
| F2 | 0.41 | 8.0 ± 0.5 | 11.30 ± 0.6 | F8 | 0.51 | 7.0 ± 0.5 | 9.40 ± 1.0 |
| F3 | 0.41 | 8.0 ± 0.6 | 15.00 ± 0.9 | F9 | 0.54 | 8.0 ± 0.4 | 14.30 ± 1.0 |
| F4 | 0.46 | 7.0 ± 1.0 | 7.51 ± 0.3 | F10 | 0.69 | 7.0 ± 0.25 | 5.05 ± 0.2 |
| F5 | 0.31 | 8.0 ± 1.0 | 11.04 ± 0.6 | F11 | 0.62 | 7.5 ± 1.0 | 11.00 ± 0.5 |
| F6 | 0.15 | 8.75 ± 0.3 | 9.18 ± 0.7 | F12 | 0.62 | 7.75 ± 0.9 | 12.24 ± 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A.; Qureshi, F.; Abbas, N.; Arshad, M.S.; Ali, E. An Evaluation of the Binding Strength of Okra Gum and the Drug Release Characteristics of Tablets Prepared from It. Pharmaceutics 2017, 9, 20. https://doi.org/10.3390/pharmaceutics9020020
Hussain A, Qureshi F, Abbas N, Arshad MS, Ali E. An Evaluation of the Binding Strength of Okra Gum and the Drug Release Characteristics of Tablets Prepared from It. Pharmaceutics. 2017; 9(2):20. https://doi.org/10.3390/pharmaceutics9020020
Chicago/Turabian StyleHussain, Amjad, Farah Qureshi, Nasir Abbas, Muhammad Sohail Arshad, and Ejaz Ali. 2017. "An Evaluation of the Binding Strength of Okra Gum and the Drug Release Characteristics of Tablets Prepared from It" Pharmaceutics 9, no. 2: 20. https://doi.org/10.3390/pharmaceutics9020020
APA StyleHussain, A., Qureshi, F., Abbas, N., Arshad, M. S., & Ali, E. (2017). An Evaluation of the Binding Strength of Okra Gum and the Drug Release Characteristics of Tablets Prepared from It. Pharmaceutics, 9(2), 20. https://doi.org/10.3390/pharmaceutics9020020