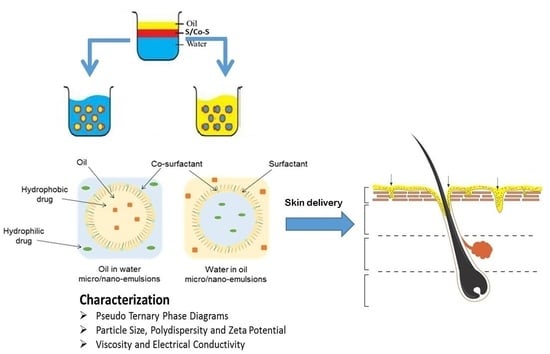
Topical Nano and Microemulsions for Skin Delivery
Abstract
:1. Introduction
2. Classification of Nano and Microsystems Used for Skin Delivery
3. Formulation of Micro and Nanoemulsions
4. Formulation Parameters: Composition and Preparation Methods
4.1. Preparation Methods
4.2. Composition
5. Physical Characterisation of Nano- and Microemulsions
5.1. Pseudo Ternary Phase Diagrams
5.2. Particle Size, Polydispersity and Zeta Potential
5.3. Viscosity and Electrical Conductivity
6. Skin Delivery from Nano and Microemulsions
Anti-Inflammatory Drugs
7. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scheuplein, R.J. Analysis of permeability data for the case of parallel diffusion pathways. Biophys. J. 1966, 6, 1–17. [Google Scholar] [CrossRef]
- Scheuplein, R.J.; Blank, I.H. Permeability of the skin. Physiol. Rev. 1971, 51, 702–747. [Google Scholar] [PubMed]
- Kasting, G.B.; Barai, N.D.; Wang, T.F.; Nitsche, J.M. Mobility of water in human stratum corneum. J. Pharm. Sci. 2003, 92, 2326–2340. [Google Scholar] [CrossRef] [PubMed]
- Illel, B.; Schaefer, H.; Wepierre, J.; Doucet, O. Follicles play an important role in percutaneous absorption. J. Pharm. Sci. 1991, 80, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Leite-Silva, V.R.; Liu, D.C.; Sanchez, W.Y.; Studier, H.; Mohammed, Y.H.; Holmes, A.; Becker, W.; Grice, J.E.; Benson, H.A.; Roberts, M.S. Effect of flexing and massage on in vivo human skin penetration and toxicity of zinc oxide nanoparticles. Nanomedicine (Lond.) 2016, 11, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Leite-Silva, V.R.; Sanchez, W.Y.; Studier, H.; Liu, D.C.; Mohammed, Y.H.; Holmes, A.M.; Ryan, E.M.; Haridass, I.N.; Chandrasekaran, N.C.; Becker, W.; et al. Human skin penetration and local effects of topical nano zinc oxide after occlusion and barrier impairment. Eur. J. Pharm. Biopharm. 2016, 104, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloid Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. 1), S131–S155. [Google Scholar] [CrossRef]
- Jores, K.; Haberland, A.; Wartewig, S.; Mader, K.; Mehnert, W. Solid lipid nanoparticles (SLN) and oil-loaded SLN studied by spectrofluorometry and Raman spectroscopy. Pharm. Res. 2005, 22, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Vyas, S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1997, 172, 33–70. [Google Scholar] [CrossRef]
- Dragicevic-Curic, N.; Scheglmann, D.; Albrecht, V.; Fahr, A. Temoporfin-loaded invasomes: Development, characterization and in vitro skin penetration studies. J. Control. Release 2008, 127, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Geusens, B.; van Gele, M.; Braat, S.; de Smedt, S.C.; Stuart, M.C.; Prow, T.W.; Sanchez, W.; Roberts, M.S.; Sanders, N.; Lambert, J. Flexible Nanosomes (SECosomes) Enable Efficient siRNA Delivery in Cultured Primary Skin Cells and in the Viable Epidermis of Ex Vivo Human Skin. Adv. Funct. Mater. 2010, 20, 4077–4090. [Google Scholar] [CrossRef]
- Mura, S.; Manconi, M.; Fadda, A.M.; Sala, M.C.; Perricci, J.; Pini, E.; Sinico, C. Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil: In vitro evaluation of drug permeation by infrared spectroscopy. Pharm. Dev. Technol. 2013, 18, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Caddeo, C.; Sinico, C.; Valenti, D.; Mostallino, M.C.; Biggio, G.; Fadda, A.M. Ex vivo skin delivery of diclofenac by transcutol containing liposomes and suggested mechanism of vesicle-skin interaction. Eur. J. Pharm. Biopharm. 2011, 78, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Blume, G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim. Biophys. Acta 1992, 1104, 226–232. [Google Scholar] [CrossRef]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Exp. Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Borowska, K.; Wolowiec, S.; Glowniak, K.; Sieniawska, E.; Radej, S. Transdermal delivery of 8-methoxypsoralene mediated by polyamidoamine dendrimer G2.5 and G3.5—In vitro and in vivo study. Int. J. Pharm. 2012, 436, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol. Physiol. 2008, 21, 246–259. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Clarification of critical differences. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Heuschkel, S.; Goebel, A.; Neubert, R.H. Microemulsions—Modern colloidal carrier for dermal and transdermal drug delivery. J. Pharm. Sci. 2008, 97, 603–631. [Google Scholar] [CrossRef] [PubMed]
- Scriven, L.E. Equilibrium bicontinuous structure. Nature 1976, 263, 123–125. [Google Scholar] [CrossRef]
- Lindman, B.; Shinoda, K.; Olsson, U.; Anderson, D.; Karlström, G.; Wennerström, H. On the demonstration of bicontinuous structures in microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 1989, 38, 205–224. [Google Scholar] [CrossRef]
- Bhatia, G.; Zhou, Y.; Banga, A.K. Adapalene microemulsion for transfollicular drug delivery. J. Pharm. Sci. 2013, 102, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Naoui, W.; Bolzinger, M.A.; Fenet, B.; Pelletier, J.; Valour, J.P.; Kalfat, R.; Chevalier, Y. Microemulsion microstructure influences the skin delivery of an hydrophilic drug. Pharm. Res. 2011, 28, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Anton, N.; Benoit, J.P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates-a review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Jasmina, H.; Džana, O.; Alisa, E.; Edina, V.; Ognjenka, R. Preparation of nanoemulsions by high-energy and low energy emulsification methods. In CMBEBIH 2017, Proceedings of the International Conference on Medical and Biological Engineering (IFMBE), Sarajevo, Bosnia and Herzegovina, 16–18 March 2017; Badnjevic, A., Ed.; Springer: Singapore, 2017. [Google Scholar]
- Sole, I.; Solans, C.; Maestro, A.; Gonzalez, C.; Gutierrez, J.M. Study of nano-emulsion formation by dilution of microemulsions. J. Colloid Interface Sci. 2012, 376, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tabor, R.; Eastoe, J.; Li, X.; Heenan, R.K.; Dong, J. Formation and stability of nanoemulsions with mixed ionic-nonionic surfactants. Phys. Chem. Chem. Phys. 2009, 11, 9772–9778. [Google Scholar] [CrossRef] [PubMed]
- Pons, R.; Carrera, I.; Caelles, J.; Rouch, J.; Panizza, P. Formation and properties of mini-emulsions formed by microemulsions dilution. Adv. Colloid Interface Sci. 2003, 106, 129–146. [Google Scholar] [CrossRef]
- Vitale, S.A.; Katz, J.L. Liquid droplet dispersions formed by homogeneous liquid—Liquid nucleation: “The ouzo effect”. Langmuir 2003, 19, 4105–4110. [Google Scholar] [CrossRef]
- Wang, L.; Mutch, K.J.; Eastoe, J.; Heenan, R.K.; Dong, J. Nanoemulsions prepared by a two-step low-energy process. Langmuir 2008, 24, 6092–6099. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Morrison, E.D.; Frethem, C.D.; Zasadzinski, J.A.; McCormick, A.V. Cryogenic electron microscopy study of nanoemulsion formation from microemulsions. Langmuir 2014, 30, 10826–10833. [Google Scholar] [CrossRef] [PubMed]
- Kreilgaard, M.; Pedersen, E.J.; Jaroszewski, J.W. NMR characterisation and transdermal drug delivery potential of microemulsion systems. J. Control. Release 2000, 69, 421–433. [Google Scholar] [CrossRef]
- Paolino, D.; Ventura, C.A.; Nistico, S.; Puglisi, G.; Fresta, M. Lecithin microemulsions for the topical administration of ketoprofen: Percutaneous adsorption through human skin and in vivo human skin tolerability. Int. J. Pharm. 2002, 244, 21–31. [Google Scholar] [CrossRef]
- Hua, L.; Weisan, P.; Jiayu, L.; Ying, Z. Preparation, evaluation, and NMR characterization of vinpocetine microemulsion for transdermal delivery. Drug Dev. Ind. Pharm. 2004, 30, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B. Overcoming the Cutaneous Barrier with Microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Progress in the use of microemulsions for transdermal and dermal drug delivery. Pharm. Dev. Technol. 2017, 22, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, A.L.; Reis, M.Y.; Silva, G.C.; Ramalho, I.M.; Guimaraes, G.P.; Silva, J.A.; Saraiva, K.L.; Damasceno, B.P. Microemulsion for topical application of pentoxifylline: In vitro release and in vivo evaluation. Int. J. Pharm. 2016, 506, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Danino, D.; Bernheim-Groswasser, A.; Talmon, Y. Digital cryogenic transmission electron microscopy: An advanced tool for direct imaging of complex fluids. Colloids Surf. A Physicochem. Eng. Asp. 2001, 183–185, 113–122. [Google Scholar] [CrossRef]
- Podlogar, F.; Gasperlin, M.; Tomsic, M.; Jamnik, A.; Rogac, M.B. Structural characterisation of water-Tween 40/Imwitor 308-isopropyl myristate microemulsions using different experimental methods. Int. J. Pharm. 2004, 276, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Stokes, J.P.; Kim, M.W.; Huang, J.S. Percolation in an oil-continuous microemulsion. Phys. Rev. Lett. 1985, 55, 1884–1887. [Google Scholar] [CrossRef] [PubMed]
- Thevenin, M.A.; Grossiord, J.L.; Poelman, M.C. Sucrose esters/cosurfactant microemulsion systems for transdermal delivery: Assessment of bicontinuous structures. Int. J. Pharm. 1996, 137, 177–186. [Google Scholar] [CrossRef]
- Lutz, R.; Aserin, A.; Wachtel, E.J.; Ben-Shoshan, E.; Danino, D.; Garti, N. A Study of the Emulsified Microemulsion by SAXS, Cryo-TEM, SD-NMR, and Electrical Conductivity. J. Dispers. Sci. Technol. 2007, 28, 1149–1157. [Google Scholar] [CrossRef]
- Acharya, D.P.; Hartley, P.G. Progress in microemulsion characterization. Curr. Opin. Colloid Interface Sci. 2012, 17, 274–280. [Google Scholar] [CrossRef]
- Hoppel, M.; Juric, S.; Ettl, H.; Valenta, C. Effect of monoacyl phosphatidylcholine content on the formation of microemulsions and the dermal delivery of flufenamic acid. Int. J. Pharm. 2015, 479, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Meinke, M.; Sterry, W.; Patzelt, A. Which skin model is the most appropriate for the investigation of topically applied substances into the hair follicles? Skin Pharmacol. Physiol. 2010, 23, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Liu, X.; Mostafa, A.; Mohammed, Y.; Grice, J.E.; Anissimov, Y.G.; Sakran, W.; Roberts, M.S. Estimating Maximal In Vitro Skin Permeation Flux from Studies Using Non-sink Receptor Phase Conditions. Pharm. Res. 2016, 33, 2180–2194. [Google Scholar] [CrossRef] [PubMed]
- Baspinar, Y.; Borchert, H.H. Penetration and release studies of positively and negatively charged nanoemulsions—Is there a benefit of the positive charge? Int. J. Pharm. 2012, 430, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Baspinar, Y.; Keck, C.M.; Borchert, H.H. Development of a positively charged prednicarbate nanoemulsion. Int. J. Pharm. 2010, 383, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.C.; Chiang, B.H.; Huang, D.W.; Li, P.H. Skin permeation of D-limonene-based nanoemulsions as a transdermal carrier prepared by ultrasonic emulsification. Ultrason. Sonochem. 2014, 21, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Campani, V.; Biondi, M.; Mayol, L.; Cilurzo, F.; Pitaro, M.; de Rosa, G. Development of nanoemulsions for topical delivery of vitamin K1. Int. J. Pharm. 2016, 511, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Dey, S.; Choudhury, S.; Mazumder, B. Topical delivery of aceclofenac as nanoemulsion comprising excipients having optimum emulsification capabilities: Preparation, characterization and in vivo evaluation. Exp. Opin. Drug Deliv. 2013, 10, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Samad, A.; Singh, S.K.; Ahsan, M.N.; Haque, M.W.; Faruk, A.; Ahmed, F.J. Nanoemulsion gel-based topical delivery of an antifungal drug: In vitro activity and in vivo evaluation. Drug Deliv. 2016, 23, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, A.; Heuschkel, S.; Jacobi, U.; Presse, G.; Neubert, R.H.; Sterry, W.; Lademann, J. Comparison of stratum corneum penetration and localization of a lipophilic model drug applied in an o/w microemulsion and an amphiphilic cream. Eur. Pharm. Biopharm. 2007, 67, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Wosicka, H.; Cal, K. Targeting to the hair follicles: Current status and potential. J. Dermatol. Sci. 2010, 57, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Knorr, F.; Richter, H.; Blume-Peytavi, U.; Vogt, A.; Antoniou, C.; Sterry, W.; Patzelt, A. Hair follicles—An efficient storage and penetration pathway for topically applied substances. Summary of recent results obtained at the Center of Experimental and Applied Cutaneous Physiology, Charite-Universitatsmedizin Berlin, Germany. Skin Pharmacol. Physiol. 2008, 21, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Kweon, J.H.; Chi, S.C.; Park, E.S. Transdermal delivery of diclofenac using microemulsions. Arch. Pharm. Res. 2004, 27, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Basil, M.; AlBaraghthi, T.; Sunoqrot, S.; Tarawneh, O. Nanoemulsion-based gel formulation of diclofenac diethylamine: Design, optimization, rheological behavior and in vitro diffusion studies. Pharm. Dev. Technol. 2016, 21, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, Y.; Kim, J.; Yoon, M.; Choi, Y.W. Formulation of microemulsion systems for transdermal delivery of aceclofenac. Arch. Pharm. Res. 2005, 28, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Isailovic, T.; Ethordevic, S.; Markovic, B.; Randelovic, D.; Cekic, N.; Lukic, M.; Pantelic, I.; Daniels, R.; Savic, S. Biocompatible Nanoemulsions for Improved Aceclofenac Skin Delivery: Formulation Approach Using Combined Mixture-Process Experimental Design. J. Pharm. Sci. 2016, 105, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Cui, Y.; Yun, B.J.; Ko, I.J.; Chi, S.C. Transdermal delivery of piroxicam using microemulsions. Arch. Pharm. Res. 2005, 28, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Ramadan, W.; Ahmed, M.A. Investigation of true nanoemulsions for transdermal potential of indomethacin: Characterization, rheological characteristics, and ex vivo skin permeation studies. J. Drug Target. 2009, 17, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Ramadan, W.; Gargum, H.M.; Singh, R. Preparation and in vivo evaluation of indomethacin loaded true nanoemulsions. Sci. Pharm. 2010, 78, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ledet, G.; Pamujula, S.; Walker, V.; Simon, S.; Graves, R.; Mandal, T.K. Development and in vitro evaluation of a nanoemulsion for transcutaneous delivery. Drug Dev. Ind. Pharm. 2014, 40, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, N.; Ghosal, S.K.; Moulik, S.P. Enhanced in vitro percutaneous absorption and in vivo anti-inflammatory effect of a selective cyclooxygenase inhibitor using microemulsion. Drug Dev. Ind. Pharm. 2005, 31, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Shafiq, S. Enhanced anti-inflammatory effects of celecoxib from a transdermally applied nanoemulsion. Die Pharm. 2009, 64, 258–259. [Google Scholar]
- Lala, R.R.; Awari, N.G. Nanoemulsion-based gel formulations of COX-2 inhibitors for enhanced efficacy in inflammatory conditions. Appl. Nanosci. 2014, 4, 143–151. [Google Scholar] [CrossRef]
- Abd, E.; Namjoshi, S.; Mohammed, Y.H.; Roberts, M.S.; Grice, J.E. Synergistic Skin Penetration Enhancer and Nanoemulsion Formulations Promote the Human Epidermal Permeation of Caffeine and Naproxen. J. Pharm. Sci. 2016, 105, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, M.; Ettl, H.; Holper, E.; Valenta, C. Influence of the composition of monoacyl phosphatidylcholine based microemulsions on the dermal delivery of flufenamic acid. Int. J. Pharm. 2014, 475, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Mahrhauser, D.S.; Kahlig, H.; Partyka-Jankowska, E.; Peterlik, H.; Binder, L.; Kwizda, K.; Valenta, C. Investigation of microemulsion microstructure and its impact on skin delivery of flufenamic acid. Int. J. Pharm. 2015, 490, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.S.; Choi, J.G.; Park, E.S.; Chi, S.C. Transdermal delivery of ketoprofen using microemulsions. Int. J. Pharm. 2001, 228, 161–170. [Google Scholar] [CrossRef]
- Kim, B.S.; Won, M.; Lee, K.M.; Kim, C.S. In vitro permeation studies of nanoemulsions containing ketoprofen as a model drug. Drug Deliv. 2008, 15, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Lee, M.K.; Hwang, K.J.; Kim, C.K. Phospholipid-based microemulsions of flurbiprofen by the spontaneous emulsification process. Int. J. Pharm. 1999, 183, 145–154. [Google Scholar] [CrossRef]
- Dasgupta, S.; Ghosh, S.K.; Ray, S.; Kaurav, S.S.; Mazumder, B. In vitro & in vivo studies on lornoxicam loaded nanoemulsion gels for topical application. Curr. Drug Deliv. 2014, 11, 132–138. [Google Scholar] [PubMed]
- Khurana, S.; Jain, N.K.; Bedi, P.M. Nanoemulsion based gel for transdermal delivery of meloxicam: Physico-chemical, mechanistic investigation. Life Sci. 2013, 92, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Sintov, A.C.; Shapiro, L. New microemulsion vehicle facilitates percutaneous penetration in vitro and cutaneous drug bioavailability in vivo. J. Control. Release 2004, 95, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Duangjit, S.; Mehr, L.M.; Kumpugdee-Vollrath, M.; Ngawhirunpat, T. Role of simplex lattice statistical design in the formulation and optimization of microemulsions for transdermal delivery. Biol. Pharm. Bull. 2014, 37, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Mahrhauser, D.; Hoppel, M.; Scholl, J.; Binder, L.; Kahlig, H.; Valenta, C. Simultaneous analysis of skin penetration of surfactant and active drug from fluorosurfactant-based microemulsions. Eur. J. Pharm. Biopharm. 2014, 88, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Todosijevic, M.N.; Savic, M.M.; Batinic, B.B.; Markovic, B.D.; Gasperlin, M.; Randelovic, D.V.; Lukic, M.Z.; Savic, S.D. Biocompatible microemulsions of a model NSAID for skin delivery: A decisive role of surfactants in skin penetration/irritation profiles and pharmacokinetic performance. Int. J. Pharm. 2015, 496, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Kriwet, K.; Muller-Goyman, C.C. Diclofenac release from phospholipid drug systems and permeation through excised human stratum corneum. Int. J. Pharm. 1995, 135, 231–242. [Google Scholar] [CrossRef]
- Dreher, F.; Walde, P.; Walther, P.; Wehrli, E. Interaction of a lecithin microemulsion gel with human stratum corneum and its effect on human stratum corneum transport. J. Control. Release 1997, 45, 131–140. [Google Scholar] [CrossRef]
- Naeem, M.; Rahman, N.U.; Tavares, G.D.; Barbosa, S.F.; Chacra, N.B.; Lobenberg, R.; Sarfraz, M.K. Physicochemical, in vitro and in vivo evaluation of flurbiprofen microemulsion. An. Acad. Bras. Cienc. 2015, 87, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, G.; Ozguney, I.; Karasulu, H.Y.; Guneri, T.; Basdemir, G. In vitro permeation of diclofenac sodium from novel microemulsion formulations through rabbit skin. Drug Dev. Res. 2005, 65, 17–25. [Google Scholar] [CrossRef]
- Sintov, A.C.; Botner, S. Transdermal drug delivery using microemulsion and aqueous systems: Influence of skin storage conditions on the in vitro permeability of diclofenac from aqueous vehicle systems. Int. J. Pharm. 2006, 311, 55–62. [Google Scholar] [CrossRef] [PubMed]
- El-Leithy, E.S.; Ibrahim, H.K.; Sorour, R.M. In vitro and in vivo evaluation of indomethacin nanoemulsion as a transdermal delivery system. Drug Deliv. 2015, 22, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Matsko, N.; Raupach, K.; El-Hagin, N.; Valenta, C. Development of sucrose stearate-based nanoemulsions and optimisation through gamma-cyclodextrin. Eur. J. Pharm. Biopharm. 2011, 79, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Samad, A.; Nazish, I.; Ahmed, F.J. Nanocarrier-based topical drug delivery for an antifungal drug. Drug Dev. Ind. Pharm. 2014, 40, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ouyang, W.Q.; Wei, Y.P.; Syed, S.F.; Hao, C.S.; Wang, B.Z.; Shang, Y.H. Effects of Carbopol(R) 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: A skin permeation study. Int. J. Nanomed. 2016, 11, 5971–5987. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Haberfeld, S.; Hartl, A.; Valenta, C. Effect of gamma-cyclodextrin on the in vitro skin permeation of a steroidal drug from nanoemulsions: Impact of experimental setup. Int. J. Pharm. 2012, 423, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Hoeller, S.; Sperger, A.; Valenta, C. Lecithin based nanoemulsions: A comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int. J. Pharm. 2009, 370, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Kweon, D.K.; Park, H.J. Investigations on skin permeation of hyaluronic acid based nanoemulsion as transdermal carrier. Carbohydr. Polym. 2011, 86, 837–843. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, J.; Baboota, S. Omega 3 fatty acid-enriched nanoemulsion of thiocolchicoside for transdermal delivery: Formulation, characterization and absorption studies. Drug Deliv. 2016, 23, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.W.; Zhao, L.; Wei, Y.M.; Ye, Y.; Xiao, S.H. Preparation and the in vitro evaluation of nanoemulsion system for the transdermal delivery of granisetron hydrochloride. Chem. Pharm. Bull. 2010, 58, 1015–1019. [Google Scholar] [CrossRef] [PubMed]


| Emulsion | Microemulsion | Nanoemulsion | |
|---|---|---|---|
| Physical description | Coarse dispersion | Colloidal dispersion | Colloidal dispersion |
| Particle size range | >500 nm | <100 nm | <100 nm |
| Polydispersity | High | Low | Low |
| Thermodynamic stability | Unstable | Stable | Unstable |
| Preparation | High energy | Low energy | Low/high energy |
| Composition: surfactant to oil ratio | Low | High | Moderate |
| Physical appearance | Creamy | Transparent | Transparent |
| Texture | Semi-solid | Fluid | Fluid |
| Therapeutic Class and Active Compound | H/L | Composition | Preparation Method | Physical Characterisation | Skin Permeation Evaluation | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Particle Size (nm) | Surface Charge (mV) | Poly Dispersity | Viscosity (mPa s) | |||||||
| NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAID) | ||||||||||
| Aceclofenac | L | Nanoemulsion NE31 (O/W) Triacetin 13.6% Water 54.6% Cremophor EL® 23.9% PEG-400 7.9% Nanoemulsion gel NG31 NE31 gelled with Carbopol 934® 1% Drug load (DL): 1.5 mg% | Spontaneous aqueous phase titration | NE31 39.48 | NE31 0.230 | NE31 339.51 ± 0.31 | Method (M) | Full thickness rat abdominal skin Receptor: methanol-PBS (pH 7.4) (3:7) | [57] | |
| Results (R) | Flux J (μg·cm−2·h−1) in 24 h NE31: 254.90 ± 1.25 NG31: 199.60 ± 6.93 Control (Hiffenac™ Gel) 43.67 ± 2.11 Enhancement ratio (ER) NE31: 5.84 NG31: 4.57 | |||||||||
| Aceclofenac (ACF) | L | Nanoemulsion L1.5 S0.5 P2A Medium chain triglycerides (MCT) Castor oil 1:1 20% Water 76% Lecithin 80 1.5% Sucrose stearate S-970 0.5% Sucrose palmitate P-1670 2% Drug load (DL): 1% w/w | High pressure homogenization | 181.2 ± 0.8 | −39.2 ± 1.5 | 0.110 ± 0.006 | 3.60 ± 0.23 | (M) | Human skin (in vivo 12 times tape stripping) | [65] |
| (R) | Amount of drug in SC strips (μg/cm2) L1.5 S0.5 P2A 39.85 ± 1.29 Control L2 P802A 28.32 ± 4.39 | |||||||||
| Lornoxicam | L | Nanoemulsion NE8 Labrafac® Tween 80 Pluronic F-68® Smix 3:1 Oil: Smix 2:8 Nanoemulsion gel NG8 NE 8 gelled with Carbopol 934® 1% Drug load (DL): 1.5% | Spontaneous aqueous phase titration | 139 ± 29 | 0.233 | 23.87 ± 1.86 | (M) | Full thickness pig abdominal skin Receptor: PBS (pH 7.4) | [79] | |
| (R) | Flux J (μg·cm−2·h−1) in 24 h NE8: 254.90 ± 1.25 NG8: 199.60 ± 6.93 Control (gel) 43.67 ± 2.11 | |||||||||
| Indomethacin | L | Nanoemulsion F6 (O/W) Labrafil® 5% Water 50% Tween 80 33.75% Transcutol-HP® 11.25% Smix ratio 3:1 Smix/oil ratio 4.00 Nanoemulsion gel NG6 F6 gelled with Carbopol 940® 1% Triethanolamine 0.5% Drug load (DL): 0.5% | Spontaneous aqueous phase titration | F6 25.53 ± 2.22 | F6 0.087 | F6 14.32 ± 1.12 | (M) | Full thickness rat abdominal skin Receptor: methanol-PBS (pH 7.4) (1:9) | [67] | |
| (R) | Flux J (μg·cm−2·h−1) F6: 73.96 ± 2.89 NG6: 61.64 ± 2.38 Control Indobene gel (Indo Gel™) 9.38 ± 0.41 ER F6: 7.88 NG6: 6.57 | |||||||||
| Naproxen and Caffeine | L, H | Nanoemulsions with penetration enhancers in oil phase E1 Eucalyptol (EU) 15.93% Water 30.97% Volpo-N10® 26.55% Ethanol 26.55% E2 Eucalyptol (EU) 14.63% Water 36.59% Volpo-N10® 24.39% Ethanol 24.39% O1 Oleic acid (OA) 15.93% Water 30.97% Volpo-N10® 26.55% Ethanol 26.55% O2 Oleic acid (OA) 14.63% Water 36.59% Volpo-N10® 24.39% Ethanol 24.39% Drug load (DL): Caffeine 3% Naproxen 2% Controls: C1C: water 100% C2C,N: water 40%, ethanol 60% C3C: water 75%, PEG-6000 25% C4N: water 50%, ethanol 25%, Volpo-N10 25% | Spontaneous aqueous phase titration and moderate agitation | Caffeine E1: 19.3 ± 4.0 E2: 16.0 ± 3.6 O1: 5.9 ± 2.4 O2: 1.2 ± 0.1 Naproxen E1: 37.8 ± 5.9 E2: 25.0 ± 3.0 O1: 11.6 ± 3.8 O2: 13.5 ± 4.5 | Caffeine/Naproxen-EU 15.3 Caffeine/Naproxen-OA 15.3 | Caffeine/Naproxen-EU 13.7 ± 4.5 15.1 ± 4.0 Caffeine/Naproxen-OA 23.7 ± 4.7 28.3 ± 4.5 | (M) | Full thickness human skin Receptor: PBS (pH 7.4) | [73] | |
| (R) | Flux J (μg·cm−2·h−1) in 24 h (Caffeine) E1: 263.6 ± 1.2 E2: 267.7 ± 24.0 O1: 118.8 ± 57.3 O2: 136.4 ± 95.2 Control (Caffeine) C1: 2.2 ± 0.8 C2: 25.6 ± 3.1 C3: 2.5 ± 0.7 C4: not identified Flux J (Naproxen) E1: 122.4 ± 27.1 E2: 86.6 ± 8.9 O1: 101.2 ± 41.7 O2: 74.0 ± 2.3 Control (Naproxen) C1: not identified C2: 23.4 ± 4.8 C3: 6.2 ± 0.3 C4: 7.3 ± 2.7 | |||||||||
| Diclofenac diethylamine (DDEA) | L | Nanoemulsion F1 Oleic acid 15% Water 30% Polysorbate 20 18.3% Ethanol 36.7% Smix 1:2 55% Nanoemulsion gel NE F1 gelled with Carbopol 971P® 0.75% added Propylene glycol 10.0% Methyl paraben 0.18% Propyl paraben 0.02% Drug load (DL): 1.16% w/w DDEA (equivalent to 1% w/w diclofenac) | Spontaneous aqueous phase titration and vortex mixing | 59.97 ± 3.22 | 0.28 ± 0.07 | 1.002 | (M) | Strat-M® membrane Receptor: PBS (pH 7.4): methanol (70:30) | [63] | |
| (R) | Flux J (μg·cm−2·h−1) in 12 h F1: 11.5 NE gel: 12.0 Controls DDEA solution: 1.71 Conventional gel: 11.7 Emulgel: 12.5 (coarse emulsion gel) | |||||||||
| Indomethacin | L | Nanoemulsion Triacetin® Capryol 90® 1:1 10% Water 40% Tween 80 25% Transcutol 25% Drug load (DL): 1% | Spontaneous aqueous phase titration and vortex mixing | 101.1 | n.a | n.a | 60 ± 2.1 | (M) | Full thickness hairless new born albino rat Receptor: PBS (pH 7.4) | [90] |
| (R) | Flux J (μg·cm−2·h−1) in 6 h 55.81 ± 4.65 No control | |||||||||
| Meloxicam (MLX) | L | Nanoemulsion gel Caprylic acid 0.95% Water 70%Tween 80 20% Propylene glycol 10% Carbopol 940® 0.05% | Spontaneous aqueous phase titration | 125 ± 1.9 | −31.85 ± 0.61 | 0.193 ± 0.01 | (M) | Abdominal rat skin Receptor: Acetate buffer (pH 6.0) | [80] | |
| (R) | Flux J (μg·cm−2·h−1) 6.407 ± 0.0911 Control (MLX solution): not identified Amount in skin layers in 24 h Tape strips: SC level Control > MLX-NE gel (1.02 folds) Epidermal level MLX-NE gel > Control (3.24 folds) Dermal level MLX-NE gel > Control (1.42 folds) | |||||||||
| Flufenamic acid | L | Nanoemulsion Potassium sorbate 0.1% γ-Cyclodextrin 1.0% Water to 100% PCL-liquid (cetearyl ethyl hexanoate, isopropyl myristate) 20% Sucrose stearate S-970 2.5% Drug load (DL): 1% | High pressure homogenization | - | - | - | - | (M) | Dermatomed pig abdominal skin (1.2 mm) Receptor: PBS (pH 7.4) | [91] |
| (R) | Flux J (μg·cm−2·h−1) γ–SN Fluf 1.83 ± 0.87 No control | |||||||||
| ANTIFUNGAL AGENTS | ||||||||||
| Amphotericin B | L | Nanoemulsions F I Sefsol 218® + DMSO 1:1 18.7% Water 44% Tween 80 Propylene glycol Smix 2:1 37.3% F III Sefsol 218® + DMSO 1:1 6% Water 64% Tween 80 Propylene glycol Smix 1:2 30% F VI Sefsol 218® + DMSO 1:1 16.8% Water 49.5% Tween 80 Propylene glycol Smix 1:3 33.6% Drug load (DL): 0.1% | Spontaneous aqueous phase titration | FI 67.33 ± 0.8 F III 252 ± 1.0 F VI 74.2 ± 1.2 | FI −37.305 F III −28.202 F VI −18.148 | FI 0.635 F III 0.468 F VI 0.453 | FI 25.4 ± 1 F III 40.7 ± 1.3 F VI 43.1 ± 1.4 | (M) | Albino Wistar rat abdominal skin Receptor: 2% DMSO in PBS (pH 7.4) | [92] |
| (R) | Flux J (μg·cm−2·h−1) F I: 18.02 ± 4.34 F III: 8.808 ± 3.55 F VI: 17.581 ± 2.56 Controls Drug solution 0.1% 5.895 ± 2.06 Fungisome® gel 0.1% 9.704 ± 5.74 | |||||||||
| Amphotericin B | L | Nanoemulsion NE (FV) Sefsol-218® 10% Water 65% Tween 80 Transcutol® Smix 1:3 25% AmpB-NE gel FV gelled with Carbopol 980® 1% 1:1 Drug load (DL): 0.1% | Spontaneous aqueous phase titration | FV 76.2 ± 1.4 AmpB-NE gel: 97.04 ± 7.4 | FV −31.48 AmpB-NE gel −39.27 ± 0.25 | FV 0.303 AmpB-NE gel: 0.19 ± 0.01 | FV 39.01 ± 1.4 AmpB-NE gel: 892 ± 9.64 | (M) | Albino rat abdominal skin Receptor: 2% DMSO in PBS (pH 7.4) | [58] |
| (R) | Flux J (μg·cm−2·h−1) FV: 15.74 ± 0.4 AmpB-NE gel 18.09 ± 0.6 Control (AmpB solution) 4.59 ± 0.01 ER FV: 8.97 AmpB-NE gel 10.42 | |||||||||
| Terbinafine (TER) Citral (CIT) | L L | Nanoemulsion (NE) CIT 4% Water 71% Cremophor EL-40® 18% 1,2-propylene glycol 6% Smix 3:1 NG1 NE gelled with Carbopol 934® 1% 1:1 (NG2 and NG3 contain 2% and 3% Carbopol 934®, respectively, at the same ratio with NE) Drug load (DL) in NE TER 1% and CIT 4% (oil phase) Controls: TER-CIT in Conventional gels (1.5% Carbopol 934®) | Spontaneous aqueous phase titration | NE 15.53 ± 3.32 NG1 14.88 ± 3.11 | NE −7.4 ± 1.8 NG1 −6.5 ± 2.3 | NE 0.074 ± 0.009 NG1 0.084 ± 0.025 | (M) | Guinea pig abdominal skin Receptor: PBS (pH 7.4) | [93] | |
| (R) | Flux J (μg·cm−2·h−1) (TER) NE: 11.30 ± 0.56 NG1: 11.50 ± 0.43 Control: 1.48 ± 0.34 Flux J (CIT) NE: 54.71 ± 1.34 NG1: 55.01 ± 1.67 Control: 10.55 ± 0.87 Amount in stratum corneum in 12 h (μg·cm−2) NE-TER: 1.65 ± 0.29 NG1-TER: 6.27 ± 1.03 Control TER: 5.63 ± 0.76 NE-CIT: 0.95 ± 0.52 NG1-CIT: 10.88 ± 5.80 Control CIT: 13.68 ± 1.91 Amount in epidermis-dermis in 12 h (μg·cm−2) NE-TER: 73.5 ± 8.23 NG1-TER: 75.25 ± 9.52 Control TER: 17.42 ± 5.63 NE-CIT: 210.71 ± 12.38 NG1-CIT: 214.64 ± 0.92 Control CIT: 39.47 ± 5.51 | |||||||||
| Fluconazole | H | Lecithin based NE PCL-liquid (cetearyl ethyl hexanoate, isopropyl myristate) 20% Potassium sorbate 0.1% γ-Cyclodextrin 1.0% Water to 100% Lipoid E-80® 2.5% Drug load (DL): 1% | High pressure homogenization | LN Fluc 156.87 ± 09.73 γ-LN Fluc 155.60 ± 07.96 | LN Fluc −24.70 ± 3.41 γ-LN Fluc −22.50 ± 2.20 | LN Fluc 0.05 ± 0.01 γ-LN Fluc 0.07 ± 0.02 | (M) | Dermatomed pig abdominal skin (1.2mm) Receptor: PBS (pH 7.4) | [91] | |
| (R) | Flux J (μg·cm−2·h−1) LN Fluc: 109.55 ± 11.30 γ-LN Fluc: 93.63 ± 3.80 No control | |||||||||
| CORTICOSTEROIDS | ||||||||||
| Fludrocortisone acetate | L | Lecithin based NE PCL-liquid (cetearyl ethyl hexanoate, isopropyl myristate) 20% Potassium sorbate 0.1% γ-Cyclodextrin 0.5% or 1.0% Water to 100% Lecithin E-80® 2.5% Drug load (DL): 1% | High pressure homogenization | γ-0.5% NE 171.03 ± 0.32 γ-1% NE 169.73 ± 2.35 | γ-0.5% NE −33.17 ± 0.75 γ-1% NE −31.73 ± 1.52 | γ-0.5% NE 0.098 ± 0.042 γ-1% NE 0.033 ± 0.049 | (M) | Dermatomed pig abdominal skin (1.2mm) Receptor: PBS (pH 7.4) | [94] | |
| (R) | Flux J (μg·cm−2·h−1)in 24 h Finite dose γ-1% NE 0.067 ± 0.047 NE Control: 0.008 ± 0.007 Infinite dose γ-1% NE 2.48 ± 0.68 NE Control: 0.09 ± 0.07 ER of γ-1% NE: finite dose 8.38 infinite dose 27.55 Control: NE without cyclodextrin Applied as finite (5mg/cm2) and infinite doses (500mg/cm2) No significant different in drug flux between γ-1% NE and γ-0.5% NE | |||||||||
| Fludrocortisone acetate (FA) Flumethasone pivalate (FP) | L | Nanoemulsion (positive charge) PCL-liquid (cetearyl ethyl hexanoate, isopropyl myristate) 20% Lipoid S-75® 4% α tocopherol 1% Phytosphingosine (PS) 0.4% or 0.6% Water to 100% Sucrose laurate L-1695 1% or Tween 80 1% Drug load (DL): 1% FA NL: FA NE with sucrose laurate L-1695 FA NT: FA NE with tween 80 FP NL: FP NE with sucrose laurate L-1695 FP NT: FP NE with tween 80 | High pressure homogenization | FA NL 161 ± 0.7 FA NL-0.4PS 215 ± 2.8 FA NL-0.6PS 254 ± 2.2 FA NT 170 ± 3.8 FA NT-0.4PS 216 ± 26.6 FA NT-0.6PS 170 ± 2.1 | FA NL −6.2 ± 0.4 FA NL-0.4PS +46 ± 0.4 FA NL-0.6PS +48 ± 0.7 FA NT −55 ± 0.7 FA NT-0.4PS +45 ± 0.7 FA NT-0.6 PS +48 ± 1.1 | FA NL 0.12–0.22 FA NL-0.4PS 0.22–0.25 FA NL-0.6 PS 0.06–0.1 FA NT 0.15–0.18 FA NT-0.4PS 0.13–0.18 FA NT-0.6 PS 0.10–0.14 | (M) | Dermatomed pig abdominal skin (1 mm) Receptor: PBS (pH 7.4) | [95] | |
| (R) | Flux J (μg·cm−2·h−1) in 48 h FA NL 0.126 ± 0.027 FA NL-0.4PS 0.150 ± 0.010 FA NL-0.6 PS 0.189 ± 0.012 FA NT 0.263 ± 0.043 FA NT-0.4PS 0.353 ± 0.018 FA NT-0.6 PS 0.377 ± 0.038 FP NT 2.290 ± 0.313 FP NT-0.4PS 2.698 ± 0.117 FP NT-0.6 PS 3.073 ± 0.104 No control Flux increased with PS concentration; Tween 80 > sucrose laurate | |||||||||
| Prednicarbate (PC) | L | Positively charged NE (PCNE) Phytosphingosine (PS) 0.6% Lecithin E-80®, Tween 80 2% Ethanol 20% α tocopherol 0.03% Potassium sorbate 0.1% Negatively charged NE (NCNE) Myristic acid 1% was used to replace PS Drug load (DL): 0.25% | High pressure homogenization | PCNE: 157 NCNE: 136 | PCNE: 50–60 NCNE: −(40–50) | 0.05–0.1 | (M) | Full thickness human skin Receptor: Ethanol-PBS (1:1) No PC detected in receptor in 24 h | [53,54] | |
| (R) | Amount PC in skin in 24 h PCNE: 18.4 ± 3.4 μg/mL NCNE : 11.7 ± 2.5 μg/mL No control Positive > negative charged NE | |||||||||
| Fludrocortisone acetate (FA) | L | Lecithin based NE PCL-liquid (cetearyl ethyl hexanoate, isopropyl myristate) 20% Lecithin E-80® 2.5% Potassium sorbate 0.1% γ-Cyclodextrin 1.0% Water to 100% Drug load (DL): 1% | High pressure homogenization | γ-LN Flud 175.82 ± 00.47 | γ-LN Flud −30.19 ± 4.12 | γ-LN Flud 0.09 ± 0.04 | (M) | Dermatomed pig abdominal skin (1.2mm thick) Receptor: PBS (pH 7.4) | [91] | |
| (R) | Flux J (μg·cm−2·h−1) (FA) γ-LN Flud: 4.53 ± 0.99 No control | |||||||||
| VITAMINS | ||||||||||
| α tocopherol (vitamin E) | L | Hyaluronic acid-based NE (L6) Methylene oxide (O) Tween 80-Span 20 (S) HA-GMS solution (A) Mass ratio O:S:A 2:3:95 Drug load (DL): 0.1% HA-GMS is water soluble amphiphile from crosslinking esterification of hyaluronic acid and glycerol α-mono stearate (stearin) | Oil/water/surfactant emulsifying system and solvent evaporation | 57.3 ± 0.2 | 0.260 | (M) | Full thickness Wistar rat dorsal skin Receptor: PBS (pH 7.4) | [96] | ||
| (R) | Flux J (μg·cm−2·h−1) in 24 h L6: 14.68 ± 4.13 Control: not detected Control: 0.1% vitamin E in ethanol solution | |||||||||
| α tocopherol (vitamin E) and Vitamin K1 (VK1) | L | Nanoemulsions α-tocopherol (α-TOC), VK1 10% Water 64% Tween 80 10% Ethanol 16% Drug load (DL): 3% or 5% | Spontaneous aqueous phase titration and Ultrasonic nebulization NE-neb-VK1 = ultrasonic nebulizer | NE-VK1 3% 254.8 ± 10.7 NE-neb-VK1 3% 259.4 ± 4.1 NE-VK1 5% 215.7 ± 2.3 NE-neb-VK1 5% 233.2 ± 0.2 | NE-VK1 3% −14.89 ± 2.68 NEs-neb-VK1 3% −16.60 ± 1.01 NE-VK1 5% −14.14 ± 0.29 NE-neb-VK1 5% −15.4 ± 0.1 | NE-VK1 3% 0.22 ± 0.05 NEs-neb-VK1 3% 0.19 ± 0.14 NE-VK1 5% 0.23 ± 0.02 NE-neb-VK1 5% 0.26 ± 0.02 | (M) | Pig ear skin (thickness 1.7–2.3 mm) Receptor: PBS : Ethanol (7:3 v/v) | [56] | |
| (R) | Amount in epidermis in 24 h (ng/mg) NEs-VK1 3%: 46.7 NEs-neb-VK1 3%: 72.8 NEs-VK1 5%: 55.6 NEs-neb-VK1 5%: 51.4 Amount in dermis in 24 h (ng/mg) NEs-neb-VK1 3%: 27.9 NEs-neb-VK1 5%: 24.8 No control | |||||||||
| MISCELLANEOUS | ||||||||||
| Thiocolchicoside (TCC) anti inflammatory, analgesic, muscle relaxant | H | Nanoemulsion C1 (W/O type) Linseed oil : Sefsol® 1:1 35.44% Water 10.81% Span 80 40.53% Transcutol P® 13.51% Smix 3:1 C3 (W/O type) Linseed oil : Sefsol® 1:1 35.19% Water 9.26% Span 80 41.67% Transcutol P® 13.89% Smix 3:1 Drug load (DL): 0.2% | Spontaneous aqueous phase titration | C1 117.73 ± 13.71 C3 131.43 ± 15.15 | C1 0.285 C3 0.311 | C1 61.12 ± 5.28 C3 65.75 ± 6.08 | (M) | Full thickness weanling pig abdominal skin Receptor: PBS (pH 7.4) | [97] | |
| (R) | Flux J (μg·cm−2·h−1) in 24 h (TCC) C1: 30.63 ± 4.18 C3: 28.01 ± 3.41 Control (TCC aqueous solution) 5.99 ± 0.73 ER C1: 5.114 C3: 4.676 Type of NE did not influence ER | |||||||||
| Curcumin natural anti-inflammatory | L | Nanoemulsion NE gel Glyceryl monooleate (GMO) Water Cremophor RH40® PEG 400 O:S:CoS 1:8:1 Water: oil phase 5:1 NE gelled with Viscolam AT 100P® 5% and added: Methyl paraben 0.2% Propyl paraben 0.05% Glycerine 5% Propylene glycol 15% Drug load (DL): 0.35% | Spontaneous aqueous phase titration with 1 h ultrasonic sonication | 85.0 ± 1.5 | 0.18 ± 0.0 | −5.9 ± 0.3 | 2000–2700 | (M) | Shed snake skin Receptor: PBS (pH 7.4) | [91] |
| (R) | Flux J (μg·cm−2·h−1) NE gel: 1.699 ± 0.050 Control gel 0.836 ± 0.004 | |||||||||
| Bovine albumin-fluorescein isothiocyanate conjugate (FITC-BSA) vaccine model | L | Nanoemulsion Squalene 37.5% Water 52.5% Span 80, Tween 80 10% Smix 1:1 Drug load (DL): 0.25% | Spontaneous aqueous phase titration with high pressure homogenization | 85.2 ± 15.5 | −45.17 ± 4.77 | 0.186 ± 0.026 | 14.6 ± 0.026 | (M) | Mouse skin Receptor: PBS (pH 7.4) | [69] |
| (R) | Flux J (μg·cm−2·h−1) in 48 h NE: 23.44 ± 17.230 Controls CE: 6.10 ± 0.977 CA: 3.15 ± 0.897 Controls CE: emulsifiers solution (10% of Smix) CA: aqueous solution | |||||||||
| Granisetron HCl (GHCl) anti emetic drug | H | Nanoemulsion with penetration enhancer NMP Isopropyl myristate (IPM) 4% Tween 85 20% Ethanol 20% N-methyl pyrrolidone (NMP) 10% Water up to 100% Drug load (DL): 2.5% | Spontaneous aqueous phase titration | 48.3 ± 1.7 | 0.27 ± 0.02 | (M) | Full thickness rat abdominal skin Receptor: saline solution | [98] | ||
| (R) | Flux J (μg·cm−2·h−1) NMP NE: 85.39 ± 2.90 Control: 71.17 ± 3.54 Amount in skin in 12 h (μg·cm−2) NMP NE: 891.8 ± 2.86 Control: 889.1 ± 2.24 NMP NE ≅ NE Control: NE without NMP | |||||||||
| Minoxidil (Min) antihypertensive vasodilator (stimulate hair growth) | H | Lecithin based NE PCL-liquid (cetearyl ethyl hexanoate, isopropyl myristate) 20% Potassium sorbate 0.1% γ-Cyclodextrin 1.0% Water to 100% Lecithin E-80® 2.5% Drug load (DL): 1% | High pressure homogenization | - | - | - | (M) | Dermatomed pig abdominal skin (1.2mm thick) Receptor: PBS (pH 7.4) | [91] | |
| (R) | Flux J (μg·cm−2·h−1) 102.56 ± 9.41 No control | |||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. https://doi.org/10.3390/pharmaceutics9040037
Nastiti CMRR, Ponto T, Abd E, Grice JE, Benson HAE, Roberts MS. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics. 2017; 9(4):37. https://doi.org/10.3390/pharmaceutics9040037
Chicago/Turabian StyleNastiti, Christofori M. R. R., Thellie Ponto, Eman Abd, Jeffrey E. Grice, Heather A. E. Benson, and Michael S. Roberts. 2017. "Topical Nano and Microemulsions for Skin Delivery" Pharmaceutics 9, no. 4: 37. https://doi.org/10.3390/pharmaceutics9040037
APA StyleNastiti, C. M. R. R., Ponto, T., Abd, E., Grice, J. E., Benson, H. A. E., & Roberts, M. S. (2017). Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics, 9(4), 37. https://doi.org/10.3390/pharmaceutics9040037