Targeting Strategies for the Combination Treatment of Cancer Using Drug Delivery Systems
Abstract
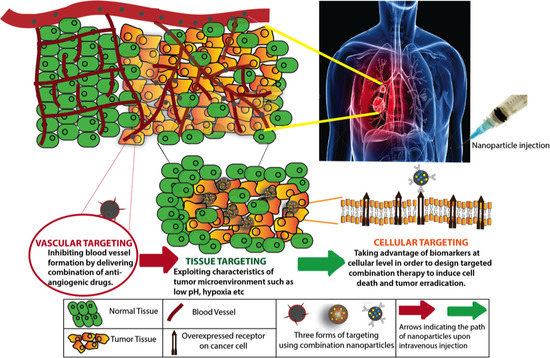
:1. Introduction
2. Targeted Nanomedicines
3. Targeting Tumor Vasculature
4. Targeting the Tumor Microenvironment
5. Cellular Level Targeting
5.1. Direct Cellular Targeting
5.2. Indirect Cellular Targeting
5.3. Dual Effect (Direct and Indirect) Cellular Targeting
6. Multi-tier (Vascular, Cellular and Tissue Combinations) Targeting
7. Conclusions and Future Direction of Active Targeting
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, I.B.; Joe, A.K. Mechanisms of disease: Oncogene addiction—A rationale for molecular targeting in cancer therapy. Nat. Clin. Pract. Oncol. 2006, 3, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Tutar, L.; Tutar, E.; Ozgur, A.; Tutar, Y. Therapeutic Targeting of microRNAs in Cancer: Future Perspectives. Drug Dev. Res. 2015, 76, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, A. History of Cancer, Ancient and Modern Treatment Methods. J. Cancer Sci. Ther. 2009, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, R.; Seidi, K.; Banimohamad-Shotorbani, B.; Jahanban-Esfahlan, A.; Yousefi, B. Combination of nanotechnology with vascular targeting agents for effective cancer therapy. J. Cell. Physiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.S.; Sherwood, S.G.; Drews, K.C.; Kester, M. Targeting cancer cells in the tumor microenvironment: Opportunities and challenges in combinatorial nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Huang, X.; Mo, J.; Zhao, W. Drug Delivery Using Nanoparticles for Cancer Stem-Like Cell Targeting. Front. Pharmacol. 2016, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev. 2017, 46, 3830–3852. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Sahu, P.K.; Beg, S.; Babu, S.M. Nanoparticles for Cancer Targeting: Current and Future Directions. Curr. Drug Deliv. 2016, 13, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Ustundag Okur, N.; Karavas, E.; Bikiaris, D.N. Surface Modified Multifunctional and Stimuli Responsive Nanoparticles for Drug Targeting: Current Status and Uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Y.; Li, C.; Wang, Y.; Sun, Y.; Wang, J. A novel anti-VEGF targeting and MRI-visible smart drug delivery system for specific diagnosis and therapy of liver cancer. Macromol. Biosci. 2013, 13, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Roger, E.; Lautram, N.; Benoit, J.P.; Passirani, C. The rise and rise of stealth nanocarriers for cancer therapy: Passive versus active targeting. Nanomedicine 2010, 5, 1415–1433. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xie, F.; Wen, X.; Chen, H.; Zhang, H.; Liu, J.; Zhang, H.; Zou, H.; Yu, Y.; Chen, Y.; et al. Therapeutic PEG-ceramide nanomicelles synergize with salinomycin to target both liver cancer cells and cancer stem cells. Nanomedicine 2017, 12, 1025–1042. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.L.; Yoshioka, Y.; Ruan, G.X.; Chen, Y.Z.; Mizuguchi, H.; Mukai, Y.; Okada, N.; Gao, J.Q.; Nakagawa, S. Optimization and internalization mechanisms of PEGylated adenovirus vector with targeting peptide for cancer gene therapy. Biomacromolecules 2012, 13, 2402–2409. [Google Scholar] [CrossRef] [PubMed]
- Balalaeva, I.V.; Zdobnova, T.A.; Krutova, I.V.; Brilkina, A.A.; Lebedenko, E.N.; Deyev, S.M. Passive and active targeting of quantum dots for whole-body fluorescence imaging of breast cancer xenografts. J. Biophotonics 2012, 5, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Sykes, E.A.; Chen, J.; Zheng, G.; Chan, W.C. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano 2014, 8, 5696–5706. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar] [PubMed]
- Gao, W.; Xiang, B.; Meng, T.T.; Liu, F.; Qi, X.R. Chemotherapeutic drug delivery to cancer cells using a combination of folate targeting and tumor microenvironment-sensitive polypeptides. Biomaterials 2013, 34, 4137–4149. [Google Scholar] [CrossRef] [PubMed]
- Angioletti-Uberti, S. Exploiting Receptors Competition to Enhance Nanoparticles Binding Selectivity. Phys. Rev. Lett. 2017, 118, 068001. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Le Breton, A.; Preat, V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, S.; Karagiannis, T.C. Transferrin receptor-mediated endocytosis: A useful target for cancer therapy. J. Membr. Biol. 2014, 247, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ling, C.; Li, W.; Jiang, H.; Zhi, Q.; Jiang, M. Molecular Mechanisms of Anti-cancer Activities of β-elemene: Targeting Hallmarks of Cancer. Anticancer Agents Med. Chem. 2016, 16, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.; Kydd, J.; Piel, B.; Rai, P. Targeting Cancer using Polymeric Nanoparticle mediated Combination Chemotherapy. Int. J. Nanomed. Nanosurg. 2016, 2. [Google Scholar] [CrossRef]
- Sneider, A.; Jadia, R.; Piel, B.; VanDyke, D.; Tsiros, C.; Rai, P. Engineering Remotely Triggered Liposomes to Target Triple Negative Breast Cancer. Oncomedicine 2017, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jadia, R.; Scandore, C.; Rai, P. Nanoparticles for Effective Combination Therapy of Cancer. Int. J. Nanotechnol. Nanomed. 2016, 1, 1–27. [Google Scholar]
- Long, J.T.; Cheang, T.Y.; Zhuo, S.Y.; Zeng, R.F.; Dai, Q.S.; Li, H.P.; Fang, S. Anticancer drug-loaded multifunctional nanoparticles to enhance the chemotherapeutic efficacy in lung cancer metastasis. J. Nanobiotechnology 2014, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, L.; Lv, P.; Zhang, P. Transferrin-conjugated doxorubicin-loaded lipid-coated nanoparticles for the targeting and therapy of lung cancer. Oncol. Lett. 2015, 9, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wang, C.Z.; Fan, H.J.; Zhang, C.J.; Zhang, H.W.; Lv, M.H.; Cui, S.D. A dual-targeting liposome conjugated with transferrin and arginine-glycine-aspartic acid peptide for glioma-targeting therapy. Oncol. Lett. 2014, 8, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Ocampo, S.M.; Simoes, B.M.; Josefa Rodriguez, M.; Cadenas, J.F.; Couleaud, P.; Spence, K.; Latorre, A.; Miranda, R.; Somoza, A.; et al. Multifunctionalized iron oxide nanoparticles for selective drug delivery to CD44-positive cancer cells. Nanotechnology 2016, 27, 065103. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, H.; Chen, C.; Zhang, X.; Tian, X.; Zhang, Y.; Shi, Z.; Liu, Q. Polymer-Lipid Hybrid Theranostic Nanoparticles Co-Delivering Ultrasmall Superparamagnetic Iron Oxide and Paclitaxel for Targeted Magnetic Resonance Imaging and Therapy in Atherosclerotic Plaque. J. Biomed. Nanotechnol. 2016, 12, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Ndong, C.; Toraya-Brown, S.; Kekalo, K.; Baker, I.; Gerngross, T.U.; Fiering, S.N.; Griswold, K.E. Antibody-mediated targeting of iron oxide nanoparticles to the folate receptor alpha increases tumor cell association in vitro and in vivo. Int. J. Nanomed. 2015, 10, 2595–2617. [Google Scholar] [CrossRef]
- Pramanik, A.K.; Siddikuzzaman; Palanimuthu, D.; Somasundaram, K.; Samuelson, A.G. Biotin Decorated Gold Nanoparticles for Targeted Delivery of a Smart-Linked Anticancer Active Copper Complex: In Vitro and In Vivo Studies. Bioconjug. Chem. 2016, 27, 2874–2885. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Rao, B.; Chen, S.; Duanmu, J. Targeting tissue factor on tumour cells and angiogenic vascular endothelial cells by factor VII-targeted verteporfin photodynamic therapy for breast cancer in vitro and in vivo in mice. BMC Cancer 2010, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Barr, M.P.; O’Byrne, K.J.; Al-Sarraf, N.; Gray, S.G. VEGF-mediated cell survival in non-small-cell lung cancer: Implications for epigenetic targeting of VEGF receptors as a therapeutic approach. Epigenomics 2015, 7, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, H.J.; Liang, Y.; Liang, X.J.; Huang, H.C.; Zhao, Y.Z.; Liao, Q.C.; Chen, Y.Q.; Leng, A.M.; Yuan, W.J.; et al. Tumor-specific expression of shVEGF and suicide gene as a novel strategy for esophageal cancer therapy. World J. Gastroenterol. 2016, 22, 5342–5352. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Liu, T.; Zhang, D.S.; Wang, Y.; Gu, H.; Di, W. Highly effective antiangiogenesis via magnetic mesoporous silica-based siRNA vehicle targeting the VEGF gene for orthotopic ovarian cancer therapy. Int. J. Nanomed. 2015, 10, 2579–2594. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, Y.; Li, Y.; Jin, Z.; Pan, Y.; Liu, G.; Fu, S.; Zhang, Y.; Feng, K.; Feng, Y. MicroRNA-128 plays a critical role in human non-small cell lung cancer tumourigenesis, angiogenesis and lymphangiogenesis by directly targeting vascular endothelial growth factor-C. Eur. J. Cancer 2014, 50, 2336–2350. [Google Scholar] [CrossRef] [PubMed]
- Toi, H.; Tsujie, M.; Haruta, Y.; Fujita, K.; Duzen, J.; Seon, B.K. Facilitation of endoglin-targeting cancer therapy by development/utilization of a novel genetically engineered mouse model expressing humanized endoglin (CD105). Int. J. Cancer 2015, 136, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shao, K.; Liu, Y.; Kuang, Y.; Li, J.; An, S.; Guo, Y.; Ma, H.; Jiang, C. Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis. ACS Nano 2013, 7, 2860–2871. [Google Scholar] [CrossRef] [PubMed]
- Giatromanolaki, A.; Koukourakis, M.I.; Koutsopoulos, A.; Mendrinos, S.; Sivridis, E. The metabolic interactions between tumor cells and tumor-associated stroma (TAS) in prostatic cancer. Cancer Biol. Ther. 2012, 13, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Deng, A.; Jiang, W.; Tian, R.; Shen, Y. Synthesis and in vitro evaluation of pH-sensitive magnetic nanocomposites as methotrexate delivery system for targeted cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Cailin, F.; Wenlan, W.; Pinghua, Y.; Jiayu, G.; Junbo, L. Preparation and properties evaluation of a novel pH-sensitive liposomes based on imidazole-modified cholesterol derivatives. Int. J. Pharm. 2017, 518, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Pires, B.R.; Amorim, I.S.; Souza, L.D.; Rodrigues, J.A.; Mencalha, A.L. Targeting Cellular Signaling Pathways in Breast Cancer Stem Cells and its Implication for Cancer Treatment. Anticancer Res. 2016, 36, 5681–5691. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.Z. Combination therapy targeting EGFR/MET crosstalk using nanotechnology improves photodynamic therapy treatment of pancreatic cancer. Mol. Cancer Ther. 2009. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, Q.; Fu, Y.; Sun, X.; Gong, T.; Zhang, Z. Coadministration of Oligomeric Hyaluronic Acid-Modified Liposomes with Tumor-Penetrating Peptide-iRGD Enhances the Antitumor Efficacy of Doxorubicin against Melanoma. ACS Appl. Mater. Interfaces 2017, 9, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Ruzzo, A.; Catalano, V.; Canestrari, E.; Giacomini, E.; Santini, D.; Tonini, G.; Vincenzi, B.; Fiorentini, G.; Magnani, M.; Graziano, F. Genetic modulation of the interleukin 6 (IL-6) system in patients with advanced gastric cancer: A background for an alternative target therapy. BMC Cancer 2014, 14, 357. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Liu, X.; Gui, R.; Wang, Z. Facile synthesis of gold nanorods/hydrogels core/shell nanospheres for pH and near-infrared-light induced release of 5-fluorouracil and chemo-photothermal therapy. Colloids Surf. B Biointerfaces 2015, 128, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ding, L.; Xu, H.J.; Shen, Z.; Ju, H.; Jia, L.; Bao, L.; Yu, J.S. Cell-specific and pH-activatable rubyrin-loaded nanoparticles for highly selective near-infrared photodynamic therapy against cancer. J. Am. Chem. Soc. 2013, 135, 18850–18858. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, J.P.; Wolburg, H.; Klumpp, A.; Probst, H.; Weller, M. Hypoxia-induced cell death in human malignant glioma cells: Energy deprivation promotes decoupling of mitochondrial cytochrome c release from caspase processing and necrotic cell death. Cell. Death Differ. 2003, 10, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Ammirante, M.; Shalapour, S.; Kang, Y.; Jamieson, C.A.; Karin, M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc. Natl. Acad. Sci. USA 2014, 111, 14776–14781. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Peng, X.; Wang, Y.; Wang, Y.; Shin, D.M.; El-Sayed, M.A.; Nie, S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yan, C.; Yan, Y.; Chen, L.; Song, L.; Zang, F.; An, Y.; Teng, G.; Gu, N.; Zhang, Y. Multi-modal Mn-Zn ferrite nanocrystals for magnetically-induced cancer targeted hyperthermia: A comparison of passive and active targeting effects. Nanoscale 2016, 8, 16902–16915. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.; Zhang, T.; Zhang, P.; Sun, J.; He, Z. Synergistic and complete reversal of the multidrug resistance of mitoxantrone hydrochloride by three-in-one multifunctional lipid-sodium glycocholate nanocarriers based on simultaneous BCRP and Bcl-2 inhibition. Int. J. Nanomed. 2016, 11, 4077–4091. [Google Scholar] [CrossRef]
- Shen, Q.; Qiu, L. Reversal of P-glycoprotein-mediated multidrug resistance by doxorubicin and quinine co-loaded liposomes in tumor cells. J. Liposome Res. 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Scarano, W.; de Souza, P.; Stenzel, M.H. Dual-drug delivery of curcumin and platinum drugs in polymeric micelles enhances the synergistic effects: A double act for the treatment of multidrug-resistant cancer. Biomater. Sci. 2015, 3, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Zahreddine, H.; Borden, K.L. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Ma, Y.; Li, L. The application of prodrug-based nano-drug delivery strategy in cancer combination therapy. Colloids Surf. B Biointerfaces 2016, 146, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jin, H.; Wang, C.; Zhang, Z.; Ruan, H.; Sun, L.; Yang, C.; Li, Y.; Qin, W.; Wang, C. Synergistic Cisplatin/Doxorubicin Combination Chemotherapy for Multidrug-Resistant Cancer via Polymeric Nanogels Targeting Delivery. ACS Appl. Mater. Interfaces 2017, 9, 9426–9436. [Google Scholar] [CrossRef] [PubMed]
- Murugan, C.; Rayappan, K.; Thangam, R.; Bhanumathi, R.; Shanthi, K.; Vivek, R.; Thirumurugan, R.; Bhattacharyya, A.; Sivasubramanian, S.; Gunasekaran, P.; et al. Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in bresat cancer cells: An improved nanomedicine strategies. Sci. Rep. 2016, 6, 34053. [Google Scholar] [CrossRef] [PubMed]
- Ziming, Y. Synergistic mediation of tumor signaling pathways in hepatocellular carcinoma therapy via dual-drug-loaded pH-responsive electrospun fibrous scaffolds. J. Mater. Chem. B 2015, 3, 3436–3446. [Google Scholar]
- Biffi, S.; Voltan, R.; Rampazzo, E.; Prodi, L.; Zauli, G.; Secchiero, P. Applications of nanoparticles in cancer medicine and beyond: Optical and multimodal in vivo imaging, tissue targeting and drug delivery. Expert Opin. Drug Deliv. 2015, 12, 1837–1849. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.E.; Wasserman, M.A.; Kibbe, M.R. Targeted Nanotherapies for the Treatment of Surgical Diseases. Ann. Surg. 2016, 263, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.; Zern, B.J.; Anselmo, A.C.; Shuvaev, V.V.; Mitragotri, S.; Muzykantov, V. Vascular targeting of nanocarriers: Perplexing aspects of the seemingly straightforward paradigm. ACS Nano 2014, 8, 4100–4132. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Cunningham, D.; Chau, I. Targeting Angiogenic Pathways in Colorectal Cancer: Complexities, Challenges and Future Directions. Curr. Drug Targets 2017, 18, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, L.; Wang, X.; Zhang, X.; Liu, M.; Zeng, W. Integrin (alphavbeta3) Targeted RGD Peptide Based Probe for Cancer Optical Imaging. Curr. Protein Pept. Sci. 2016, 17, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Guyot, M.; Hilmi, C.; Ambrosetti, D.; Merlano, M.; Lo Nigro, C.; Durivault, J.; Grepin, R.; Pages, G. Targeting the pro-angiogenic forms of VEGF or inhibiting their expression as anti-cancer strategies. Oncotarget 2017, 8, 9174–9188. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.D.; Gutierrez, M.M.; Volker, K.W.; Howard, D.L. Thermal Effect of J-Plasma(R) Energy in a Porcine Tissue Model: Implications for Minimally Invasive Surgery. Surg. Technol. Int. 2017, 30, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, C.; Wang, W.; Liu, J.; Liu, Q.; Huang, F.; Chu, L.; Gao, H.; Li, C.; Kong, D.; et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci. Rep. 2016, 6, 21225. [Google Scholar] [CrossRef] [PubMed]
- Somani, R.R.; Bhanushali, U.V. Targeting angiogenesis for treatment of human cancer. Indian J. Pharm. Sci. 2013, 75, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Cruz, A.; Fonte, P.; Pinto, I.M.; Neves-Petersen, M.T.; Sarmento, B. A new paradigm for antiangiogenic therapy through controlled release of bevacizumab from PLGA nanoparticles. Sci. Rep. 2017, 7, 3736. [Google Scholar] [CrossRef] [PubMed]
- Pham, E.; Yin, M.; Peters, C.G.; Lee, C.R.; Brown, D.; Xu, P.; Man, S.; Jayaraman, L.; Rohde, E.; Chow, A.; et al. Preclinical Efficacy of Bevacizumab with CRLX101, an Investigational Nanoparticle-Drug Conjugate, in Treatment of Metastatic Triple-Negative Breast Cancer. Cancer Res. 2016, 76, 4493–4503. [Google Scholar] [CrossRef] [PubMed]
- Keefe, S.M.; Hoffman-Censits, J.; Cohen, R.B.; Mamtani, R.; Heitjan, D.; Eliasof, S.; Nixon, A.; Turnbull, B.; Garmey, E.G.; Gunnarsson, O.; et al. Efficacy of the nanoparticle-drug conjugate CRLX101 in combination with bevacizumab in metastatic renal cell carcinoma: Results of an investigator-initiated phase I-IIa clinical trial. Ann. Oncol. 2016, 27, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Fu, F.; Xiong, Z.; Shen, M.; Shi, X. Dendrimer-entrapped gold nanoparticles modified with RGD peptide and alpha-tocopheryl succinate enable targeted theranostics of cancer cells. Colloids Surf. B Biointerfaces 2015, 133, 36–42. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Alves, C.S.; Oliveira, N.; Rodrigues, J.; Zhu, J.; Banyai, I.; Tomas, H.; Shi, X. RGD peptide-modified multifunctional dendrimer platform for drug encapsulation and targeted inhibition of cancer cells. Colloids Surf. B Biointerfaces 2015, 125, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Dickreuter, E.; Cordes, N. The cancer cell adhesion resistome: mechanisms, targeting and translational approaches. Biol. Chem. 2017, 398, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Thao, L.Q.; Lee, C.; Kim, B.; Lee, S.; Kim, T.H.; Kim, J.O.; Lee, E.S.; Oh, K.T.; Choi, H.G.; Yoo, S.D.; et al. Doxorubicin and paclitaxel co-bound lactosylated albumin nanoparticles having targetability to hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2017, 152, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Rao, B.; Chen, S.; Duanmu, J. Selective and effective killing of angiogenic vascular endothelial cells and cancer cells by targeting tissue factor using a factor VII-targeted photodynamic therapy for breast cancer. Breast Cancer Res. Treat. 2011, 126, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, J.; Liang, C.; Feng, L.; Dong, Z.; Song, X.; Song, G.; Liu, Z. Drug-induced co-assembly of albumin/catalase as smart nano-theranostics for deep intra-tumoral penetration, hypoxia relieve, and synergistic combination therapy. J. Control. Release 2016, 263, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, M.; Mao, H.; Wang, N.; Zhang, B.; Zhao, X.; Qian, J.; Xing, C. Albumin-based nanoparticles as methylprednisolone carriers for targeted delivery towards the neonatal Fc receptor in glomerular podocytes. Int. J. Mol. Med. 2017, 39, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Chen, W.; Cooper, H.M.; Xu, Z.P. A Facile Way of Modifying Layered Double Hydroxide Nanoparticles with Targeting Ligand-Conjugated Albumin for Enhanced Delivery to Brain Tumour Cells. ACS Appl. Mater. Interfaces 2017, 9, 20444–20453. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, X.; Wang, C.; Feng, L.; Li, Y.; Liu, Z. Drug-Induced Self-Assembly of Modified Albumins as Nano-theranostics for Tumor-Targeted Combination Therapy. ACS Nano 2015, 9, 5223–5233. [Google Scholar] [CrossRef] [PubMed]
- Kibria, G.; Hatakeyama, H.; Sato, Y.; Harashima, H. Anti-tumor effect via passive anti-angiogenesis of PEGylated liposomes encapsulating doxorubicin in drug resistant tumors. Int. J. Pharm. 2016, 509, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Adochite, R.C.; Moshnikova, A.; Carlin, S.D.; Guerrieri, R.A.; Andreev, O.A.; Lewis, J.S.; Reshetnyak, Y.K. Targeting breast tumors with pH (low) insertion peptides. Mol. Pharm. 2014, 11, 2896–2905. [Google Scholar] [CrossRef] [PubMed]
- Weerakkody, D.; Moshnikova, A.; El-Sayed, N.S.; Adochite, R.C.; Slaybaugh, G.; Golijanin, J.; Tiwari, R.K.; Andreev, O.A.; Parang, K.; Reshetnyak, Y.K. Novel pH-Sensitive Cyclic Peptides. Sci. Rep. 2016, 6, 31322. [Google Scholar] [CrossRef] [PubMed]
- Adochite, R.C.; Moshnikova, A.; Golijanin, J.; Andreev, O.A.; Katenka, N.V.; Reshetnyak, Y.K. Comparative Study of Tumor Targeting and Biodistribution of pH (Low) Insertion Peptides (pHLIP((R)) Peptides) Conjugated with Different Fluorescent Dyes. Mol. Imaging Biol. 2016, 18, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Tapmeier, T.T.; Moshnikova, A.; Beech, J.; Allen, D.; Kinchesh, P.; Smart, S.; Harris, A.; McIntyre, A.; Engelman, D.M.; Andreev, O.A.; et al. The pH low insertion peptide pHLIP Variant 3 as a novel marker of acidic malignant lesions. Proc. Natl. Acad. Sci. USA 2015, 112, 9710–9715. [Google Scholar] [CrossRef] [PubMed]
- Karabadzhak, A.G.; An, M.; Yao, L.; Langenbacher, R.; Moshnikova, A.; Adochite, R.C.; Andreev, O.A.; Reshetnyak, Y.K.; Engelman, D.M. pHLIP-FIRE, a cell insertion-triggered fluorescent probe for imaging tumors demonstrates targeted cargo delivery in vivo. ACS Chem. Biol. 2014, 9, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Reshetnyak, Y.K. Imaging Tumor Acidity: pH-Low Insertion Peptide Probe for Optoacoustic Tomography. Clin. Cancer Res. 2015, 21, 4502–4504. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yin, Q.; Su, J.; Sun, H.; Meng, Q.; Chen, Y.; Chen, L.; Huang, Y.; Gu, W.; Xu, M.; et al. Inhibition of metastasis and growth of breast cancer by pH-sensitive poly (beta-amino ester) nanoparticles co-delivering two siRNA and paclitaxel. Biomaterials 2015, 48, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ji, Y.; Chen, Q.; Zhu, X.; Zhang, X.; Tan, Z.; Tian, Q.; Yang, X.; Zhang, Z. In vitro and in vivo chemo-phototherapy of magnetic TiO2 drug delivery system formed by pH-sensitive coordination bond. J. Biomater. Appl. 2016, 31, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Thurn, K.T.; Paunesku, T.; Wu, A.; Brown, E.M.; Lai, B.; Vogt, S.; Maser, J.; Aslam, M.; Dravid, V.; Bergan, R.; et al. Labeling TiO2 nanoparticles with dyes for optical fluorescence microscopy and determination of TiO2-DNA nanoconjugate stability. Small 2009, 5, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Li, Y.; Shimizu, H. Enhanced degradation in nanocomposites of TiO2 and biodegradable polymer. Environ. Sci. Technol. 2008, 42, 4551–4554. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lv, G.; Pan, C.; Song, M.; Wu, C.; Guo, D.; Wang, X.; Chen, B.; Gu, Z. Poly(lactic acid) (PLA) based nanocomposites–A novel way of drug-releasing. Biomed. Mater. 2007, 2, L1–L4. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, B.; Cheng, J.; Wang, J.; Xu, W.; Liu, L.; Xia, G.; Wei, H.; Wang, X.; Yang, M.; et al. Biocompatibility of Fe3O4/DNR magnetic nanoparticles in the treatment of hematologic malignancies. Int. J. Nanomed. 2010, 5, 1079–1084. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J. Biomed. Mater. Res. A 2007, 80, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Luo, G.F.; Qiu, W.X.; Lei, Q.; Liu, L.H.; Zheng, D.-W.; Hong, S.; Cheng, S.X.; Zhang, X.-Z. Tumor-Triggered Drug Release with Tumor-Targeted Accumulation and Elevated Drug Retention To Overcome Multidrug Resistance. Chem. Mater. 2016, 28, 6742–6752. [Google Scholar] [CrossRef]
- Nedrow, J.R.; Josefsson, A.; Park, S.; Back, T.; Hobbs, R.F.; Brayton, C.; Bruchertseifer, F.; Morgenstern, A.; Sgouros, G. Pharmacokinetics, microscale distribution, and dosimetry of alpha-emitter-labeled anti-PD-L1 antibodies in an immune competent transgenic breast cancer model. EJNMMI Res. 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Dhule, S.S.; Penfornis, P.; He, J.; Harris, M.R.; Terry, T.; John, V.; Pochampally, R. The combined effect of encapsulating curcumin and C6 ceramide in liposomal nanoparticles against osteosarcoma. Mol. Pharm. 2014, 11, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Lam, S.K.; Zheng, C.Y.; Ho, J.C. The Effect of Tumor Microenvironment on Autophagy and Sensitivity to Targeted Therapy in EGFR-Mutated Lung Adenocarcinoma. J. Cancer 2015, 6, 382–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Atkinson, K.; Zhang, T. Combination of chemotherapy and cancer stem cell targeting agents: Preclinical and clinical studies. Cancer Lett. 2017, 396, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, J.; Cao, W.; Yang, J.; Lian, H.; Li, X.; Sun, Y.; Wang, Y.; Wang, S.; He, Z. Dual targeting folate-conjugated hyaluronic acid polymeric micelles for paclitaxel delivery. Int. J. Pharm. 2011, 421, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, S.K.; Salzano, G.; Sarisozen, C.; Torchilin, V. Anti-cancer activity of doxorubicin-loaded liposomes co-modified with transferrin and folic acid. Eur. J. Pharm. Biopharm. 2016, 105, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Aberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Zhang, R.; Chen, Z.; Zhu, L. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J. Control. Release 2014, 196, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Baradia, D.; Vhora, I.; Rathi, M.; Misra, A. cRGD grafted liposomes containing inorganic nano-precipitate complexed siRNA for intracellular delivery in cancer cells. J. Control. Release 2014, 182, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Xu, Y.; Qiu, L. Enhanced combination therapy effect on paclitaxel-resistant carcinoma by chloroquine co-delivery via liposomes. Int. J. Nanomed. 2015, 10, 6615–6632. [Google Scholar] [CrossRef]
- Schroeder, A.; Sigal, A.; Turjeman, K.; Barenholz, Y. Using PEGylated nano-liposomes to target tissue invaded by a foreign body. J. Drug Target. 2008, 16, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Li, Y.; Yang, X.; Huang, Y.; Wu, H.; Huang, Y.; Lin, J.; Li, Y.; Hou, Z.; Zhang, Q. Development of both methotrexate and mitomycin C loaded PEGylated chitosan nanoparticles for targeted drug codelivery and synergistic anticancer effect. ACS Appl. Mater. Interfaces 2014, 6, 11413–11423. [Google Scholar] [CrossRef] [PubMed]
- Lin, J. Tumor-targeted co-delivery of mitomycin C and 10-hydroxycamptothecin via micellar nanocarriers for enhanced anticancer efficacy. RSC Adv. 2015, 5, 23022–23033. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Pan, J.; Sarisozen, C.; Luther, E.; Torchilin, V. Enhanced Cytotoxicity of Folic Acid-Targeted Liposomes Co-Loaded with C6 Ceramide and Doxorubicin: In Vitro Evaluation on HeLa, A2780-ADR, and H69-AR Cells. Mol. Pharm. 2016, 13, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.; Surapaneni, S.K.; Tikoo, K.; Singh, C.; Burman, R.; Gill, M.S.; Suresh, S. Folic acid functionalized long-circulating co-encapsulated docetaxel and curcumin solid lipid nanoparticles: In vitro evaluation, pharmacokinetic and biodistribution in rats. Drug Deliv. 2016, 23, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Malarvizhi, G.L.; Retnakumari, A.P.; Nair, S.; Koyakutty, M. Transferrin targeted core-shell nanomedicine for combinatorial delivery of doxorubicin and sorafenib against hepatocellular carcinoma. Nanomedicine 2014, 10, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Sarisozen, C.; Abouzeid, A.H.; Torchilin, V.P. The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur. J. Pharm. Biopharm. 2014, 88, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Zhang, S.; Sun, H. Co-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatment. Oncol. Rep. 2017, 37, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Abetov, D.; Mustapova, Z.; Saliev, T.; Bulanin, D.; Batyrbekov, K.; Gilman, C.P. Novel Small Molecule Inhibitors of Cancer Stem Cell Signaling Pathways. Stem Cell. Rev. 2015, 11, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.S.; Jang, G.B.; Lee, H.Y.; Nam, J.S. Targeting cancer stem cells by using the nanoparticles. Int. J. Nanomed. 2015, 10, 251–260. [Google Scholar] [CrossRef]
- Li, S.Y.; Sun, R.; Wang, H.X.; Shen, S.; Liu, Y.; Du, X.J.; Zhu, Y.H.; Jun, W. Combination therapy with epigenetic-targeted and chemotherapeutic drugs delivered by nanoparticles to enhance the chemotherapy response and overcome resistance by breast cancer stem cells. J. Control. Release 2015, 205, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Arabi, L.; Badiee, A.; Mosaffa, F.; Jaafari, M.R. Targeting CD44 expressing cancer cells with anti-CD44 monoclonal antibody improves cellular uptake and antitumor efficacy of liposomal doxorubicin. J. Control. Release 2015, 220, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Hybrid nanoparticles coated with hyaluronic acid lipoid for targeted co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. J. Mater. Chem. B 2017, 5, 6762–6775. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Wang, Y.; Chen, M. Hyaluronic acid-coated PEI-PLGA nanoparticles mediated co-delivery of doxorubicin and miR-542-3p for triple negative breast cancer therapy. Nanomedicine 2016, 12, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Assanhou, A.G.; Li, W.; Zhang, L.; Xue, L.; Kong, L.; Sun, H.; Mo, R.; Zhang, C. Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials 2015, 73, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Wang, B.; Bao, L.; Zhao, Y.S.; Zhang, S.M.; Zhang, S.Q. Overexpression of ILK promotes temozolomide resistance in glioma cells. Mol. Med. Rep. 2017, 15, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lu, Q.; Hu, X. Down-regulation of P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by honokiol. Cancer Lett. 2006, 243, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.K.; Kim, H.P.; Han, S.W.; Hur, H.S.; Oh, D.Y.; Im, S.A.; Bang, Y.J.; Kim, T.Y. Combination of EGFR and MEK1/2 inhibitor shows synergistic effects by suppressing EGFR/HER3-dependent AKT activation in human gastric cancer cells. Mol. Cancer Ther. 2009, 8, 2526–2536. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Qian, J.; Cao, S.; Yang, Z.; Pang, Z.; Pan, S.; Fan, L.; Xi, Z.; Jiang, X.; Zhang, Q. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials 2012, 33, 5115–5123. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, H.; Li, Y.; Wu, F.; Li, Y.; Bao, Y.; Yan, X.; Huang, Z.; Xu, P. Hyaluronic acid and Arg-Gly-Asp peptide modified Graphene oxide with dual receptor-targeting function for cancer therapy. J. Biomater. Appl. 2017, 32, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.M.; Kokkoli, E. Dual-ligand alpha5beta1 and alpha6beta4 integrin targeting enhances gene delivery and selectivity to cancer cells. J. Control. Release 2017, 251, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.; Liu, Q.; Li, W.; Zhang, T.; Zou, M.; Li, H.; Wu, T.; Cheng, S.; Su, Z.; et al. One-Step Self-Assembling Nanomicelles for Pirarubicin Delivery To Overcome Multidrug Resistance in Breast Cancer. Mol. Pharm. 2016, 13, 3934–3944. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Beitler, J.J.; Wang, H.; Lee, M.J.; Huang, W.; Koenig, L.; Nannapaneni, S.; Amin, A.R.; Bonner, M.; Shin, H.J.; et al. Honokiol enhances paclitaxel efficacy in multi-drug resistant human cancer model through the induction of apoptosis. PLoS ONE 2014, 9, e86369. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Tian, Y.; Zhang, H.; Jia, K.; Feng, T.; Sun, D.; Gao, Z.; Xu, F.; Hou, Z.; Li, Y.; et al. Metformin reverses multidrug resistance in human hepatocellular carcinoma Bel7402/5fluorouracil cells. Mol. Med. Rep. 2014, 10, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ling, G.; Sun, J.; Zhang, T.; Yuan, Y.; Sun, Y.; Wang, Z.; He, Z. Multifunctional nanoassemblies for vincristine sulfate delivery to overcome multidrug resistance by escaping P-glycoprotein mediated efflux. Biomaterials 2011, 32, 5524–5533. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Dong, X.; Qi, M.; Song, X.; Sun, J. Dual pH/redox responsive and CD44 receptor targeting hybrid nano-chrysalis based on new oligosaccharides of hyaluronan conjugates. Carbohydr. Polym. 2017, 157, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, T.; Chaki, S.; Futaki, S.; Nakase, I.; Akaji, K.; Kawakami, T.; Maruyama, K.; Kamiya, H.; Harashima, H. Transferrin-modified liposomes equipped with a pH-sensitive fusogenic peptide: An artificial viral-like delivery system. Biochemistry 2004, 43, 5618–5628. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Zhong, T.; Zhao, Y.; Zhang, W.Q.; Ren, W.; Huang, D.; Zhang, S.; Guo, Y.; Yao, X.; et al. The antitumor activity of tumor-homing peptide-modified thermosensitive liposomes containing doxorubicin on MCF-7/ADR: In vitro and in vivo. Int. J. Nanomed. 2015, 10, 2229–2248. [Google Scholar] [CrossRef]
- Zou, Y.; Song, Y.; Yang, W.; Meng, F.; Liu, H.; Zhong, Z. Galactose-installed photo-crosslinked pH-sensitive degradable micelles for active targeting chemotherapy of hepatocellular carcinoma in mice. J. Control. Release 2014, 193, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Ramasamy, T.; Kim, S.Y.; Kim, J.; Ku, S.K.; Youn, Y.S.; Kim, J.R.; Jeong, J.H.; Choi, H.G.; Yong, C.S.; et al. PEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic co-delivery of axitinib and celastrol in multi-targeted cancer therapy. Acta Biomater. 2016, 39, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yu, M.Z.; Wang, J.C.; Hou, W.J.; Gao, L.Y.; Ma, X.F.; Pei, X.W.; Niu, Y.J.; Liu, X.Y.; Qiu, C.; et al. Synergistic inhibition of breast cancer by co-delivery of VEGF siRNA and paclitaxel via vapreotide-modified core-shell nanoparticles. Biomaterials 2014, 35, 5028–5038. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, M.; Pan, L.; Shi, J. Tumor vascular-targeted co-delivery of anti-angiogenesis and chemotherapeutic agents by mesoporous silica nanoparticle-based drug delivery system for synergetic therapy of tumor. Int. J. Nanomed. 2016, 11, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Li, J.Q.; Wang, Z.Z.; Dong, D.W.; Qi, X.R. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials 2014, 35, 5226–5239. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, H.; Mackay, R.M.; Elgy, C.; Morgan, C.; Griffiths, M.; Clark, H.; Skipp, P.; Madsen, J. Nanoparticles in the lung and their protein corona: The few proteins that count. Nanotoxicology 2016, 10, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, K.; Zhang, L.; Hu, G.; Wan, J.; Tang, J.; Yin, S.; Duan, J.; Qin, M.; Wang, N.; et al. Significantly enhanced tumor cellular and lysosomal hydroxychloroquine delivery by smart liposomes for optimal autophagy inhibition and improved antitumor efficiency with liposomal doxorubicin. Autophagy 2016, 12, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Li, S.; Hao, Y.; Chen, L.; Zhang, X. Antibody h-R3-dendrimer mediated siRNA has excellent endosomal escape and tumor targeted delivery ability, and represents efficient siPLK1 silencing and inhibition of cell proliferation, migration and invasion. Oncotarget 2016, 7, 13782–13796. [Google Scholar] [CrossRef] [PubMed]
- Tawiah, K.D.; Porciani, D.; Burke, D.H. Toward the Selection of Cell Targeting Aptamers with Extended Biological Functionalities to Facilitate Endosomal Escape of Cargoes. Biomedicines 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, L.; Tan, X.; Li, F.; Zhao, M.; Peng, S. Lipid rafts-mediated endocytosis and physiology-based cell membrane traffic models of doxorubicin liposomes. Biochim. Biophys. Acta 2016, 1858, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, J.Y.; Wang, X.C.; Yue, H.X.; Hu, M.N.; Yu, X.; Xu, H. [Preparation and evaluation of doxorubicin hydrochloride liposomes modified by poly(2-ethyl-2-oxazoline)-cholesteryl methyl carbonate]. Yao Xue Xue Bao 2015, 50, 1174–1179. [Google Scholar] [PubMed]
- Rombouts, K.; Martens, T.F.; Zagato, E.; Demeester, J.; De Smedt, S.C.; Braeckmans, K.; Remaut, K. Effect of covalent fluorescence labeling of plasmid DNA on its intracellular processing and transfection with lipid-based carriers. Mol. Pharm. 2014, 11, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, M.; Malamas, A.; Shin, T.; Jin, E.; Sun, Y.; Lu, Z.R. Multifunctional cationic lipid-based nanoparticles facilitate endosomal escape and reduction-triggered cytosolic siRNA release. Mol. Pharm. 2014, 11, 2734–2744. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kydd, J.; Jadia, R.; Velpurisiva, P.; Gad, A.; Paliwal, S.; Rai, P. Targeting Strategies for the Combination Treatment of Cancer Using Drug Delivery Systems. Pharmaceutics 2017, 9, 46. https://doi.org/10.3390/pharmaceutics9040046
Kydd J, Jadia R, Velpurisiva P, Gad A, Paliwal S, Rai P. Targeting Strategies for the Combination Treatment of Cancer Using Drug Delivery Systems. Pharmaceutics. 2017; 9(4):46. https://doi.org/10.3390/pharmaceutics9040046
Chicago/Turabian StyleKydd, Janel, Rahul Jadia, Praveena Velpurisiva, Aniket Gad, Shailee Paliwal, and Prakash Rai. 2017. "Targeting Strategies for the Combination Treatment of Cancer Using Drug Delivery Systems" Pharmaceutics 9, no. 4: 46. https://doi.org/10.3390/pharmaceutics9040046
APA StyleKydd, J., Jadia, R., Velpurisiva, P., Gad, A., Paliwal, S., & Rai, P. (2017). Targeting Strategies for the Combination Treatment of Cancer Using Drug Delivery Systems. Pharmaceutics, 9(4), 46. https://doi.org/10.3390/pharmaceutics9040046