Suitability of Remediated PFAS-Affected Soil in Cement Pastes and Mortars
Abstract
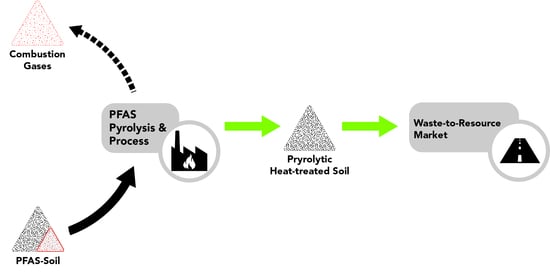
:1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Analysis
2.3. Fine Aggregate Replacement (FAR)
2.4. Supplementary Cementitious Material (SCM)
3. Results and Discussions
3.1. Compositional Differences
3.2. Replacing Sand with Heat-Treated Soil in Mortar Mixes
3.2.1. Particle Size Distribution
3.2.2. Fresh Properties
3.2.3. Mechanical Performance
3.3. Heat-Treated Soil as Supplementary Cementitious Material
3.3.1. Impact of the Hydration State of the Soil on the Mechanical Performance
3.3.2. Effects of SCM Percentage on the Mechanical Performance
3.3.3. Compositional Differences of the Hydrated Samples
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gagg, C.R. Cement and concrete as an engineering material: An historic appraisal and case study analysis. Eng. Fail. Anal. 2014, 40, 114–140. [Google Scholar] [CrossRef]
- CO2—What about It. Available online: https://www.slideshare.net/lesecq/co2-what-about-it (accessed on 17 March 2020).
- Davidson, E. Defining the trend: Cement Consumption versus Gross Domestic Product. Glob. Cem. Mag. 2014, 8–14. [Google Scholar]
- Andrew, R.M. Global CO2 emissions from cement production, 1928–2018. Earth Syst. Sci. Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef] [Green Version]
- Lord, M. Zero Carbon Industry Plan: Rethinking Cement; Zero Carbon Australia; Beyond Zero Emissions Inc.: Melbourne, Australia, 2017. [Google Scholar]
- Scrivener, K. Options for the future of cement. Indian Concr. J. 2014, 88, 11–21. [Google Scholar]
- Naqi, A.; Jang, J.G. Recent Progress in Green Cement Technology Utilizing Low-Carbon Emission Fuels and Raw Materials: A Review. Sustainability 2019, 11, 537. [Google Scholar] [CrossRef] [Green Version]
- Courland, R. Concrete planet. The Strange and Fascinating Story of the World’s Most Common Man-Made Material; Promethius Books: Buffalo, NY, USA, 2011. [Google Scholar]
- AustralianGovernment. Per- and Poly-Fluoroalkyl Substances (PFASs). Available online: http://www.environment.gov.au/protection/chemicals-management/pfas#a4 (accessed on 31 March 2020).
- Lechner, M.; Knapp, H. Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from Soil to Plant and Distribution to the Different Plant Compartments Studied in Cultures of Carrots (Daucus carota ssp. Sativus), Potatoes (Solanum tuberosum), and Cucumbers (Cucumis Sativus). J. Agric. Food Chem. 2011, 59, 11011–11018. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Ehlers, S.; Fürst, P.; Schafft, H.; Lahrssen-Wiederholt, M. Transfer of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) From Contaminated Feed Into Milk and Meat of Sheep: Pilot Study. Arch. Environ. Contam. Toxicol. 2012, 63, 288–298. [Google Scholar] [CrossRef]
- Squadrone, S.; Ciccotelli, V.; Prearo, M.; Favaro, L.; Scanzio, T.; Foglini, C.; Abete, M.C. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA): Emerging contaminants of increasing concern in fish from Lake Varese, Italy. Environ. Monit. Assess. 2015, 187, 438. [Google Scholar] [CrossRef]
- Nakayama, S.; Harada, K.; Inoue, K.; Sasaki, K.; Seery, B.; Saito, N.; Koizumi, A. Distributions of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) in Japan and their toxicities. Environ. Sci. Int. J. Environ. Physiol. Toxicol. 2005, 12, 293–313. [Google Scholar]
- Steenland, K.; Tinker, S.; Shankar, A.; Ducatman, A. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ. Health Persp. 2010, 118, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Bost, P.C.; Strynar, M.J.; Reiner, J.L.; Zweigenbaum, J.A.; Secoura, P.L.; Lindstrom, A.B.; Dye, J.A. U.S. domestic cats as sentinels for perfluoroalkyl substances: Possible linkages with housing, obesity, and disease. Environ. Res. 2016, 151, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, Y.; Wang, P.; Wang, T.; Liu, S.; Johnson, A.C.; Sweetman, A.J.; Baninla, Y. Pollution pathways and release estimation of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in central and eastern China. Sci. Total Environ. 2017, 580, 1247–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, J.A.; Abrams, S.; Bradburne, T.; Bryant, D.; Burns, M.; Cassidy, D.; Cherry, J.; Chiang, S.-Y.; Cox, D.; Crimi, M.; et al. PFAS Experts Symposium: Statements on regulatory policy, chemistry and analytics, toxicology, transport/fate, and remediation for per- and polyfluoroalkyl substances (PFAS) contamination issues. Remediat. J. 2019, 29, 31–48. [Google Scholar] [CrossRef]
- FEATURE: Sands of Time—Clock Ticking for Construction During Aggregates Shortage. Available online: https://www.imeche.org/news/news-article/feature-sands-of-time-clock-ticking-for-construction-during-aggregates-shortage (accessed on 17 March 2020).
- Industry Professionals Refute Authors’ Sand Shortage Claim? Available online: https://www.quarrymagazine.com/2019/10/11/industry-professionals-refute-authors-sand-shortage-claim/ (accessed on 17 March 2020).
- Footprints in the Sand. Available online: https://www.worldcement.com/special-reports/16062015/footprints-in-the-sand-829/ (accessed on 17 March 2020).
- Why the World Is Running Out of Sand. Available online: https://www.bbc.com/future/article/20191108-why-the-world-is-running-out-of-sand (accessed on 17 March 2020).
- We’re Running Out of Sand and Cities Are to Blame. Available online: https://www.forbes.com/sites/lauriewinkless/2019/08/22/were-running-out-of-sand-and-cities-are-to-blame/#4e5f10e21240 (accessed on 17 March 2020).
- PFAS Soil Stockpiles Sitting in Melbourne’s Inner-West. Available online: https://www.theage.com.au/national/victoria/pfas-soil-stockpiles-sitting-in-melbourne-s-inner-west-20200309-p5488p.html (accessed on 17 March 2020).
- Jain, P.; Kim, H.; Townsend, T.G. Heavy metal content in soil reclaimed from a municipal solid waste landfill. Waste Manag. 2005, 25, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tiller, K. Urban soil contamination in Australia. Soil Res. 1992, 30, 937–957. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Fehervari, A.; Gallage, C.; MacLeod, A.; Garcez, E.; Zhang, J.; Antic, A.; Gates, W.; Collins, F. Workability and Fresh Properties of a Low CO2 Footprint Concrete. In Proceedings of the Concrete 2019 Concrete in Practice—Progress through Knowledge Biennial National Conference of the Concrete Institute of Australia, Sydney, Australia, 8–11 September 2019. [Google Scholar]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Avet, F.; Snellings, R.; Alujas Diaz, A.; Ben Haha, M.; Scrivener, K. Development of a new rapid, relevant and reliable (R3) test method to evaluate the pozzolanic reactivity of calcined kaolinitic clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.; Scian, A.N.; Irassar, E. Assessment of Pozzolanic Activity of Different Calcined Clays. Cem. Concr. Compos. 2013, 37, 319–327. [Google Scholar] [CrossRef]
- Scrivener, K. Cement Chemistry and Sustainable Cementitious Materials. Online Course, edX. 2019. Available online: https://www.edx.org/course/cement-chemistry-and-sustainable-cementitious-mate?source=aw&awc=6798_1590375041_5d6891d65617f1b609b1c5aca4bdb7c5&utm_source=aw&utm_medium=affiliate_partner&utm_content=text-link&utm_term=422873_My+Mooc (accessed on 18 March 2019).
- Geng, H.; Chen, W.; Li, Q.; Shui, Z.; Yuan, B. Effect of Pre-dispersing Metakaolin in Water on the Properties, Hydration, and Metakaolin Distribution in Mortar. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Scrivener, K.; Avet, F.; Maraghechi, H.; Zunino, F.; Ston, J.; Hanpongpun, W.; Favier, A. Impacting factors and properties of limestone calcined clay cements (LC3). Green Mater. 2019, 7, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Martirena, F.; Díaz, E.; Rocha, D.; Maraghechi, H.; Scrivener, K.L. Performance of Concrete Made with a Calcined Clay—Limestone-Portland Cement Exposed to Natural Conditions. In Proceedings of the Sixth International Conference on Durability of Concrete Structures, Leeds, UK, 18–20 July 2018; pp. 244–247. [Google Scholar]
- Paiva, H.; Velosa, A.; Cachim, P.; Ferreira, V. Effect of pozzolans with different physical and chemical characteristics on concrete properties. Mater. Constr. 2016, 66. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Sakthivel, T.; Santhanam, M.; Gettu, R.; Pillai, R.G. Mechanical properties and durability performance of concretes with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 136–151. [Google Scholar] [CrossRef]
- Standards Australia, AS. 3972. General Purpose and Blended; Cements; Standards Australia: Sydney, Australia, 2010. [Google Scholar]
- ASTM. C305 Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM. C109/C109M Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 75 mm Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Standards Australia, AS. 1012.3.1. Methods of Testing Concrete. Method 3: Determination of Properties Related to the consistency of concrete—Slump test; Standards Australia: Sydney, Australia, 2014. [Google Scholar]
- Standards Australia, AS. 1012.8.1. Methods of Testing Concrete. Method for Making and Curing Concrete—Compression and Indirect Tensile Test Specimens; Standards Australia: Sydney, Australia, 2014. [Google Scholar]
- Renex Technology. Available online: http://www.renexgroup.com/ (accessed on 9 April 2020).
- ASTM. D6913/D6913M—17 Standard Test Methods for Particle-Size Distribution (Gradation) of Soils Using Sieve Analysis; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Standards Australia, AS. 1141.5. Methods for Sampling and Testing Aggregates, Method 5: Particle Density and Water Absorption of Fine Aggregate; Standards Australia: Sydney, Australia, 2000. [Google Scholar]
- Msinjili, N.S.; Gluth, G.J.G.; Sturm, P.; Vogler, N.; Kühne, H.-C. Comparison of calcined illitic clays (brick clays) and low-grade kaolinitic clays as supplementary cementitious materials. Mater. Struct. 2019, 52, 94. [Google Scholar] [CrossRef] [Green Version]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Pearson: London, UK, 2001. [Google Scholar]
- Żbik, M.S.; Raftery, N.A.; Smart, R.S.C.; Frost, R.L. Kaolinite platelet orientation for XRD and AFM applications. Appl. Clay Sci. 2010, 50, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Kłosek-Wawrzyn, E.; Małolepszy, J.; Murzyn, P. Sintering Behavior of Kaolin with Calcite. Procedia Eng. 2013, 57, 572–582. [Google Scholar] [CrossRef] [Green Version]
- Mohsen, Q.; El-maghraby, A. Characterization and assessment of Saudi clays raw material at different area. Arab. J. Chem. 2010, 3, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Alouani, M.E.; Alehyen, S.; Achouri, M.E.; Taibi, M. Preparation, Characterization, and Application of Metakaolin-Based Geopolymer for Removal of Methylene Blue from Aqueous Solution. J. Chem. 2019. [Google Scholar] [CrossRef]
- Kenne Diffo, B.B.; Elimbi, A.; Cyr, M.; Dika Manga, J.; Tchakoute Kouamo, H. Effect of the rate of calcination of kaolin on the properties of metakaolin-based geopolymers. J. Asian Ceram. Soc. 2015, 3, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Śrondoń, J. X-Ray Powder Diffraction Identification of Illitic Materials. Clays Clay Miner. 1984, 32, 337–349. [Google Scholar] [CrossRef]
- Pentrak, M.; Madejova, J.; Andrejkovičová, S.; Uhlik, P.; Komadel, P. Stability of kaolin sand from the Vyšný Petrovec deposit (south Slovakia) in an acid environment. Geol. Carpathica 2012, 63. [Google Scholar] [CrossRef]
- Li, Y.-S.; Church, J.S.; Woodhead, A.L. Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. J. Magn. Magn. Mater. 2012, 324, 1543–1550. [Google Scholar] [CrossRef]
- Namduri, H.; Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Sperinck, S.; Raiteri, P.; Marks, N.; Wright, K. Dehydroxylation of kaolinite to metakaolin—A molecular dynamics study. J. Mater. Chem. 2011, 21, 2118–2125. [Google Scholar] [CrossRef] [Green Version]
- Brindley, G.W.; Nakahira, M. The Kaolinite-Mullite Reaction Series: II, Metakaolin. J. Am. Ceram. Soc. 1959, 42, 314–318. [Google Scholar] [CrossRef]
- Malhotra, V.M. Results of a Laboratory Study—Superplasticizers in Concrete; Canada Center For Mineral and Energy Technology Department of Energy, Mines and Resources: Ottawa, ON, Canada, 1978.
- Muhit, I.B. Dosage Limit Determination of Superplasticizing Admixture and Effect Evaluation on Properties of Concrete. Int. J. Sci. Eng. Res. 2013, 4, 1–5. [Google Scholar]
- Mageswari, M.; Vidivelli, B. The Use of Sawdust Ash as Fine Aggregate Replacement in Concrete. Mater. Sci. 2009, 720–726. [Google Scholar]
- Rahman, S.; Farnaz, T.; Islam, T. Experimental Investigation of Concrete by Partial Replacement of Sand with Red Soil. In Proceedings of the 5th International Conference on Engineering Research, Innovation and Education (ICERIE 2019), Shahjalal University of Science and Technology (SUST), Sylhet, Bangladesh, 25–27 January 2019. [Google Scholar]
- Kalhara, N.; Perera, A.; Perera, A.; Lankathilake, N.; Ranasingh, T. Suitability of Soil Washed Sand as Fine Aggregates to Replace River Sand in the Concrete. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 46, 25–33. [Google Scholar]
- Prakash, K.S.; Rao, C.H. Study on Compressive Strength of Quarry Dust as Fine Aggregate in Concrete. Adv. Civ. Eng. 2016. [Google Scholar] [CrossRef] [Green Version]
- Elimbi, A.; Tchakoute, H.K.; Njopwouo, D. Effects of calcination temperature of kaolinite clays on the properties of geopolymer cements. Constr. Build. Mater. 2011, 25, 2805–2812. [Google Scholar] [CrossRef]
- Garg, N.; Skibsted, J. Dissolution kinetics of calcined kaolinite and montmorillonite in alkaline conditions: Evidence for reactive Al (V) sites. J. Am. Ceram. Soc. 2019, 102, 7720–7734. [Google Scholar] [CrossRef]
- Sargent, P. The development of alkali-activated mixtures for soil stabilisation. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Cambridge, UK, 2015; pp. 555–604. [Google Scholar] [CrossRef]
- Dodson, V.H. Pozzolans and the Pozzolanic Reaction. In Concrete Admixtures; Springer: Boston, MA, USA, 1990. [Google Scholar] [CrossRef]
- Dunstan, E.R. How Does Pozzolanic Reaction Make Concrete ‘Green”? In Proceedings of the World of Coal Ash (WOCA) Conference, Denver, CO, USA, 9–12 May 2011. [Google Scholar]
- Madejová, J.; Komadel, P. Baseline Studies of the Clay Minerals Society Source Clays: Infrared Methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]











| Clinker Constituents (%) | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | TiO2 | K2O | MnO | P2O3 | SO3 |
| 19.9 | 4.7 | 3.38 | 1.3 | 63.9 | 0.17 | 0.25 | 0.45 | 0.08 | 0.06 | 2.5 | |
| Gypsum (%) | 3.3 | ||||||||||
| Loss on ignition | 3 | ||||||||||
| Bulk density (kg/m3) | ~1400 | ||||||||||
| Specific gravity | ~3.0 | ||||||||||
| pH | ~12 | ||||||||||
| Particle size | ~90% less than 35 μm and ~30% less than 7 μm | ||||||||||
| Mix Designation | Binder (wt%) | w:b Ratio | SP (wt% of Binder) | Aggregate (wt%) | Aggregate:Binder | |||
|---|---|---|---|---|---|---|---|---|
| OPC | SCM | Concrete Sand | FAR | |||||
| Mortars | Reference | 100 | 0 | 0.45 | 0 | 100 | 0 | 1:2 |
| 15% FAR | 100 | 0 | 0.45 | 0 | 85 | 15 | 1:2 | |
| 30% FAR | 100 | 0 | 0.45 | 0 | 70 | 30 | 1:2 | |
| 60% FAR | 100 | 0 | 0.45 | 0 | 40 | 60 | 1:2 | |
| 100% FAR | 100 | 0 | 0.45 | 0 | 0 | 100 | 1:2 | |
| 30% FAR with SP | 100 | 0 | 0.45 | 0.45 | 70 | 30 | 1:2 | |
| 60% FAR with SP | 100 | 0 | 0.45 | 1.00 | 40 | 60 | 1:2 | |
| 100% FAR with SP | 100 | 0 | 0.45 | 2.38 | 0 | 100 | 1:2 | |
| 15% SCM | 85 | 15 | 0.45 | 0 | 100 | 0 | 1:2 | |
| 30% SCM | 70 | 30 | 0.45 | 0 | 100 | 0 | 1:2 | |
| 45% SCM | 55 | 45 | 0.45 | 0 | 100 | 0 | 1:2 | |
| Pastes | Reference | 100 | 0 | 0.45 | 0 | 0 | 0 | - |
| 15% SCM | 85 | 15 | 0.45 | 0 | 0 | 0 | - | |
| 30% SCM | 70 | 30 | 0.45 | 0 | 0 | 0 | - | |
| 45% SCM | 55 | 45 | 0.45 | 0 | 0 | 0 | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehervari, A.; Gates, W.P.; Gallage, C.; Collins, F. Suitability of Remediated PFAS-Affected Soil in Cement Pastes and Mortars. Sustainability 2020, 12, 4300. https://doi.org/10.3390/su12104300
Fehervari A, Gates WP, Gallage C, Collins F. Suitability of Remediated PFAS-Affected Soil in Cement Pastes and Mortars. Sustainability. 2020; 12(10):4300. https://doi.org/10.3390/su12104300
Chicago/Turabian StyleFehervari, Andras, Will P. Gates, Chathuranga Gallage, and Frank Collins. 2020. "Suitability of Remediated PFAS-Affected Soil in Cement Pastes and Mortars" Sustainability 12, no. 10: 4300. https://doi.org/10.3390/su12104300
APA StyleFehervari, A., Gates, W. P., Gallage, C., & Collins, F. (2020). Suitability of Remediated PFAS-Affected Soil in Cement Pastes and Mortars. Sustainability, 12(10), 4300. https://doi.org/10.3390/su12104300