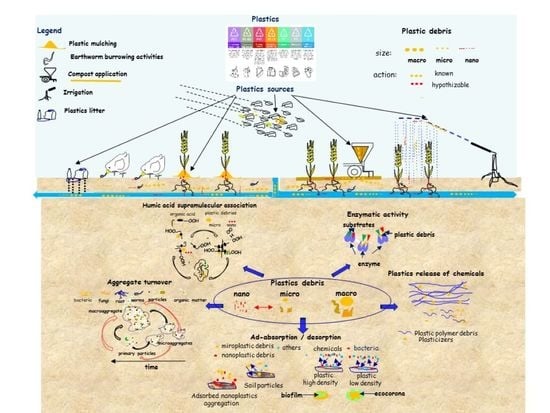
Soil Pollution from Micro- and Nanoplastic Debris: A Hidden and Unknown Biohazard
Abstract
:1. Introduction
2. Preliminary Aspects
3. Sources of Soil Contamination
3.1. Landfills
3.2. Flooded Areas, Rise up of Saline Waters in Coastal Soils and Eolian Transport
3.3. Soils Irrigated with Waste Waters or Fertilized with Sewage Sludge or Composts
3.4. Soil under Plastic Mulching
3.5. Techno-Soil with Remediated Sediments
4. Microplastics and Nanoplastics Fate in Soil
5. Effects of Microplastics and Nanoplastics on Soil Properties
5.1. Soil Chemical-Physical Properties
5.2. Soil Active Extracellular Molecules
5.3. Soil Microbial Community
5.4. Soil Fauna
5.5. Soil Pedogenesis
5.6. Plants
Biological Indicators of Microplastics and Nanoplastics in Soil
6. Analyses of Soil Microplastics and Nanoplastics
6.1. MPS and NPs Sources and Relevance of Ageing Processes
6.2. Microplastics Characterization
6.3. Nanoplastic Characterization
6.4. Eco-Corona and Plastisphere Characterization
7. The Reduction of Microplastic and Nanoplastic Inputs to Agricultural Soils
7.1. Biodegradable and Bio-Based Plastics
7.2. Development of Clean Up and Bioremediation Technologies
8. Socio-Economic Impacts of MPs and NPs Soil Pollution
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment—Sources, fates and effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Rist, S.; Carney Almroth, B.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Chen, Q.L.; An, X.L.; Yang, X.R.; Christie, P.; Ke, X.; Wu, L.H.; Zhu, Y.G. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol. Biochem. 2018, 116, 302–310. [Google Scholar] [CrossRef]
- Lenz, R.W.; Marchessault, R.H. Bacterial polyesters: Biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules 2005, 6, 1–8. [Google Scholar] [CrossRef]
- Somleva, M.N.; Peoples, O.P.; Snell, K.D. PHA Bioplastics, Biochemicals, and Energy from Crops. Plant Biotechnol. J. 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [Green Version]
- Lambert, S.; Wagner, M. Formation of microscopic particles during the degradation of different polymers. Chemosphere 2016, 161, 510–517. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, K.N.; Karapanagioti, H.K. Surface properties of beached plastic pellets. Mar. Environ. Res. 2012, 81, 70–77. [Google Scholar] [CrossRef]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of Various Plastics in the Environment. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2019; Volume 78, pp. 71–92. [Google Scholar]
- Hüffer, T.; Weniger, A.K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J.; Kraak, M.H.S.; Parsons, J.R. Preface; Springer: Berlin/Heidelberg, Germany, 2012; Volume 220, ISBN 9781461434139. [Google Scholar]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.B.; Quinn, B. Microplastic collection techniques. In Microplastic Pollutants; Elsevier Science: Amsterdam, The Netherland, 2017; pp. 179–202. [Google Scholar]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggård, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef]
- Nasser, F.; Lynch, I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J. Proteom. 2016, 137, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Pulido-Reyes, G.; Leganes, F.; Fernández-Piñas, F.; Rosal, R. Bio-nano interface and environment: A critical review. Environ. Toxicol. Chem. 2017, 36, 3181. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the ‘plastisphere’: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Redmile-Gordon, M.A.; Brookes, P.C.; Evershed, R.P.; Goulding, K.W.T.; Hirsch, P.R. Measuring the soil-microbial interface: Extraction of extracellular polymeric substances (EPS) from soil biofilms. Soil Biol. Biochem. 2014, 72, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Kettler, K.; Veltman, K.; van de Meent, D.; van Wezel, A.; Hendriks, A.J. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ. Toxicol. Chem. 2014, 33, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Arias-Andres, M.; Klümper, U.; Rojas-Jimenez, K.; Grossart, H.P. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. Pollut. 2018, 237, 261. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 15, 339–344. [Google Scholar] [CrossRef]
- Maris, J.; Bourdon, S.; Brossard, J.M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical recycling: Compatibilization of mixed thermoplastic wastes. Polym. Degrad. Stab. 2018, 147, 245–266. [Google Scholar] [CrossRef]
- Allsopp, M.; Walters, A.; Santillo, D.; Johnston, P. Plastic Debris in the World’s Oceans. 2018. Available online: https://www.greenpeace.to/greenpeace/wp-content/uploads/2011/05/plastic_ocean_report.pdf (accessed on 20 August 2020).
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Wong, M.H.; Wu, S.C.; Deng, W.J.; Yu, X.Z.; Luo, Q.; Leung, A.O.W.; Wong, C.S.C.; Luksemburg, W.J.; Wong, A.S. Export of toxic chemicals—A review of the case of uncontrolled electronic-waste recycling. Environ. Pollut. 2007, 149, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Sheavly, S.B. Marine Debris-An Overview of a Critical Issue for Our Oceans. In Proceedings of the Sixth Meeting of the UN Open-Ended Informal Consultative Processes on Oceans and the Law of the Sea, New York, NY, USA, 6–10 June 2005. [Google Scholar]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Microplastics in Spanish Table Salt. Sci. Rep. 2017, 7, 8620. [Google Scholar] [CrossRef] [PubMed]
- Biber, N.F.A.; Foggo, A.; Thompson, R.C. Characterising the deterioration of different plastics in air and seawater. Mar. Pollut. Bull. 2019, 141, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P.G. A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ. Sci. Process. Impacts 2016, 18, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Bayo, J.; Olmos, S.; López-Castellanos, J.; Alcolea, A. Microplastics and microfibers in the sludge of a municipal wastewater treatment plant. Int. J. Sustain. Dev. Plan. 2016, 11, 812–821. [Google Scholar] [CrossRef] [Green Version]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in sewage sludge: Effects of treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Qian, L.; Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, L. Treatment of municipal sewage sludge in supercritical water: A review. Water Res. 2016, 89, 118–131. [Google Scholar] [CrossRef]
- Crutchik, D.; Franchi, O.; Caminos, L.; Jeison, D.; Belmonte, M.; Pedrouso, A.; Val del Rio, A.; Mosquera-Corral, A.; Campos, J.L. Polyhydroxyalkanoates (PHAs) production: A feasible economic option for the treatment of sewage sludge in municipalwastewater treatment plants? Water 2020, 12, 1118. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, Y.; Wang, J.; Pang, H.; Li, Y. Buried straw layer plus plastic mulching reduces soil salinity and increases sunflower yield in saline soils. Soil Tillage Res. 2016, 155, 363–370. [Google Scholar] [CrossRef]
- Fan, Y.; Ding, R.; Kang, S.; Hao, X.; Du, T.; Tong, L.; Li, S. Plastic mulch decreases available energy and evapotranspiration and improves yield and water use efficiency in an irrigated maize cropland. Agric. Water Manag. 2017, 179, 122–131. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, F.; Jia, Z.; Ren, X.; Cai, T. Response of soil water, temperature, and maize (Zea may L.) production to different plastic film mulching patterns in semi-arid areas of northwest China. Soil Tillage Res. 2017, 166, 113–121. [Google Scholar] [CrossRef]
- Hilborn, K.T.; Helper, P.R.; Cooper, G.R.C. Plastic film aids control of lettuce diseases. Maine Farm Res. 1957, 5, 11–17. [Google Scholar]
- Reynolds, S.G. The effect of mulches on southern blight (Sclerotium rolfsii) in dwarf bean (Phaseolus vulgaris). Trop. Agric. 1970, 47, 137–144. [Google Scholar]
- Hawthorne, B.T. Effect of mulching on the incidence of Sclerotinia minor on lettuce. New Zeal. J. Exp. Agric. 1975, 3, 273–274. [Google Scholar] [CrossRef]
- Research, T.M. Agricultural Films (LDPE, LLDPE, HDPE, EVA/EBA, Reclaims and Others) Market for Greenhouse, Mulching and Silage Applications-Global Industry Analysis, Size, Share, Growth, Trends and Forecast. Trends Forecast. 2013–2019. 2013. Available online: https://rahul28feb86.files.wordpress.com/2013/10/agricultural-films-market-for-greenhouse-mulching-and-silage-applications-global-industry-analysis-size-share-growth-trends-and-forecast-2013-20191.pdf (accessed on 20 August 2020).
- Ramos, L.; Berenstein, G.; Hughes, E.A.; Zalts, A.; Montserrat, J.M. Polyethylene film incorporation into the horticultural soil of small periurban production units in Argentina. Sci. Total Environ. 2015, 523, 74–81. [Google Scholar] [CrossRef] [PubMed]
- US EPA Guidelines for Using Mulches and Weed Barriers US EPA. Available online: https://cms.agr.wa.gov/getmedia/51d0a8b0-d22a-4ed6-b668-3ae84a6a83c1/3007_MulchesWeedBarriers (accessed on 29 July 2019).
- Wang, J.; Luo, Y.; Teng, Y.; Ma, W.; Christie, P.; Li, Z. Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use of plastic film. Environ. Pollut. 2013, 180, 265–273. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Zumstein, M.T.; Schintlmeister, A.; Nelson, T.F.; Baumgartner, R.; Woebken, D.; Wagner, M.; Kohler, H.P.E.; McNeill, K.; Sander, M. Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Melgarejo, P.; Legua, P.; Pérez-Sarmiento, F.; Martínez-Font, R.; Martínez-Nicolás, J.J.; Giordani, E.; Tozzi, F.; Hernández, F. Effect of a new remediated substrate on bioactive compounds and antioxidant characteristics of pomegranate (Punica granatum L.) cultivar ‘Purple Queen’. Arch. Agron. Soil Sci. 2019, 65, 1565–1574. [Google Scholar] [CrossRef]
- Mallikarjuna, V.; Bindu Mani, T. Soil Stabilization Using Plastic Waste. Int. J. Res. Eng. Technol. 2016, 5, 391–394. [Google Scholar] [CrossRef]
- César, M.E.F.; Mariani, P.D.S.C.; Innocentini-Mei, L.H.; Cardoso, E.J.B.N. Particle size and concentration of poly(ε-caprolactone) and adipate modified starch blend on mineralization in soils with differing textures. Polym. Test. 2009, 28, 680–687. [Google Scholar] [CrossRef]
- Chinaglia, S.; Tosin, M.; Degli-Innocenti, F. Biodegradation rate of biodegradable plastics at molecular level. Polym. Degrad. Stab. 2018, 147, 237–244. [Google Scholar] [CrossRef]
- Tosin, M. Effect of the Composting Substrate on Biodégradation of Solid Materials under Controlled Composting Conditions. J. Environ. Polym. Degrad. 1996, 4, 55–63. [Google Scholar] [CrossRef]
- Deepika, R.C.; Janani, R.; Vignesh, R.; Charu Deepika, R.; Manigandan, P.; Janani, R. Screening of Plastic Degrading Microbes from Various Dumped Soil Samples. Int. Res. J. Eng. Technol. 2016, 3, 2493–2498. [Google Scholar]
- Shovitri, M.; Nafi’Ah, R.; Antika, T.R.; Alami, N.H.; Kuswytasari, N.D.; Zulaikha, E. Soil burial method for plastic degradation performed by Pseudomonas PL-01, Bacillus PL-01, and indigenous bacteria. AIP Conf. Proc. 2017, 1854, 1854. [Google Scholar]
- Ng, E.L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Wang, H.; Adeleye, A.S.; Huang, Y.; Li, F.; Keller, A.A. Heteroaggregation of nanoparticles with biocolloids and geocolloids. Adv. Colloid Interface Sci. 2015, 226, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Velzeboer, I.; Quik, J.T.K.; van de Meent, D.; Koelmans, A.A. Rapid settling of nanoparticles due to heteroaggregation with suspended sediment. Environ. Toxicol. Chem. 2014, 33, 1766–1773. [Google Scholar] [CrossRef]
- Advances in Polymer Nanocomposites—1st Edition. Available online: https://www.elsevier.com/books/advances-in-polymer-nanocomposites/gao/978-1-84569-940-6 (accessed on 2 July 2020).
- Lambert, S.; Sinclair, C.; Boxall, A. Occurrence, degradation, and effect of polymer-based materials in the environment. Rev. Environ. Contam. Toxicol. 2014, 227, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Klemchuk, P.P. Degradable plastics: A critical review. Polym. Degrad. Stab. 1990, 27, 183–202. [Google Scholar] [CrossRef]
- Schöler, A.; Jacquiod, S.; Vestergaard, G.; Schulz, S.; Schloter, M. Analysis of soil microbial communities based on amplicon sequencing of marker genes. Biol. Fertil. Soils 2017, 53, 485–489. [Google Scholar] [CrossRef]
- Vestergaard, G.; Schulz, S.; Schöler, A.; Schloter, M. Making big data smart—how to use metagenomics to understand soil quality. Biol. Fertil. Soils 2017, 53, 479–484. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Mendoza Vega, J.; Ku Quej, V.; de los Angeles Chi, J.; Sanchez del Cid, L.; Chi, C.; Escalona Segura, G.; Gertsen, H.; Salánki, T.; van der Ploeg, M.; et al. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef]
- Cornelis, G.; Pang, L.; Doolette, C.; Kirby, J.K.; McLaughlin, M.J. Transport of silver nanoparticles in saturated columns of natural soils. Sci. Total Environ. 2013, 463–464, 120–130. [Google Scholar] [CrossRef]
- Wuithschick, M.; Witte, S.; Kettemann, F.; Rademann, K.; Polte, J. Illustrating the formation of metal nanoparticles with a growth concept based on colloidal stability. Phys. Chem. Chem. Phys. 2015, 17, 19895–19900. [Google Scholar] [CrossRef] [Green Version]
- Pachapur, V.L.; Dalila Larios, A.; Cledón, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Behavior and characterization of titanium dioxide and silver nanoparticles in soils. Sci. Total Environ. 2016, 563–564, 933–943. [Google Scholar] [CrossRef]
- Ceccherini, M.T.; Ascher, J.; Pietramellara, G.; Vogel, T.M.; Nannipieri, P. Vertical advection of extracellular DNA by water capillarity in soil columns. Soil Biol. Biochem. 2007, 39, 158–163. [Google Scholar] [CrossRef]
- Siegfried, M.; Koelmans, A.A.; Besseling, E.; Kroeze, C. Export of microplastics from land to sea. A modelling approach. Water Res. 2017, 127, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- De Souza MacHado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zheng, L.; Li, J. A critical review on the sources and instruments of marine microplastics and prospects on the relevant management in China. Waste Manag. Res. 2018, 36, 898–911. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Peng, J.; Wang, J.; Wang, K.; Bao, S. Occurrence of microplastics in the beach sand of the Chinese inner sea: The Bohai Sea. Environ. Pollut. 2016, 214, 722–730. [Google Scholar] [CrossRef]
- Vermaire, J.C.; Pomeroy, C.; Herczegh, S.M.; Haggart, O.; Murphy, M. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. FACETS 2017, 2, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic transport in soil by earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef]
- Moreno, M.M.; Moreno, A. Effect of different biodegradable and polyethylene mulches on soil properties and production in a tomato crop. Sci. Hortic. (Amst.) 2008, 116, 256–263. [Google Scholar] [CrossRef]
- Atuanya, E.I.; Aborisade, W.T.; Nwogu, N.A. Impact of Plastic Enriched Composting on Soil Structure, Fertility and Growth of Maize Plants. Eur. J. Appl. Sci. 2012, 4, 105–109. [Google Scholar]
- Kasirajan, S.; Ngouajio, M. Erratum: Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2013, 33, 501–539. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.C.F.; Gomes, S.I.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Hazard assessment of nickel nanoparticles in soil—The use of a full life cycle test with Enchytraeus crypticus. Environ. Toxicol. Chem. 2017, 36, 2934–2941. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic Disguising as Soil Carbon Storage. Environ. Sci. Technol. 2018, 52, 6079–6080. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Qian, C.; Liu, X.Y.; Yu, H.Q. Two-dimensional correlation spectroscopic analysis on the interaction between humic acids and TiO2 nanoparticles. Environ. Sci. Technol. 2014, 48, 11119–11126. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.Y.; Yu, H.Q. Temperature–dependent conformational variation of chromophoric dissolved organic matter and its consequent interaction with phenanthrene. Environ. Pollut. 2017, 222, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Polizzotto, M.L.; Guan, D.; Wu, J.; Shen, Q.; Ran, W.; Wang, B.; Yu, G. Exploring the interactions and binding sites between Cd and functional groups in soil using two-dimensional correlation spectroscopy and synchrotron radiation based spectromicroscopies. J. Hazard. Mater. 2017, 326, 18–25. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, Z.Y.; Qian, C.; Yu, H.Q. Induced structural changes of humic acid by exposure of polystyrene microplastics: A spectroscopic insight. Environ. Pollut. 2018, 233, 1–7. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, J.; Zheng, P.; Tsang, D.C.W.; Qiu, R. The roles of humic substances in the interactions of phenanthrene and heavy metals on the bentonite surface. J. Soils Sediments 2015, 15, 1463–1472. [Google Scholar] [CrossRef]
- Chen, C.S.; Le, C.; Chiu, M.H.; Chin, W.C. The impact of nanoplastics on marine dissolved organic matter assembly. Sci. Total Environ. 2018, 634, 316–320. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Asuri, P.; Karajanagi, S.S.; Vertegel, A.A.; Dordick, J.S.; Kane, R.S. Enhanced stability of enzymes adsorbed onto nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- Awet, T.T.; Kohl, Y.; Meier, F.; Straskraba, S.; Grün, A.L.; Ruf, T.; Jost, C.; Drexel, R.; Tunc, E.; Emmerling, C. Effects of polystyrene nanoparticles on the microbiota and functional diversity of enzymes in soil. Environ. Sci. Eur. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Bento, C.P.M.; Chen, H.; Zhang, H.; Xue, S.; Lwanga, E.H.; Zomer, P.; Ritsema, C.J.; Geissen, V. Influence of microplastic addition on glyphosate decay and soil microbial activities in Chinese loess soil. Environ. Pollut. 2018, 242, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Pietramellara, G.; Ascher, J.; Borgogni, F.; Ceccherini, M.T.; Guerri, G.; Nannipieri, P. Extracellular DNA in soil and sediment: Fate and ecological relevance. Biol. Fertil. Soils 2009, 45, 219–235. [Google Scholar] [CrossRef]
- Nagler, M.; Insam, H.; Pietramellara, G.; Ascher-Jenull, J. Extracellular DNA in natural environments: Features, relevance and applications. Appl. Microbiol. Biotechnol. 2018, 102, 6343–6356. [Google Scholar] [CrossRef] [Green Version]
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.A.; Schmidt, S. DNA uptake by Arabidopsis induces changes in the expression of CLE peptides which control root morphology. Plant Signal. Behav. 2010, 5, 1112–1114. [Google Scholar] [CrossRef] [Green Version]
- Mazzoleni, S.; Bonanomi, G.; Incerti, G.; Chiusano, M.L.; Termolino, P.; Mingo, A.; Senatore, M.; Giannino, F.; Cartenì, F.; Rietkerk, M.; et al. Inhibitory and toxic effects of extracellular self-DNA in litter: A mechanism for negative plant-soil feedbacks? New Phytol. 2015, 205, 1195–1210. [Google Scholar] [CrossRef] [Green Version]
- Hawes, M.; Allen, C.; Turgeon, B.G.; Curlango-Rivera, G.; Minh Tran, T.; Huskey, D.A.; Xiong, Z. Root Border Cells and Their Role in Plant Defense. Annu. Rev. Phytopathol. 2016, 54, 143–161. [Google Scholar] [CrossRef]
- D’Alvise, P.W.; Sjøholm, O.R.; Yankelevich, T.; Jin, Y.; Wuertz, S.; Smets, B.F. TOL plasmid carriage enhances biofilm formation and increases extracellular DNA content in Pseudomonas putida KT2440. FEMS Microbiol. Lett. 2010, 312, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietramellara, G.; Ascher, J.; Ceccherini, M.T.; Nannipieri, P.; Wenderoth, D. Adsorption of pure and dirty bacterial DNA on clay minerals and their transformation frequency. Biol. Fertil. Soils 2007, 43, 731–739. [Google Scholar] [CrossRef]
- Das, T.; Sharma, P.K.; Busscher, H.J.; Van Der Mei, H.C.; Krom, B.P. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl. Environ. Microbiol. 2010, 76, 3405–3408. [Google Scholar] [CrossRef] [Green Version]
- Beall, G.W.; Sowersby, D.S.; Roberts, R.D.; Robson, M.H.; Lewis, L.K. Analysis of oligonucleotide DNA binding and sedimentation properties of montmorillonite clay using ultraviolet light spectroscopy. Biomacromolecules 2009, 10, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Kim, D.; Chae, Y.; An, Y.J. Mixture Toxicity of Nickel and Microplastics with Different Functional Groups on Daphnia magna. Environ. Sci. Technol. 2017, 51, 12852–12858. [Google Scholar] [CrossRef]
- Vedolin, M.C.; Teophilo, C.Y.S.; Turra, A.; Figueira, R.C.L. Spatial variability in the concentrations of metals in beached microplastics. Mar. Pollut. Bull. 2018, 129, 487–493. [Google Scholar] [CrossRef]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic Bag Derived-Microplastics as a Vector for Metal Exposure in Terrestrial Invertebrates. Environ. Sci. Technol. 2017, 8, 4714–4721. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Lau, P.C.Y.; Lee, J.H.; Ellen, R.P.; Cvitkovitch, D.G. Natural genetic transformation of streptococcus mutans growing in biofilms. J. Bacteriol. 2001, 183, 897–908. [Google Scholar] [CrossRef] [Green Version]
- Rossi, G.; Barnoud, J.; Monticelli, L. Polystyrene nanoparticles perturb lipid membranes. J. Phys. Chem. Lett. 2014, 5, 241–246. [Google Scholar] [CrossRef]
- Henriques, I.D.S.; Love, N.G. The role of extracellular polymeric substances in the toxicity response of activated sludge bacteria to chemical toxins. Water Res. 2007, 41, 4177–4185. [Google Scholar] [CrossRef] [PubMed]
- Leriche, V.; Briandet, R.; Carpentier, B. Ecology of mixed biofilms subjected daily to a chlorinated alkaline solution: Spatial distribution of bacterial species suggests a protective effect of one species to another. Environ. Microbiol. 2003, 5, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Mezgebe, B.; Aly Hassan, A.; Sahle-Demessie, E.; Sorial, G.A.; Bennett-Stamper, C. Experimental and modeling studies of sorption of ceria nanoparticle on microbial biofilms. Bioresour. Technol. 2014, 161, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wu, Y.; Esquivel-Elizondo, S.; Sørensen, S.J.; Rittmann, B.E. How Microbial Aggregates Protect against Nanoparticle Toxicity. Trends Biotechnol. 2018, 36, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.J.; Wang, J.J.; Liu, S.C.; Sun, X.D.; Yuan, X.Z.; Wang, S.G. Role of extracellular polymeric substances in the acute inhibition of activated sludge by polystyrene nanoparticles. Environ. Pollut. 2018, 179, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maaß, S.; Daphi, D.; Lehmann, A.; Rillig, M.C. Transport of microplastics by two collembolan species. Environ. Pollut. 2017, 225, 456–459. [Google Scholar] [CrossRef]
- Drake, H.L.; Horn, M.A. As the Worm Turns: The Earthworm Gut as a Transient Habitat for Soil Microbial Biomes. Annu. Rev. Microbiol. 2007, 61, 169–189. [Google Scholar] [CrossRef]
- Ponge, J.F.; Arpin, P.; Sondag, F.; Delecour, F. Soil fauna and site assessment in beech stands of the Belgian Ardennes. Can. J. For. Res. 1997, 27, 2053–2064. [Google Scholar] [CrossRef]
- Trap, J.; Bonkowski, M.; Plassard, C.; Villenave, C.; Blanchart, E. Ecological importance of soil bacterivores for ecosystem functions. Plant Soil 2016, 398, 1–24. [Google Scholar] [CrossRef]
- Bottone, E.J.; Perez, A.A.; Gordon, R.E.; Qureshi, M.N. Differential binding capacity and internalisation of bacterial substrates as factors in growth rate of Acanthamoeba spp. J. Med. Microbiol. 1994, 40, 148–154. [Google Scholar] [CrossRef]
- Pace, M.; Bailiff, M. Evaluation of a fluorescent microsphere technique for measuring grazing rates of phagotrophic microorganisms. Mar. Ecol. Prog. Ser. 1987, 40, 185–193. [Google Scholar] [CrossRef]
- Koutny, M.; Amato, P.; Muchova, M.; Ruzicka, J.; Delort, A.M. Soil bacterial strains able to grow on the surface of oxidized polyethylene film containing prooxidant additives. Int. Biodeterior. Biodegrad. 2009, 63, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Frydkjær, C.K.; Iversen, N.; Roslev, P. Ingestion and Egestion of Microplastics by the Cladoceran Daphnia magna: Effects of Regular and Irregular Shaped Plastic and Sorbed Phenanthrene. Bull. Environ. Contam. Toxicol. 2017, 99, 655–661. [Google Scholar] [CrossRef]
- Zhu, B.K.; Fang, Y.M.; Zhu, D.; Christie, P.; Ke, X.; Zhu, Y.G. Exposure to nanoplastics disturbs the gut microbiome in the soil oligochaete Enchytraeus crypticus. Environ. Pollut. 2018, 239, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Hrda, K.; Pouzar, M.; Knotek, P. Study of zinc oxide nanoparticles and zinc chloride toxicity to annelid Enchytraeus crypticus in modified agar-based media. Environ. Sci. Pollut. Res. 2018, 25, 22702–22709. [Google Scholar] [CrossRef]
- Bicho, R.C.; Santos, F.C.F.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Multigenerational effects of copper nanomaterials (CuONMs) are different of those of CuCl2: Exposure in the soil invertebrate Enchytraeus crypticus. Sci. Rep. 2017, 7, 8457. [Google Scholar] [CrossRef] [Green Version]
- Topuz, E.; van Gestel, C.A.M. The effect of soil properties on the toxicity and bioaccumulation of Ag nanoparticles and Ag ions in Enchytraeus crypticus. Ecotoxicol. Environ. Saf. 2017, 144, 330–337. [Google Scholar] [CrossRef]
- Hayashi, Y.; Heckmann, L.H.; Simonsen, V.; Scott-Fordsmand, J.J. Time-course profiling of molecular stress responses to silver nanoparticles in the earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2013, 98, 219–226. [Google Scholar] [CrossRef]
- Yang, X.; Huerta, L.; Wanga, E.; Bemani, A.; Gertisen, H.; Salanki, T.; Guo, X.; Fu, H.; Xue, S.; Ritsema, C.J.; et al. Biogenic transport of glyphosate decay and soil microbial activity in Chinese loess soil. Environ. Pollut. 2019, 245, 829–835. [Google Scholar] [CrossRef]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC—Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Ehlers, S.M.; Ellrich, J.A. First record of ‘plasticrusts’ and ‘pyroplastic’ from the Mediterranean Sea. Mar. Pollut. Bull. 2020, 151, 110845. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Wallerstein, C.; Arnold, R.; Webb, D. Marine pollution from pyroplastics. Sci. Total Environ. 2019, 694, 133610. [Google Scholar] [CrossRef] [PubMed]
- Sandermann, H. Plant metabolism of xenobiotics. Trends Biochem. Sci. 1992, 2, 82–84. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Q.; Yin, N.; Tu, C.; Luo, Y. Uptake and accumulation of microplastics in an edible plant. Kexue Tongbao/Chinese Sci. Bull. 2019, 64, 928–934. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.D.; Yuan, X.Z.; Jia, Y.; Feng, L.J.; Zhu, F.P.; Dong, S.S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.L.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020. Available online: https://www.nature.com/articles/s41565-020-0707-4 (accessed on 29 August 2020). [CrossRef] [PubMed]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Heal. Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef]
- Bandmann, V.; Müller, J.D.; Köhler, T.; Homann, U. Uptake of fluorescent nano beads into BY2-cells involves clathrin-dependent and clathrin-independent endocytosis. FEBS Lett. 2012, 586, 3626–3632. [Google Scholar] [CrossRef] [Green Version]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Gómez, C.; León, V.M.; Calles, S.; Gomáriz-Olcina, M.; Vethaak, A.D. The adverse effects of virgin microplastics on the fertilization and larval development of sea urchins. Mar. Environ. Res. 2017, 130, 69–76. [Google Scholar] [CrossRef]
- Trotter, B.; Ramsperger, A.F.R.M.; Raab, P.; Haberstroh, J.; Laforsch, C. Plastic waste interferes with chemical communication in aquatic ecosystems. Sci. Rep. 2019, 9, 5889. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, D.; Wan, W.; Wen, B. Hexabromocyclododecanes in soils and plants from a plastic waste treatment area in North China: Occurrence, diastereomer- and enantiomer-specific profiles, and metabolization. Environ. Sci. Pollut. Res. 2017, 24, 21625–21635. [Google Scholar] [CrossRef] [PubMed]
- 10780, U. Compost–Classificazione, Requisiti e Modalità di Impiego. 1998. Available online: http://store.uni.com/catalogo/uni-107801998?___store=en&josso_back_to=http%3A%2F%2Fstore.uni.com%2Fjosso-security-check.php&josso_cmd=login_optional&josso_partnerapp_host=store.uni.com&___from_store=it (accessed on 20 August 2020).
- ISO 11269-2. Soil Quality–Determination of the Effects of Pollutants on Soil Flora–Part2: Effects of Contaminated Soil on the Emergence and Early Growth of Higher Plants. 2012. Available online: https://www.iso.org/standard/51382.html (accessed on 1 July 2020).
- ISO 11268-1:2012(fr), Qualité du sol—Effets des Polluants vis-à-vis des Vers de terre—Partie 1: Détermination de la Toxicité Aiguë vis-à-vis de Eisenia Fetida/Eisenia Andrei. Available online: https://www.iso.org/obp/ui/#iso:std:iso:11268:-1:ed-2:v1:fr (accessed on 1 July 2020).
- Huerta Lwanga, E.; Thapa, B.; Yang, X.; Gertsen, H.; Salánki, T.; Geissen, V.; Garbeva, P. Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: A potential for soil restoration. Sci. Total Environ. 2018, 624, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, B.M.; Champakalakshmi, R.; Sakharkar, M.K.; Lim, C.S.; Sakharkar, K.R. Biodegradation of Low Density Polythene (LDPE) by Pseudomonas Species. Indian J. Microbiol. 2012, 52, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royer, S.J.; Ferrón, S.; Wilson, S.T.; Karl, D.M. Production of methane and ethylene from plastic in the environment. PLoS ONE 2018, 13, e0200574. [Google Scholar] [CrossRef] [PubMed]
- Heribert, I. Soil volatile organic compounds as tracers for microbial activities in soils. In Omics in Soil Science; Nannipieri, P., Pietramellara, G., Renella, G., Eds.; Caister Academic Press: Norfolk, UK, 2014; pp. 127–138. [Google Scholar]
- Serrano, A.; Gallego, M. Sorption study of 25 volatile organic compounds in several Mediterranean soils using headspace-gas chromatography-mass spectrometry. J. Chromatogr. A 2006, 1118, 261–270. [Google Scholar] [CrossRef]
- Ardisson, G.B.; Tosin, M.; Barbale, M.; Degli-Innocenti, F. Biodegradation of plastics in soil and effects on nitrification activity. A laboratory approach. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Endo, S.; Takizawa, R.; Okuda, K.; Takada, H.; Chiba, K.; Kanehiro, H.; Ogi, H.; Yamashita, R.; Date, T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar. Pollut. Bull. 2005, 50, 1103–1114. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef] [PubMed]
- Magrì, D.; Sánchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P.; et al. Laser ablation as a versatile tool to mimic polyethylene terephthalate nanoplastic pollutants: Characterization and toxicology assessment. ACS Nano 2018, 12, 7690–7700. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; Macleod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mylläri, V.; Ruoko, T.P.; Järvelä, P. The effects of UV irradiation to polyetheretherketone fibres—Characterization by different techniques. Polym. Degrad. Stab. 2014, 109, 278–284. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A Procedure for Measuring Microplastics using Pressurized Fluid Extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rist, S.; Baun, A.; Hartmann, N.B. Ingestion of micro- and nanoplastics in Daphnia magna—Quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut. 2017, 228, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef] [Green Version]
- Löder, M.G.J.; Gerdts, G. Methodology used for the detection and identification of microplastics—A critical appraisal. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 201–227. ISBN 9783319165103. [Google Scholar]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef] [Green Version]
- Enders, K.; Lenz, R.; Stedmon, C.A.; Nielsen, T.G. Abundance, size and polymer composition of marine microplastics ≥10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar. Pollut. Bull. 2015, 100, 70–81. [Google Scholar] [CrossRef]
- Imhof, H.K.; Laforsch, C.; Wiesheu, A.C.; Schmid, J.; Anger, P.M.; Niessner, R.; Ivleva, N.P. Pigments and plastic in limnetic ecosystems: A qualitative and quantitative study on microparticles of different size classes. Water Res. 2016, 98, 64–74. [Google Scholar] [CrossRef]
- Schwaferts, C.; Niessner, R.; Elsner, M.; Ivleva, N.P. Methods for the analysis of submicrometer-and nanoplastic particles in the environment. TrAC—Trends Anal. Chem. 2019, 112, 52–65. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018, 616–617, 1056–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felsing, S.; Kochleus, C.; Buchinger, S.; Brennholt, N.; Stock, F.; Reifferscheid, G. A new approach in separating microplastics from environmental samples based on their electrostatic behavior. Environ. Pollut. 2018, 234, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Crichton, E.M.; Noël, M.; Gies, E.A.; Ross, P.S. A novel, density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments. Anal. Methods 2017, 9, 1419–1428. [Google Scholar] [CrossRef]
- Fu, W.; Min, J.; Jiang, W.; Li, Y.; Zhang, W. Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci. Total Environ. 2020, 721, 137561. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Käppler, A.; Windrich, F.; Löder, M.G.J.; Malanin, M.; Fischer, D.; Labrenz, M.; Eichhorn, K.J.; Voit, B. Identification of microplastics by FTIR and Raman microscopy: A novel silicon filter substrate opens the important spectral range below 1300 cm−1 for FTIR transmission measurements. Anal. Bioanal. Chem. 2015, 407, 6791–6801. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef]
- Watteau, F.; Dignac, M.F.; Bouchard, A.; Revallier, A.; Houot, S. Microplastic Detection in Soil Amended with Municipal Solid Waste Composts as Revealed by Transmission Electronic Microscopy and Pyrolysis/GC/MS. Front. Sustain. Food Syst. 2018, 2. [Google Scholar] [CrossRef] [Green Version]
- Primpke, S.; Lorenz, C.; Rascher-Friesenhausen, R.; Gerdts, G. An automated approach for microplastics analysis using focal plane array (FPA) FTIR microscopy and image analysis. Anal. Methods 2017, 9, 1499–1511. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, D.; Trombini, C.; Vassura, I. Analysis of Polystyrene in Polluted Sediments by Pyrolysis-Gas Chromatography-Mass Spectrometry. J. Chromatogr. Sci. 1998, 36, 600–604. [Google Scholar] [CrossRef] [Green Version]
- Dignac, M.F.; Houot, S.; Francou, C.; Derenne, S. Pyrolytic study of compost and waste organic matter. Org. Geochem. 2005, 36, 1054–1071. [Google Scholar] [CrossRef]
- Duemichen, E.; Eisentraut, P.; Celina, M.; Braun, U. Automated thermal extraction-desorption gas chromatography mass spectrometry: A multifunctional tool for comprehensive characterization of polymers and their degradation products. J. Chromatogr. A 2019, 1592, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.; Henry, T.; Gutierrez, T. Agglomeration of nano- and microplastic particles in seawater by autochthonous and de novo-produced sources of exopolymeric substances. Mar. Pollut. Bull. 2018, 130, 258–267. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the aquatic environment. Critical review. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 325–340. ISBN 9783319165103. [Google Scholar]
- Renner, G.; Schmidt, T.C.; Schram, J. Analytical methodologies for monitoring micro(nano)plastics: Which are fit for purpose? Curr. Opin. Environ. Sci. Health 2018, 1, 55–61. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Bäuerlein, P.S.; Koelmans, A.A.; Dekker, S.C.; Van Wezel, A.P. Closing the gap between small and smaller: Towards a framework to analyse nano- and microplastics in aqueous environmental samples. Environ. Sci. Nano 2018, 5, 1640–1649. [Google Scholar] [CrossRef]
- Planken, K.L.; Cölfen, H. Analytical ultracentrifugation of colloids. Nanoscale 2010, 2, 1849–1869. [Google Scholar] [CrossRef]
- Baalousha, M.; Stolpe, B.; Lead, J.R. Flow field-flow fractionation for the analysis and characterization of natural colloids and manufactured nanoparticles in environmental systems: A critical review. J. Chromatogr. A 2011, 1218, 4078–4103. [Google Scholar] [CrossRef]
- Tiede, K.; Boxall, A.B.A.; Tiede, D.; Tear, S.P.; David, H.; Lewis, J. A robust size-characterisation methodology for studying nanoparticle behaviour in ‘real’ environmental samples, using hydrodynamic chromatography coupled to ICP-MS. J. Anal. At. Spectrom. 2009, 24, 964–972. [Google Scholar] [CrossRef]
- Correia, M.; Loeschner, K. Detection of nanoplastics in food by asymmetric flow field-flow fractionation coupled to multi-angle light scattering: Possibilities, challenges and analytical limitations. Anal. Bioanal. Chem. 2018, 410, 5603–5613. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigalke, M.; Filella, M.; Fischer, D.; Muntwyler, A.; Scheurer, M.; Watts, B. Micro-and nanoplastic analysis in soils. Chimia (Aarau) 2018, 72, 901. [Google Scholar] [CrossRef]
- Matysiak, M.; Kapka-Skrzypczak, L.; Brzóska, K.; Gutleb, A.C.; Kruszewski, M. Proteomic approach to nanotoxicity. J. Proteom. 2016, 137, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Corrigendum to “The soil microbiome—From metagenomics to metaphenomics” [Curr Opin Micrbiol 43 (June 2018)162-168](S1369527417302205)(10.1016/j.mib.2018.01.013). Curr. Opin. Microbiol. 2019, 49, 104. [Google Scholar] [CrossRef] [PubMed]
- Debeljak, P.; Pinto, M.; Proietti, M.; Reisser, J.; Ferrari, F.F.; Abbas, B.; Van Loosdrecht, M.C.M.; Slat, B.; Herndl, G.J. Extracting DNA from ocean microplastics: A method comparison study. Anal. Methods 2017, 9, 1521–1523. [Google Scholar] [CrossRef] [Green Version]
- Dussud, C.; Hudec, C.; George, M.; Fabre, P.; Higgs, P.; Bruzaud, S.; Delort, A.M.; Eyheraguibel, B.; Meistertzheim, A.L.; Jacquin, J.; et al. Colonization of non-biodegradable and biodegradable plastics by marine microorganisms. Front. Microbiol. 2018, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Hörsch, P.; Gorenflo, A.; Fuder, C.; Deleage, A.; Frimmel, F.H. Biofouling of ultra- and nanofiltration membranes fordrinking water treatment characterized by fluorescence in situ hybridization (FISH). Desalination 2005, 172, 41–52. [Google Scholar] [CrossRef]
- Lee, J.W.; Nam, J.H.; Kim, Y.H.; Lee, K.H.; Lee, D.H. Bacterial communities in the initial stage of marine biofilm formation on artificial surfaces. J. Microbiol. 2008, 46, 174–182. [Google Scholar] [CrossRef]
- Elifantz, H.; Horn, G.; Ayon, M.; Cohen, Y.; Minz, D. Rhodobacteraceae are the key members of the microbial community of the initial biofilm formed in Eastern Mediterranean coastal seawater. FEMS Microbiol. Ecol. 2013, 85, 348–357. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Cho, K.H.; Hwang, K.; Kim, E.H.; Kim, M.; Hong, S.G.; Lee, H.K. Succession of bacterial community structure during the early stage of biofilm development in the Antarctic marine environment. Korean J. Microbiol. 2016, 52, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Environmental factors support the formation of specific bacterial assemblages on microplastics. Front. Microbiol. 2018, 8, 2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wackett, L.P. Bio-based and biodegradable plastics: An annotated selection of World Wide Web sites relevant to the topics in microbial biotechnology. Microb. Biotechnol. 2019, 12, 1492–1493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tao, J.; Yam, R.C.M.; Mok, A.C.K.; Li, R.K.Y.; Song, C. Biodegradation behavior of polycaprolactone/rice husk ecocomposites in simulated soil medium. Polym. Degrad. Stab. 2008, 93, 1571–1576. [Google Scholar] [CrossRef]
- Adhikari, D.; Mukai, M.; Kubota, K.; Kai, T.; Kaneko, N.; Araki, K.S.; Kubo, M. Degradation of Bioplastics in Soil and Their Degradation Effects on Environmental Microorganisms. J. Agric. Chem. Environ. 2016, 05, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Alshehrei, F. Biodegradation of Synthetic and Natural Plastic by Microorganisms. J. Appl. Environ. Microbiol. 2017, 5, 8–19. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- NF U52-001. Biodegradable Mulching Film: Test Methods and Criteria. 2005. Available online: https://www.boutique.afnor.org/standard/nf-u52-001/biodegradable-materials-for-use-in-agriculture-and-horticulture-mulching-products-requirements-and-test-methods/article/633557/fa136042 (accessed on 1 July 2020).
- UNI 11462. Materiali Plastici Biodegradabili in Suolo–Tipi, Requisitie Metodi di Prova. 2012. Available online: https://store.uni.com/catalogo/uni-11462-2012 (accessed on 1 July 2020).
- UNI 11495. Materiali Termoplastici Biodegradabili per Uso in Agricoltura e Orticoltura–Film per Pacciamatura–Requisiti e Metodi di Prova. 2013. Available online: https://store.uni.com/catalogo/uni-11495-2013 (accessed on 1 July 2020).
- Chenon, P.; Badin, A.-L.; Nassr, N.; Kremer, L.; Thevenin, N. Ecotoxicological impacts of biodegradable polymers in agricultural soils. In Proceedings of the ORBIT 2012 Conference, Rennes, France, 12–15 June 2012. [Google Scholar]
- ISO 17556. Plastics–Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon dioxide Evolved. 2012. Available online: https://www.iso.org/standard/56089.html (accessed on 1 July 2020).
- ISO 11268-1. Soil Quality–Effects of Pollutants on Earthworms–Part1: Determination of Acute Toxicity to Eiseniafetida/Eisenia Andrei. 2012. Available online: https://www.iso.org/standard/53527.html (accessed on 1 July 2020).
- Yaradoddi, J.S.; Hugar, S.; Nagaraj, R.; Banapurmath, N.R.; Hunashyal, A.M.; Sulochana, M.B.; Shettar, A.S.; Ganachari, S.V. Alternative and Renewable Bio-based and Biodegradable Plastics. In Handbook of Ecomaterials; Martínez, L., Kharissova, O.K.B., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–20. [Google Scholar]
- Muniyasamy, S.; Ofosu, O.; John, M.J.; Anandjiwala, R.D. Mineralization of poly(lactic acid) (PLA), Poly(3-hydroxybutyrate-co-valerate) (PHBV) and PLA/PHBV blend in compost and soil environments. J. Renew. Mater. 2016, 4, 133–145. [Google Scholar] [CrossRef]
- Sekhar, V.C.; Nampoothiri, K.M.; Mohan, A.J.; Nair, N.R.; Bhaskar, T.; Pandey, A. Microbial degradation of high impact polystyrene (HIPS), an e-plastic with decabromodiphenyl oxide and antimony trioxide. J. Hazard. Mater. 2016, 318, 347–354. [Google Scholar] [CrossRef]
- Muenmee, S.; Chiemchaisri, W.; Chiemchaisri, C. Microbial consortium involving biological methane oxidation in relation to the biodegradation of waste plastics in a solid waste disposal open dump site. Int. Biodeterior. Biodegrad. 2015, 102, 172–181. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Wu, W.M.; Zhao, J.; Jiang, L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science (80-. ) 2016, 351, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, P.; Howe, C.J.; Bertocchini, F. Polyethylene bio-degradation by caterpillars of the wax moth Galleria mellonella. Curr. Biol. 2017, R292–R293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanga, C. Life Science Archives (LSA) Isolation and Identification of Polyethylene Biodegradation Bacterial from the Guts of Plastic Bags—Eating Damp Wood TermiteS; JPS Scientific Publications: Tiruvannamalai, India, 2016; Volume 2, pp. 490–499. [Google Scholar]
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Rorrer, N.A.; Kearns, F.L.; Silveira, R.L.; Pollard, B.C.; Dominick, G.; Duman, R.; El Omari, K.; et al. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurley, R.R.; Nizzetto, L. Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 185, 907–917. [Google Scholar] [CrossRef]
- vom Saal, F.S.; Hughes, C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005, 113, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Scalenghe, R. Resource or waste? A perspective of plastics degradation in soil with a focus on end-of-life options. Heliyon 2018, e00941. [Google Scholar] [CrossRef] [Green Version]
- Galloway, T.S. Micro- and nano-plastics and human health. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 343–366. ISBN 9783319165103. [Google Scholar]
- Bouwmeester, H.; Hollman, P.C.H.; Peters, R.J.B. Potential Health Impact of Environmentally Released Micro- and Nanoplastics in the Human Food Production Chain: Experiences from Nanotoxicology. Environ. Sci. Technol. 2015, 8932–8947. [Google Scholar] [CrossRef]
- Salvati, A.; Åberg, C.; dos Santos, T.; Varela, J.; Pinto, P.; Lynch, I.; Dawson, K.A. Experimental and theoretical comparison of intracellular import of polymeric nanoparticles and small molecules: Toward models of uptake kinetics. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.M.A.; Gong, C.; Xu, Y.G.; Chang, Y.; Shi, H. Factors controlling permeability of the blood-brain barrier. Cell. Mol. Life Sci. 2016, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; El Hadri, H.; Reynaud, S.; Deniau, E.; Grassl, B. Asymmetrical flow field flow fractionation methods to characterize submicron particles: Application to carbon-based aggregates and nanoplastics. Anal. Bioanal. Chem. 2017, 409, 6761–6769. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathan, S.I.; Arfaioli, P.; Bardelli, T.; Ceccherini, M.T.; Nannipieri, P.; Pietramellara, G. Soil Pollution from Micro- and Nanoplastic Debris: A Hidden and Unknown Biohazard. Sustainability 2020, 12, 7255. https://doi.org/10.3390/su12187255
Pathan SI, Arfaioli P, Bardelli T, Ceccherini MT, Nannipieri P, Pietramellara G. Soil Pollution from Micro- and Nanoplastic Debris: A Hidden and Unknown Biohazard. Sustainability. 2020; 12(18):7255. https://doi.org/10.3390/su12187255
Chicago/Turabian StylePathan, Shamina Imran, Paola Arfaioli, Tommaso Bardelli, Maria Teresa Ceccherini, Paolo Nannipieri, and Giacomo Pietramellara. 2020. "Soil Pollution from Micro- and Nanoplastic Debris: A Hidden and Unknown Biohazard" Sustainability 12, no. 18: 7255. https://doi.org/10.3390/su12187255
APA StylePathan, S. I., Arfaioli, P., Bardelli, T., Ceccherini, M. T., Nannipieri, P., & Pietramellara, G. (2020). Soil Pollution from Micro- and Nanoplastic Debris: A Hidden and Unknown Biohazard. Sustainability, 12(18), 7255. https://doi.org/10.3390/su12187255