Ultraviolet Fluorescence in the Assessment of Quality in the Mixing of Granular Material
Abstract
:1. Introduction
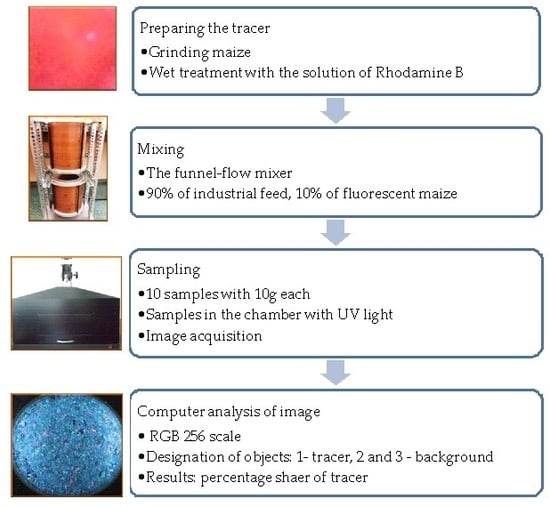
2. Materials and Methods
3. Results
3.1. Results Obtained by Means of Computer Image Analysis and the Weighing Method
3.2. Results of Statistical Comparative Analysis
4. Discussion
Funding
Conflicts of Interest
References
- Neumann, K.D. Work of the Mixer is crucial for additives. Feed Mag. 2000, 10, 371–383. [Google Scholar]
- Tasirin, S.M.; Kamarudin, S.K.; Hweage, A.M.A. Mixing behavior of binary polymer particles in bubbling fluidized bed. J. Phys. Sci. 2008, 19, 13–29. [Google Scholar]
- Jarray, A.; Shi, H.; Scheper, B.J.; Habibi, M.; Luding, S. Cohesion-driven mixing and segregation of dry granular media. Sci. Rep. 2019, 9, 13480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinekötter, R.; Gericke, H. Mixing of Solids: Particle Technology, 1st ed.; Springer: Berlin, Germany, 2000; ISBN 978-94-015-9580-3. [Google Scholar]
- Cleary, P.W.; Sinnott, M.D. Assessing mixing characteristics of particle-mixing and granulation devices. Particuol. Simul. Modeling Part. Syst. 2008, 6, 419–444. [Google Scholar] [CrossRef]
- Sarkar, A.; Wassgren, C.R. Simulation of a continuous granular mixer: Effect of operating conditions on flow and mixing. Chem. Eng. Sci. 2009, 64, 2672–2682. [Google Scholar] [CrossRef]
- Królczyk, J.B. Metrological changes in the surface morphology of cereal grains in the mixing process. Int. Agrophys. 2016, 30, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Matuszek, D.; Królczyk, J.B. Aspects of safety in production of feeds—A review. Anim. Nutr. Feed Technol. 2017, 17, 367–385. [Google Scholar] [CrossRef]
- Walczyński, S.; Korol, W. Long-term monitoring of homogeneity of compound feed in the government supervision. Krmiva Zagreb 2008, 50, 311–317. [Google Scholar]
- Zawiślak, K.; Sobczak, P.; Wełdycz, A. Mixing as CCP in the production of industrial feed. J. Cent. Eur. Agric. 2012, 13, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Bothe, D. Evaluating the quality of a mixture: Degree of homogeneity and scale of segregation. In Micro and Macro Mixing. Heat and Mass Transfer; Bockhorn, H., Mewes, D., Peukert, W., Warnecke, H.J., Eds.; Springer: Berlin, Germany, 2010; pp. 17–35. [Google Scholar] [CrossRef]
- Liu, P.Y.; Yang, R.Y.; Yu, A.B. Self-diffusion of wet particles in rotating drums. Phys. Fluids 2013, 25, 063301. [Google Scholar] [CrossRef]
- Li, H.; McCarthy, J.J. Controlling Cohesive Particle Mixing and Segregation. Phys. Rev. Lett. 2003, 90, 184301. [Google Scholar] [CrossRef] [PubMed]
- Cegielska-Radziejewska, R.; Stupe, K.; Szablewsk, T. Microflora and mycotoxin contamination in poultry feed mixtures from western Poland. Ann. Agric. Environ. Med. 2013, 20, 30–35. [Google Scholar] [PubMed]
- Sobczak, P.; Zawiślak, Z.; Żukiewicz-Sobczak, W.; Mazur, J.; Nadulski, R.; Kozak, M. The assessment of microbiological purity of selected components of animal feeds and mixtures which underwent thermal processing. J. Cent. Eur. Agric. 2016, 17, 303–314. [Google Scholar] [CrossRef]
- Kwiatek, K.; Kukier, E. Microbiological contamination of animal feed. Vet. Med. 2008, 64, 24–26. [Google Scholar]
- Królczyk, J.; Matuszek, D.; Tukiendorf, M. Modelling of quality changes in a multicomponent granular mixture during mixing. Electon. J. Pol. Agric. Univ. 2010, 13. [Google Scholar]
- Królczyk, J.B. An attempt to predict quality changes in a ten-component granular system. Teh. Vjesn. 2014, 21, 255–261. [Google Scholar]
- Chen, M.; Liu, M.; Li, T.; Tang, Y.; Liu, R.; Wen, Y.; Liu, B.; Shao, Y. A novel mixing index and its application in particle mixing behavior study in multiple-spouted bed. Powder Technol. 2018, 339, 167–181. [Google Scholar] [CrossRef]
- Eisenberg, S.; Eisenberg, D. Closer to perfection. Feed Manag. 1992, 11, 8–20. [Google Scholar]
- Zawiślak, K.; Grochowicz, J.; Sobczak, P. The Analysis of mixing degree of granular products with the use of Microtracers. TEKA Kom. Mot. Energ. Roln. OL Pan 2011, 11, 335–342. [Google Scholar]
- Matuszek, D. The analysis of homogeneity of industrial fodder for cattle. J. Res. Appl. Agric. Eng. 2013, 58, 118–121. [Google Scholar]
- Królczyk, J.B. Industrial conditions of the granular material manufacturing process. App Mech. Mater. 2014, 693, 267–272. [Google Scholar] [CrossRef]
- Królczyk, J.B. Analysis of kinetics of multicomponent, heterogeneous granular mixtures—laminar and turbulent flow approach. Chem. Process. Eng. 2016, 37, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.K.; Holt, D.; Leung, J.C.; Cooney, C.L.; Raju, G.K.; Hansen, P. Real time and noninvasive monitoring of dry powder blend homogeneity. AIChE J. 2001, 47, 2618–2622. [Google Scholar] [CrossRef]
- Lai, C.K.; Cooney, C.L. Application of a fluorescence sensor for miniscale on-line monitoring of powder mixing kinetics. J. Pharm. Sci. 2004, 93, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Karumanchi, V.; Taylor, M.K.; Ely, K.J.; Stagner, W.C. Monitoring powder blend homogeneity using light-induced fluorescence. AAPS Pharmscitech. 2011, 12, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- Durão, P.; Fauteux-Lefebvre, C.; Guay, J.M.; Batzoglou, N.; Gosselin, R. Using multiple process analytical technology probes to monitor multivitamin blends in a tableting feed frame. Talanta 2017, 164, 7–15. [Google Scholar] [CrossRef]
- Necemer, M.; Kump, P.; Rajcevic, M.; Jacimovic, R.; Budic, B.; Ponikvar, M. Determination of sulfur and chlorine in fodder by X-ray fluorescence spectral analysis and comparison with other analytical methods. Spectrochim. Acta 2003, 58, 1367–1373. [Google Scholar] [CrossRef]
- Nadeem, H.; Heindel, T.J. Review of noninvasive methods to characterize granular mixing. Powder Technol. 2018, 332, 331–350. [Google Scholar] [CrossRef] [Green Version]
- Realpe, A.; Barrios, K.; Rozo, M. Assessment of homogenization degree of powder mixing in a cylinder rotating under cascading regime. Int. J. Eng. Technol. 2015, 7, 394–404. [Google Scholar]
- Berthiaux, H.; Mosorov, V.; Tomczak, L.; Gatumel, C.; Demeyre, J.F. Principal component analysis for characterising homogeneity in powder mixing using image processing techniques. Chem. Eng. Process. Process. Intensif. 2006, 45, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Muerza, S.; Berthiaux, H.; Massol-Chaudeur, S.; Thomas, G. A dynamic study of static mixing using on-line image analysis. Powder Technol. 2002, 128, 195–204. [Google Scholar] [CrossRef]
- Dal Grande, F.; Santomaso, A.; Canu, P. Improving local composition measurements of binary mixtures by image analysis. Powder Technol. 2008, 187, 205–213. [Google Scholar] [CrossRef]
- Yang, S.C. Density effect on mixing and segregation processes in a vibrated binary granular mixture. Powder Technol. 2006, 164, 65–74. [Google Scholar] [CrossRef]
- Tai, C.H.; Hsiau, S.S.; Kruelle, C.A. Density segregation in a vertically vibrated granular bed. Powder Technol. 2010, 204, 255–262. [Google Scholar] [CrossRef]
- Hu, G.; Gong, X.; Huang, H.; Li, Y. Effects of geometric parameters and operating conditions on granular flow in a modified rotating cone. Ind. Eng. Chem. Res. 2007, 46, 9263–9268. [Google Scholar] [CrossRef]
- Liu, X.; Gong, J.; Zhang, Z.; Wu, W. An image analysis technique for the particle mixing and heat transfer process in a pan coater. Powder Technol. 2016, 295, 161–166. [Google Scholar] [CrossRef]
- Kingston, T.A.; Heindel, T.J. Granular mixing optimization and the influence of operating conditions in a double screw mixer. Powder Technol. 2014, 266, 144–155. [Google Scholar] [CrossRef]
- Matuszek, D.; Wojtkiewicz, K. Application of fluorescent markers for homogeneity assessment of grain mixtures based on maize content. Chem. Process. Eng.-Inz. 2017, 38, 505–512. [Google Scholar] [CrossRef]
- Matuszek, D.; Biłos, Ł. Use of fluorescent tracers for the assessment of the homogeneity of multicomponent granular feed mixtures. Przem. Chem. 2017, 96, 2356–2359. [Google Scholar] [CrossRef]
- Matuszek, B.D. The use of UV-induced fluorescence for the assessment of homogeneity of granular mixtures. Open Chem. 2019, 17, 485–491. [Google Scholar] [CrossRef]
- Matuszek, D. Modelling selected parameters of granular elements in the mixing process. Int. Agrophys. 2015, 29, 75–81. [Google Scholar] [CrossRef]
- Kwiatek, K.; Przeniosło-Siwczyńska, M. Instructions for Testing the Homogeneity of Medicated Feeds and Intermediate Products on the Basis of Testing the Degree of Mixing of the Active Substance; Państwowy Instytut Weterynaryjny: Puławy, Poland, 2007. [Google Scholar]
- Weinekötter, R.; Reh, L. Characterization of particulate mixtures by in-line measurments. Part. Part. Syst. Char. 1994, 11, 284–290. [Google Scholar] [CrossRef]
- Boss, J.; Krótkiewicz, M.; Tukiendorf, M. The application of picture analysis as a method to evaluate the quality of granular mixture during funnel-flow mixing. Agric. Eng. 2002, 4, 27–32. [Google Scholar]
- Arratia, P.E.; Duong, N.H.; Muzzio, F.J.; Godpole, P.; Lange, A.; Reynolds, S. Characterizing mixing and lubrication in the Bohle bin blender. Powder Technol. 2006, 161, 202–208. [Google Scholar] [CrossRef]
- Brone, D.; Muzzio, F.J. Enhanced mixing in double-cone blenders. Powder Technol. 2010, 110, 179–189. [Google Scholar] [CrossRef]






| Type of Component | Percentage of Component [%] | ||
|---|---|---|---|
| Mixture 1 | Mixture 2 | Mixture 3 | |
| Fodder chalk | 9 | 1.5 | 7.1 |
| Barley | - | 30 | - |
| Maize | 8 | 9 | 7 |
| Triticale | - | 20 | - |
| Wheat | - | 20 | - |
| Soya meal | 65.55 | 12 | 72 |
| Rape meal | - | 5 | - |
| Dry maize decoction | 5.45 | - | 4.3 |
| Sodium chloride | 2.5 | 0.5 | 2 |
| Phosphate | 3.5 | 1 | 2.8 |
| Premix | 2.5 | 1 | 2 |
| Lysine | 2.5 | - | 1.8 |
| Methionine | 0.5 | - | 0.4 |
| Threonine | 0.35 | - | 0.3 |
| Phytase | 0.05 | - | 0.05 |
| Grindazyn | 0.05 | - | 0.05 |
| Luctarom (aroma) | 0.05 | - | 0.05 |
| Neubaciol | - | - | 0.15 |
| Number of components | 13 | 10 | 14 |
| Fineness degree M [mm] | 0.64 | 0.61 | 0.63 |
| Series of Tests | Method 1 1 | Method 2 2 | Difference 3 |
|---|---|---|---|
| Mixture 1 | |||
| 1 | 8.00 ± 0.88 | 8.12 ± 0.94 | 0.30 ± 0.20 |
| 2 | 9.04 ± 0.79 | 9.05 ± 0.73 | 0.33 ± 0.18 |
| 3 | 8.98 ± 1.14 | 9.25 ± 1.06 | 0.41 ± 0.17 |
| mean, % | 8.67 ± 1.08 | 8.81 ± 1.06 | 0.34 ± 0.19 |
| CV, % | 10.83 ± 1.62 | 10.37 ± 1.66 | 0.55 ± 0.09 |
| Mixture 2 | |||
| 1 | 10.04 ± 1.20 | 10.03 ± 1.21 | 0.27 ± 0.17 |
| 2 | 10.38 ± 1.59 | 10.24 ± 1.58 | 0.26 ± 0.07 |
| 3 | 9.90 ± 1.27 | 9.85 ± 1.18 | 0.26 ± 0.09 |
| mean, % | 10.10 ± 1.41 | 10.04 ± 1.37 | 0.26 ± 0.12 |
| CV, % | 13.46 ± 1.32 | 13.17 ± 1.60 | 0.41 ± 0.16 |
| Mixture 3 | |||
| 1 | 9.34 ± 0.93 | 9.51 ± 1.04 | 0.41 ± 0.23 |
| 2 | 8.66 ± 0.92 | 8.84 ± 1.05 | 0.43 ± 0.23 |
| 3 | 9.45 ± 0.76 | 9.35 ± 0.91 | 0.29 ± 0.16 |
| mean, % | 9.15 ± 0.96 | 9.23 ± 1.06 | 0.38 ± 0.22 |
| CV, % | 9.55 ± 1.10 | 10.87 ± 0.87 | 0.56 ± 0.01 |
| Series of Tests | Method 1 1 | Method 2 2 | Difference 3 |
|---|---|---|---|
| Mixture 1 | |||
| 1 | 9.13 ± 0.87 | 9.37 ± 0.78 | 0.53 ± 0.20 |
| 2 | 9.49 ± 0.93 | 9.42 ± 0.96 | 052 ± 0.30 |
| 3 | 8.94 ± 1.09 | 8.89 ± 0.97 | 0.44 ± 0.17 |
| mean, % | 9.18 ± 1.01 | 9.23 ± 0.96 | 0.50 ± 0.23 |
| CV, % | 10.50 ± 1.19 | 9.83 ± 1.07 | 0.44 ± 0.09 |
| Mixture 2 | |||
| 1 | 9.39 ± 0.76 | 9.13 ± 0.79 | 0.38 ± 0.26 |
| 2 | 9.22 ± 0.75 | 9.21 ± 0.84 | 0.42 ± 0.26 |
| 3 | 9.56 ± 0.81 | 9.53 ± 0.89 | 0.33 ± 0.14 |
| mean, % | 9.39 ± 0.80 | 9.29 ± 0.87 | 0.38 ± 0.23 |
| CV, % | 8.25 ± 0.16 | 9.04 ± 0.28 | 0.58 ± 0.11 |
| Mixture 3 | |||
| 1 | 9.35 ± 0.75 | 9.61 ± 0.85 | 0.62 ± 0.26 |
| 2 | 9.09 ± 1.02 | 9.21 ± 0.94 | 0.64 ± 0.26 |
| 3 | 9.13 ± 0.92 | 9.19 ± 0.77 | 0.50 ± 0.25 |
| mean, % | 9.19 ± 0.92 | 9.33 ± 0.89 | 0.59 ± 0.30 |
| CV, % | 9.75 ± 1.33 | 9.15 ± 0.76 | 0.49 ± 0.06 |
| Mixture No. | t | p |
|---|---|---|
| 1 | −0.49398 | 0.62318 |
| 2 | 0.18586 | 0.85320 |
| 3 | −0.33460 | 0.73913 |
| Mixture No. | Statistical Test Result | p |
|---|---|---|
| 1 1 | −0.16586 | 0.86884 |
| 22 | 0.34004 | 0.73383 |
| 3 2 | −0.73183 | 0.46427 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matuszek, D.B. Ultraviolet Fluorescence in the Assessment of Quality in the Mixing of Granular Material. Sustainability 2020, 12, 1546. https://doi.org/10.3390/su12041546
Matuszek DB. Ultraviolet Fluorescence in the Assessment of Quality in the Mixing of Granular Material. Sustainability. 2020; 12(4):1546. https://doi.org/10.3390/su12041546
Chicago/Turabian StyleMatuszek, Dominika Barbara. 2020. "Ultraviolet Fluorescence in the Assessment of Quality in the Mixing of Granular Material" Sustainability 12, no. 4: 1546. https://doi.org/10.3390/su12041546
APA StyleMatuszek, D. B. (2020). Ultraviolet Fluorescence in the Assessment of Quality in the Mixing of Granular Material. Sustainability, 12(4), 1546. https://doi.org/10.3390/su12041546