The Effect of Untreated and Acidified Biochar on NH3-N Emissions from Slurry Digestate
Abstract
:1. Introduction
2. Materials and Methods
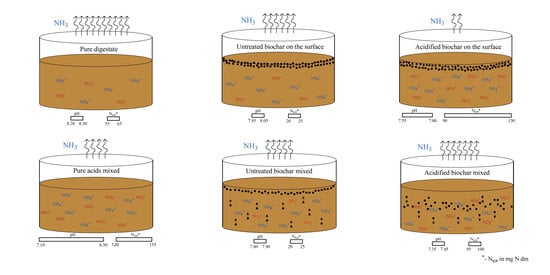
2.1. Experimental Design
2.2. Materials
2.3. Experimental Set-Up
2.4. Measurements and Analyses
2.4.1. NH3 Concentration and Digestate pH
2.4.2. Nutrient Concentration
2.4.3. BC Examination
2.5. Flux Calculation
2.6. Statistical Analysis
3. Results
3.1. NH3-N Emissions
3.2. Cumulative NH3-N Emissions
3.3. pH of Digestate
3.4. Concentration of NH4+-N, NO3−-N and Ntot
3.5. Structural Characterization of BC
4. Discussion
4.1. Effect of Untreated BC on NH3-N Emissions
4.2. Effect of Acidification on BC Characteristics and NH3−-N Emissions
4.3. Effect of Conditioner Treatments on N Concentration in Digestate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, R.; Tian, H.; Pan, S.; Prior, S.A.; Feng, Y.; Batchelor, W.D.; Chen, J.; Yang, J. Global ammonia emissions from synthetic nitrogen fertilizer applications in agricultural systems: Empirical and process-based estimates and uncertainty. Glob. Chang. Biol. 2019, 25, 314–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchis, E.; Calvet, S.; Galán, E.; Estellés, F. Meta-analysis of environmental effects on gaseous emissions from dairy cattle houses. Biosyst. Eng. 2019, 178, 176–183. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; Haan, C.D. Livestock’s Long Shadow—Environmental Issues and Options; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; ISBN 9789251055717. [Google Scholar]
- Sommer, S.G.; Petersen, S.O.; Søgaard, H.T. Greenhouse Gas Emission from Stored Livestock Slurry. J. Environ. Qual. 2000, 29, 744–751. [Google Scholar] [CrossRef]
- Groenestein, C.M.; Smits, M.C.J.; Huijsmans, J.F.M.; Oenema, O. Measures to Reduce Ammonia Emissions from Livestock Manures: Now, Soon and Later. Available online: https://edepot.wur.nl/189371 (accessed on 25 August 2020).
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci. Pollut. Res. 2013, 20, 8092–8131. [Google Scholar] [CrossRef] [PubMed]
- Krupa, S.V. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: A review. Environ. Pollut. 2003, 124, 179–221. [Google Scholar] [CrossRef]
- Ti, C.; Xia, L.; Chang, S.X.; Yan, X. Potential for mitigating global agricultural ammonia emission: A meta-analysis. Environ. Pollut. 2018, 245, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Erisman, J.W.; Bleeker, A.; Hensen, A.; Vermeulen, A. Agricultural air quality in Europe and the future perspectives. Atmos. Environ. 2008, 42, 3209–3217. [Google Scholar] [CrossRef] [Green Version]
- Kai, P.; Pedersen, P.; Jensen, J.E.; Hansen, M.N.; Sommer, S.G. A whole-farm assessment of the efficacy of slurry acidification in reducing ammonia emissions. Eur. J. Agron. 2008, 28, 148–154. [Google Scholar] [CrossRef]
- Sørensen, P.; Amato, M. Remineralisation and residual effects of N after application of pig slurry to soil. Eur. J. Agron. 2002, 16, 81–95. [Google Scholar] [CrossRef]
- Perazzolo, F.; Mattachini, G.; Tambone, F.; Calcante, A.; Provolo, G. Nutrient losses from cattle co-digestate slurry during storage. J. Agric. Eng. 2016, 47, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Brockmann, D.; Hanhoun, M.; Négri, O.; Hélias, A. Environmental assessment of nutrient recycling from biological pig slurry treatment—Impact of fertilizer substitution and field emissions. Bioresour. Technol. 2014, 163, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.; Cowling, E.B.; Cosby, B.J. The Nitrogen Cascade. BioScience 2003, 53, 341. [Google Scholar] [CrossRef]
- Carozzi, M.; Ferrara, R.M.; Rana, G.; Acutis, M. Evaluation of mitigation strategies to reduce ammonia losses from slurry fertilisation on arable lands. Sci. Total Environ. 2013, 449, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Rodhe, L.; Casimir, J.; Sindhöj, E. Possibilities and Bottlenecks for Implementing Slurry Acidification Techniques in the Baltic Sea Region (revised). Available online: http://balticslurry.eu/wp-content/uploads/2016/06/Report-2.1-Possibilities-and-bottlenecks-REVISED.pdf (accessed on 7 June 2020).
- Stenglein, R.; Clanton, C.; Schmidt, D.; Jacobson, L.; Janni, K. Covers for Mitigating Odour and Gas Emissions in Animal Agriculture: An Overview [Fact sheet]. Available online: http://www.extension.org/sites/default/files/CoversoverviewFINAL_0.pdf (accessed on 23 August 2020).
- Viguria, M.; Sanz-Cobeña, A.; López, D.M.; Arriaga, H.; Merino, P. Ammonia and greenhouse gases emission from impermeable covered storage and land application of cattle slurry to bare soil. Agric. Ecosyst. Environ. 2015, 199, 261–271. [Google Scholar] [CrossRef]
- Madejováa, J.; Jankovič, Ľ.; Slaný, M.; Hronský, V. Conformation heterogeneity of alkylammonium surfactants self-assembled on montmorillonite: Effect of head-group structure and temperature. Appl. Surf. Sci. 2020, 503, 144125. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Hristov, A.N.; Arogo, J.; Sheffield, R.E. A review of ammonia emission mitigation techniques for concentrated animal feeding operations. Biosyst. Eng. 2008, 100, 453–469. [Google Scholar] [CrossRef]
- Andersen, D.; Harmon, J.; Hoff, S.; Rieck-Hinz, A. Manure Storages—Impermeable Covers Overview [Fact sheet]. Available online: www.agronext.iastate.edu/ampat (accessed on 17 June 2020).
- Thorman, R.E.; Webb, J.; Yamulki, S.; Chadwick, D.R.; Bennett, G.; McMillan, S.; Kingston, H.; Donovan, N.; Misselbrook, T.H. The effect of solid manure incorporation on nitrous oxide emissions. In Proceedings of the 13th International Conference of the FAO RAMIRAN: Potential for Simple Technology Solutions in Organic Manure Management; Ambrozia NT Ltd.: Albena, Bulgaria, 2008; pp. 170–175. [Google Scholar]
- Loide, V.; Saue, T.; Võsa, T.; Tamm, K. The effect of acidified slurry on crop uptake and leaching of nutrients from a loamy topsoil. Acta Agric. Scand. Sect. B Soil Plant Sci. 2020, 70, 31–38. [Google Scholar] [CrossRef]
- Misselbrook, T.; Hunt, J.; Perazzolo, F.; Provolo, G. Greenhouse gas and ammonia emissions from slurry storage: Impacts of temperature and potential mitigation through covering (pig slurry) or acidification (cattle slurry). J. Environ. Qual. 2016, 45, 1520–1530. [Google Scholar] [CrossRef]
- Yoon, H.; Lim, J.H.; Chung, H.K. Ammonia removal model based on the equilibrium and mass transfer principles. Bull. Korean Chem. Soc. 2008, 29, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Carew, R. Ammonia emissions from livestock industries in Canada: Feasibility of abatement strategies. Environ. Pollut. 2010, 158, 2618–2626. [Google Scholar] [CrossRef]
- Hartung, J.; Phillips, V.R. Control of Gaseous Emissions from Livestock Buildings and Manure Stores. J. Agric. Eng. Res. 1994, 57, 173–189. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, I.; Burchill, W.; Healy, M.G.; Fenton, O.; Krol, D.J.; Lanigan, G.J. Mitigation of ammonia and greenhouse gas emissions from stored cattle slurry using acidifiers and chemical amendments. J. Clean. Prod. 2019, 237, 117822. [Google Scholar] [CrossRef]
- Maurer, D.L.; Koziel, J.A.; Harmon, J.D.; Hoff, S.J.; Rieck-Hinz, A.M.; Andersen, D.S. Summary of performance data for technologies to control gaseous, odor, and particulate emissions from livestock operations: Air management practices assessment tool (AMPAT). Data Br. 2016, 7, 1413–1429. [Google Scholar] [CrossRef] [Green Version]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, Climate Change and Soil: A Review to Guide Future Research. CSIRO L. Water Rep. 2009, 64. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into Multiple and Multilevel Structures of Biochars and Their Potential Environmental Applications: A Critical Review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Li, S.; Barreto, V.; Li, R.; Chen, G.; Hsieh, Y.P. Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2018, 133, 136–146. [Google Scholar] [CrossRef]
- Saarnio, S.; Räty, M.; Hyrkäs, M.; Virkajärvi, P. Biochar addition changed the nutrient content and runoff water quality from the top layer of a grass field during simulated snowmelt. Agric. Ecosyst. Environ. 2018, 265, 156–165. [Google Scholar] [CrossRef]
- Maurer, D.L.; Koziel, J.A.; Kalus, K.; Andersen, D.S.; Opalinski, S. Pilot-scale testing of non-activated biochar for swine manure treatment and mitigation of ammonia, hydrogen sulfide, odorous volatile organic compounds (VOCs), and greenhouse gas emissions. Sustainability 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Sun, H.; Xue, L.; Liu, Y.; Gao, Q.; Lu, K.; Yang, L. Biochar applied at an appropriate rate can avoid increasing NH3 volatilization dramatically in rice paddy soil. Chemosphere 2017, 168, 1277–1284. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Van Zwieten, L.; Singh, B.P.; Smillie, R.; Harden, S.; Fornasier, F. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag. 2017, 61, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Wu, S.; Kipkemoi Kirui, W.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, H.; Xiao, H.; Shi, W.; Müller, K.; Van Zwieten, L.; Wang, H. Wheat straw biochar application increases ammonia volatilization from an urban compacted soil giving a short-term reduction in fertilizer nitrogen use efficiency. J. Soils Sediments 2019, 19, 1624–1631. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Holly, M.A.; Larson, R.A. Thermochemical Conversion of Biomass Storage Covers to Reduce Ammonia Emissions from Dairy Manure. Water. Air. Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Peiris, C.; Nayanathara, O.; Navarathna, C.M.; Jayawardhana, Y.; Nawalage, S.; Burk, G.; Karunanayake, A.G.; Madduri, S.B.; Vithanage, M.; Kaumal, M.N.; et al. The influence of three acid modifications on the physicochemical characteristics of tea-waste biochar pyrolyzed at different temperatures: A comparative study. RSC Adv. 2019, 9, 17612–17622. [Google Scholar] [CrossRef] [Green Version]
- Bremner, J.M.; Keeney, D.R. Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal. Chim. Acta 1965, 32, 485–495. [Google Scholar] [CrossRef]
- FAO Standard Operating Procedure for Soil Calcium Carbonate Equivalent: Titrimetric Method. Available online: http://www.fao.org/3/ca8620en/CA8620EN.pdf (accessed on 13 July 2020).
- Niskanen, R.; Mäntylahti, V. Determination of soil specific surface area by water vapor adsorption: II. Dependence of soil specific surface areas on clay and organic carbon content. Agric. Food Sci. 1987, 59, 67–72. [Google Scholar] [CrossRef]
- Gámiz, B.; Hall, K.; Spokas, K.A.; Cox, L. Understanding activation effects on low-temperature biochar for optimization of herbicide sorption. Agronomy 2019, 9, 588. [Google Scholar] [CrossRef] [Green Version]
- Venterea, R.T.; Parkin, T.B. USDA-ARS GRACEnet Project Protocols Chapter 3. Chamber-Based Trace Gas Flux Measurements 4. Flux 2010, 2010, 1–39. [Google Scholar]
- R Foundation for Statistical Computing. R Development Core Team R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Publishing Company: Reading, MA, USA, 1977. [Google Scholar]
- Dray, S.; Dufour, A.B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Mia, S.; Dijkstra, F.A.; Singh, B. Aging Induced Changes in Biochar’s Functionality and Adsorption Behavior for Phosphate and Ammonium. Environ. Sci. Technol. 2017, 51, 8359–8367. [Google Scholar] [CrossRef] [PubMed]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Chang, R.; Sohi, S.P.; Jing, F.; Liu, Y.; Chen, J. A comparative study on biochar properties and Cd adsorption behavior under effects of ageing processes of leaching, acidification and oxidation. Environ. Pollut. 2019, 254, 113123. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, Y.; Hirano, S.; Mihashi, A.; Kobayashi, Y.; Zhai, S.; Sugiyama, J. Prediction of Lignin Contents from Infrared Spectroscopy: Chemical Digestion and Lignin/Biomass Ratios of Cryptomeria japonica. Appl. Biochem. Biotechnol. 2019, 188, 1066–1076. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Oancea, A.; Grasset, O.; Le Menn, E.; Bollengier, O.; Bezacier, L.; Le Mouélic, S.; Tobie, G. Laboratory infrared reflection spectrum of carbon dioxide clathrate hydrates for astrophysical remote sensing applications. Icarus 2012, 221, 900–910. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. Encycl. Anal. Chem. 2006, 1–23. [Google Scholar] [CrossRef]
- Das, D.D.; Schnitzer, M.I.; Monreal, C.M.; Mayer, P. Chemical composition of acid-base fractions separated from biooil derived by fast pyrolysis of chicken manure. Bioresour. Technol. 2009, 100, 6524–6532. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Saxena, A.; Song, B.; Ward, B.B.; Beveridge, T.J.; Myneni, S.C.B. Elucidation of functional groups on gram-positive and gram-negative bacterial surfaces using infrared spectroscopy. Langmuir 2004, 20, 11433–11442. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrologic properties of biochars produced at different temperatures. Biomass Bioenergy 2012, 41, 34–43. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, K.; Chen, B. Linking hydrophobicity of biochar to the water repellency and water holding capacity of biochar-amended soil. Environ. Pollut. 2019, 253, 779–789. [Google Scholar] [CrossRef]
- García-González, M.C.; Vanotti, M.B.; Szogi, A.A. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of aeration. J. Environ. Manage. 2015, 152, 19–26. [Google Scholar] [CrossRef]
- Vithanage, M.; Rajapaksha, A.U.; Zhang, M.; Thiele-Bruhn, S.; Lee, S.S.; Ok, Y.S. Acid-activated biochar increased sulfamethazine retention in soils. Environ. Sci. Pollut. Res. 2015, 22, 2175–2186. [Google Scholar] [CrossRef]
- Cibati, A.; Foereid, B.; Bissessur, A.; Hapca, S. Assessment of Miscanthus × giganteus derived biochar as copper and zinc adsorbent: Study of the effect of pyrolysis temperature, pH and hydrogen peroxide modification. J. Clean. Prod. 2017, 162, 1285–1296. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.Y.; Ni, N.; Nkoh, J.N.; Li, J.Y.; Xu, R.K.; Qian, W. Beneficial dual role of biochars in inhibiting soil acidification resulting from nitrification. Chemosphere 2019, 234, 43–51. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef]
- Rechberger, M.V.; Kloss, S.; Rennhofer, H.; Tintner, J.; Watzinger, A.; Soja, G.; Lichtenegger, H.; Zehetner, F. Changes in biochar physical and chemical properties: Accelerated biochar aging in an acidic soil. Carbon N. Y. 2017, 115, 209–219. [Google Scholar] [CrossRef]
- Shen, W.; Li, Z.; Liu, Y. Surface Chemical Functional Groups Modification of Porous Carbon. Recent Patents Chem. Eng. 2008, 1, 27–40. [Google Scholar] [CrossRef]
- Huff, M.D.; Lee, J.W. Biochar-surface oxygenation with hydrogen peroxide. J. Environ. Manage. 2016, 165, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Iriarte-Velasco, U.; Sierra, I.; Zudaire, L.; Ayastuy, J.L. Preparation of a porous biochar from the acid activation of pork bones. Food Bioprod. Process. 2016, 98, 341–353. [Google Scholar] [CrossRef]
- Petit, C.; Kante, K.; Bandosz, T.J. The role of sulfur-containing groups in ammonia retention on activated carbons. Carbon N. Y. 2010, 48, 654–667. [Google Scholar] [CrossRef]
- Ro, K.; Lima, I.; Reddy, G.; Jackson, M.; Gao, B. Removing Gaseous NH3 Using Biochar as an Adsorbent. Agriculture 2015, 5, 991–1002. [Google Scholar] [CrossRef] [Green Version]
- Chadwick, D.; Sommer, S.; Thorman, R.; Fangueiro, D.; Cardenas, L.; Amon, B.; Misselbrook, T. Manure management: Implications for greenhouse gas emissions. Anim. Feed Sci. Technol. 2011, 166–167, 514–531. [Google Scholar] [CrossRef]
- Soliman, M.; Eldyasti, A. Ammonia-Oxidizing Bacteria (AOB): Opportunities and Applications—A review; Springer: Dordrecht, The Netherlands, 2018; Volume 17, ISBN 1115701894. [Google Scholar] [CrossRef]
- Šimek, M.; Jíšová, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Kupper, T.; Häni, C.; Neftel, A.; Kincaid, C.; Bühler, M.; Amon, B.; VanderZaag, A. Ammonia and greenhouse gas emissions from slurry storage—A review. Agric. Ecosyst. Environ. 2020, 300, 106963. [Google Scholar] [CrossRef]
- Lan, Z.M.; Chen, C.R.; Rashti, M.R.; Yang, H.; Zhang, D.K. Stoichiometric ratio of dissolved organic carbon to nitrate regulates nitrous oxide emission from the biochar-amended soils. Sci. Total Environ. 2017, 576, 559–571. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y.; Liu, T.; Huang, Q.; Yang, S.; Wang, W.; Jin, P. The simultaneous adsorption of nitrate and phosphate by an organic-modified aluminum-manganese bimetal oxide: Adsorption properties and mechanisms. Appl. Surf. Sci. 2019, 478, 539–551. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alsewaileh, A.S.; Usman, A.R.; Al-Wabel, M.I. Effects of pyrolysis temperature on nitrate-nitrogen (NO3−-N) and bromate (BrO3−) adsorption onto date palm biochar. J. Environ. Manage. 2019, 237, 289–296. [Google Scholar] [CrossRef] [PubMed]






| Abbreviation | Treatment description |
|---|---|
| Control | Digestate |
| BCm | Untreated BC mixed into digestate |
| BCs | Untreated BC on the digestate surface |
| BCm + H2SO4 | BC treated with sulfuric acid mixed into digestate |
| BCs + H2SO4 | BC treated with sulfuric acid on the digestate surface |
| BCm + H3PO4 | BC treated with phosphoric acid mixed into digestate |
| BCs + H3PO4 | BC treated with phosphoric acid on the digestate surface |
| BCm + H2O2 | BC treated with hydrogen peroxide mixed into digestate |
| BCs + H2O2 | BC treated with hydrogen peroxide on the digestate surface |
| H2SO4 | Sulfuric acid mixed into digestate |
| H3PO4 | Phosphoric acid mixed into digestate |
| H2O2 | Hydrogen peroxide mixed into digestate |
| Parameter | Digestate | BC | BC + H2SO4 | BC + H3PO4 | BC + H2O2 |
|---|---|---|---|---|---|
| pH | 8.2 | 6.40 | 1.55 | 2.65 | 4.80 |
| Specific surface area, m2 g−1 | - | 126.14 | 161.48 | 145.92 | 166.31 |
| Acid neutralization capacity (CaCO3, %) | - | 3.80 | 0.00 | 0.49 | 3.86 |
| Dry matter (%) | 4.06 | 100 | 100 | 100 | 100 |
| Ctot (%) | 40.37 | 50.29 | 51.18 | 53.57 | 51.77 |
| Ntot (%) | 6.87 | 2.79 | 2.81 | 2.96 | 2.85 |
| C/N | 5.88 | 18.03 | 18.22 | 18.10 | 18.16 |
| NH4+-N (mg kg−1) | 46,390 | 80 | 400 | 230 | 220 |
| NO3−-N (mg kg−1) | 3520 | 60 | 80 | 90 | 80 |
| Factor | Df | Sum Sq. | Mean Sq. | F Value | Pr(>F) | R2 | ω2 | Sig |
|---|---|---|---|---|---|---|---|---|
| BC | 1 | 48 | 47.70 | 46.127 | 1.07 × 10−11 | 0.0039 | 0.0038 | *** |
| BC application method | 1 | 251 | 251.33 | 243.025 | <2 × 10−16 | 0.0207 | 0.2066 | *** |
| Acids | 3 | 206 | 68.68 | 66.411 | <2 × 10−16 | 0.0170 | 0.0167 | *** |
| Time passed | 287 | 446 | 1.55 | 1.502 | 1.60 × 10−7 | 0.0368 | 0.0122 | *** |
| BC:acids | 3 | 101 | 33.80 | 32.686 | <2 × 10−16 | 0.0083 | 0.0081 | *** |
| BC application method:acids | 3 | 104 | 34.72 | 33.573 | <2 × 10−16 | 0.0086 | 0.0083 | *** |
| BC:time passed | 287 | 439 | 1.53 | 1.478 | 4.85 × 10−7 | 0.0362 | 0.0117 | *** |
| BC application method:time passed | 287 | 374 | 1.30 | 1.262 | 0.002165 | 0.0309 | 0.0064 | ** |
| Acid:time passed | 861 | 1087 | 1.26 | 1.221 | 2.86 × 10−5 | 0.0897 | 0.0162 | *** |
| BC:acid:time passed | 861 | 1069 | 1.24 | 1.200 | 0.000121 | 0.0882 | 0.0147 | *** |
| BC application method:acids:time passed | 861 | 838 | 0.97 | 0.941 | 0.879399 | 0.0691 | 0.0000 | |
| Residuals | 6912 | 7148 | 1.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covali, P.; Raave, H.; Escuer-Gatius, J.; Kaasik, A.; Tõnutare, T.; Astover, A. The Effect of Untreated and Acidified Biochar on NH3-N Emissions from Slurry Digestate. Sustainability 2021, 13, 837. https://doi.org/10.3390/su13020837
Covali P, Raave H, Escuer-Gatius J, Kaasik A, Tõnutare T, Astover A. The Effect of Untreated and Acidified Biochar on NH3-N Emissions from Slurry Digestate. Sustainability. 2021; 13(2):837. https://doi.org/10.3390/su13020837
Chicago/Turabian StyleCovali, Pavel, Henn Raave, Jordi Escuer-Gatius, Allan Kaasik, Tõnu Tõnutare, and Alar Astover. 2021. "The Effect of Untreated and Acidified Biochar on NH3-N Emissions from Slurry Digestate" Sustainability 13, no. 2: 837. https://doi.org/10.3390/su13020837
APA StyleCovali, P., Raave, H., Escuer-Gatius, J., Kaasik, A., Tõnutare, T., & Astover, A. (2021). The Effect of Untreated and Acidified Biochar on NH3-N Emissions from Slurry Digestate. Sustainability, 13(2), 837. https://doi.org/10.3390/su13020837