Determination of Air Pollutants: Application of a Low-Cost Method for Preparation of VOC Mixtures at Known Concentration
Abstract
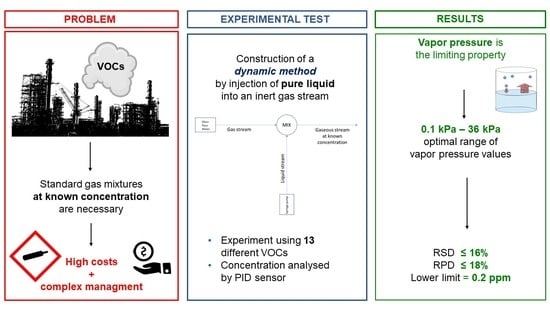
:1. Introduction
- −
- Under-oxygenated atmospheres with the danger of asphyxiation (inert gases);
- −
- Explosive atmospheres (flammable gases);
- −
- Over-oxygenated atmospheres (oxygen), in which case the slightest flame or spark can ignite any combustible substance present.
2. Materials and Methods
2.1. Equations
2.2. Materials
- −
- Syringes: Hamilton™ 80384: glass liquid syringe with a maximum capacity of 10 µL, with an internal diameter equal to 0.485 mm, suitable for syringe pump use;
- −
- Reagents: Several VOCs, in pure liquid form, from different classes of compounds were chosen and are listed in Table 1 where producers and main properties are provided too, calculated at 293 K [20]. These VOCs were selected due to their potential presence in odorous emissions, their potential odorous/toxicological impact [21], and their different chemical–physical parameters, as shown in Table 1;
- −
- Mass flow meter: Alicat™ Portable Calibration Unit Mass Flow Meters, an instrument used to set the desired flow rate of the diluting gas;
- −
- Nitrogen: SAPIO® liquid evaporated pure nitrogen, used as diluent gas;
- −
- Syringe pump: NE-300 Just Infusion™ Syringe Pump. It allows obtaining the continuous injection of liquid inside the mixing node. By setting the diameter of the syringe used and the required injection rate, it maintains the proper linear velocity of the pump to control the set point liquid flow;
- −
- Nalophan: Nalo Kalle™. This is the constituent material of the bags used to sample the gaseous mixture flows to be analysed. It is a transparent material, with characteristics that make it particularly suitable for containing gas mixtures: it is impermeable, inert, odourless, and resistant to traction, tearing, and impact [3].
2.3. Analysis
3. Results and Discussion
3.1. Gross Results
3.2. Repeatability and Accuracy
3.3. Low Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engewald, W.; Dettmer-Wilde, K. Practical Gas Chromatography; Springer: Berlin Heidelberg, Germany, 2014; ISBN 978-3-642-54639-6. [Google Scholar] [CrossRef]
- ASTM D7360; Standard Test Method for Analysis of Benzene by Gas Chromatography with External Calibration. ASTM: West Conshohocken, PA, USA, 2016.
- EN 13725:2022; Determination of Odour Concentration by Dynamic Olfactometry and Odour Emission Rate. CEN-CENELEC Management Centre: Brussels, Belgium, 2022.
- Papurello, D. Direct injection mass spectrometry technique for the odorant losses at ppb(v) level from nalophanTM sampling bags. Int. J. Mass Spectrom. 2019, 436, 137–146. [Google Scholar] [CrossRef]
- Lakshmi HimaBindu, M.R.; Angala Parameswari, S.; Gopinath, C. A review on GC-MS and method development and validation. Int. J. Pharm. Qual. Assur. 2013, 4, 42–51. [Google Scholar]
- Fernandes, Y.; Bry, A.; de Persis, S. Identification and quantification of gases emitted during abuse tests by overcharge of a commercial Li-ion battery. J. Power Sources 2018, 389, 106–119. [Google Scholar] [CrossRef]
- Liu, D.; Nyord, T.; Rong, L.; Feilberg, A. Real-time quantification of emissions of volatile organic compounds from land spreading of pig slurry measured by PTR-MS and wind tunnels. Sci. Total Environ. 2018, 639, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- EN 14470-2; Fire Safety Storage Cabinets—Part 2: Safety cabinets for pressurised gas cylinders. CEN-CENELEC Management Centre: Brussels, Belgium, 2006. [CrossRef]
- European Industrial Gases Association Aisbl. Hazards of Oxygen-Deficient Atmospheres; European Industrial Gases Association Aisbl: Bruxelles, Belgium, 2018. [Google Scholar]
- ISO 11625:2007; Gas Cylinders—Safe Handling. ISO: Geneva, Switzerland, 2007.
- Invernizzi, M.; Roveda, L.; Polvara, E.; Sironi, S. Lights and Shadows of the Voc Emission Quantification. Chem. Eng. Trans. 2021, 85, 109–114. [Google Scholar] [CrossRef]
- European Union Publications Office. The VOC Solvent Emission Directive EUR-Lex.; European Union Publications Office: Luxembourg, 2010. [Google Scholar]
- ISO 6145-2:2014; Gas analysis—Preparation of calibration gas mixtures using dynamic methods—Part 2: Piston pumps. ISO: Geneva, Switzerland, 2014.
- Platonov, I.A.; Rodinkov, O.V.; Gorbacheva, A.R.; Moskvin, L.N.; Kolesnichenko, I.N. Methods and devices for the preparation of standard gas mixtures. J. Anal. Chem. 2018, 73, 109–127. [Google Scholar] [CrossRef]
- Barratt, R.S. The preparation of standard gas mixtures a review. Analyst 1981, 106, 817–849. [Google Scholar] [CrossRef]
- ISO 6145-4:2004; Gas analysis—Preparation of calibration gas mixtures using dynamic volumetric methods—Part 4: Continuous syringe injection method. ISO: Geneva, Switzerland, 2004.
- Rhoderick, G.C.; Yen, J.H. Development of a NIST Standard Reference Material Containing Thirty Volatile Organic Compounds at 5 nmol/mol in Nitrogen. Anal. Chem. 2006, 78, 3125–3132. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalik, A.; Makhlouf, G.; Hassan, M.A. A new implementation of EN1839 method T mixture preparation to prepare calibration gas mixtures for anesthetic gas monitors. J. Clin. Monit. Comput. 2021, 35, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Täffner, T.; Bischoff, M.; Niemeyer, B. Test gas generation from pure liquids: An application-oriented overview of methods in a nutshell. Int. J. Chem. Eng. 2012, 2012, 417029. [Google Scholar] [CrossRef]
- Perry, R.; Green, D.W. Perry’s Chemical Engineers’ Handbook; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Spinazzè, A.; Polvara, E.; Cattaneo, A.; Invernizzi, M.; Cavallo, D.M.; Sironi, S. Dynamic Olfactometry and Oil Refinery Odour Samples: Application of a New Method for Occupational Risk Assessment. Toxics 2022, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alibakhshi, A. Enthalpy of vaporization, its temperature dependence and correlation with surface tension: A theoretical approach. Fluid Phase Equilib. 2017, 432, 62–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, D.; Dou, Z.; Veilleux, J.C.; Shi, G.H.; Collins, D.S.; Vlachos, P.P.; Ardekani, A.M. The Interface Motion and Hydrodynamic Shear of the Liquid Slosh in Syringes. Pharm. Res. 2021, 38, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Grob, K. Split and Splitless Injection for Quantitative Gas Chromatography; Wiley: Hoboken, NJ, USA, 2001; ISBN 3527298797. [Google Scholar]
- Hu, W.; Huang, B.; Weindorf, D.C.; Chen, Y. Metals Analysis of Agricultural Soils via Portable X-ray Fluorescence Spectrometry. Bull. Environ. Contam. Toxicol. 2014, 92, 420–426. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air: Compendium Method TO-15; Report No. EPA/625/R-96/010b; U.S. Environmental Protection Agency: Washington, DC, USA, 1999.





| Compound | Vapour Pressure @ 293 K(Pa) | Viscosity (mm2/s) | Density (kg/m3) | Surface Tension (mn/m) | Purity (%) | Producer |
|---|---|---|---|---|---|---|
| Toluene | 2900 | 0.6 | 867 | 28 | 99.0% | Sigma-Aldrich® |
| Pentane | 57,000 | 0.4 | 626 | 16 | ≥99.0% | Sigma-Aldrich® |
| Hexane | 16,000 | 0.5 | 660 | 18 | ≥95.0% | Sigma-Aldrich® |
| Heptane | 4703 | 0.6 | 684 | 20 | 99.0% | Sigma-Aldrich® |
| 2-Methylpentane | 22,700 | 0.6 | 653 | 17 | ≥95.0% | Sigma-Aldrich® |
| Isooctane | 5300 | 0.7 | 690 | 22 | ≥99.5% | Sigma-Aldrich® |
| Decane | 127 | 1.3 | 730 | 24 | 99.5% | Sigma-Aldrich® |
| Dodecane | 12 | 2.0 | 750 | 25 | ≥99.0% | Sigma-Aldrich® |
| n-Butanol | 625 | 3.7 | 810 | 70 | 99.8% | Sigma-Aldrich® |
| Cyclopentane | 36,660 | 0.6 | 751 | 23 | 98.0% | Sigma-Aldrich® |
| Cyclohexane | 10,384 | 1.3 | 779 | 25 | 99.5% | Sigma-Aldrich® |
| Methyl ethyl ketone (MEK) | 9867 | 0.5 | 805 | 25 | ≥99.0% | Sigma-Aldrich® |
| Dimethyl disulphide (DMDS) | 3000 | 0.6 | 1′060 | 44 | ≥99.0% | Sigma-Aldrich® |
| Compound | PID Response Factor (Lamp Type 10.6 eV) |
|---|---|
| Toluene | 0.56 |
| Pentane | 7 |
| Hexane | 3 |
| Heptane | 2.2 |
| 2-Methylpentane | 3 |
| Isooctane | 1.1 |
| Decane | 1.2 |
| Dodecane | 1 |
| n-Butanol | 3.9 |
| Cyclopentane | 10 |
| Cyclohexane | 1.3 |
| Methyl ethyl ketone (MEK) | 0.96 |
| Dimethyl disulphide (DMDS) | 0.2 |
| Compound | m | q | R2 |
|---|---|---|---|
| n-Butanol | 1.09 | 0.6 | 0.999 |
| Cyclohexane | 1.00 | 3.4 | 0.999 |
| Cyclopentane | 0.94 | 0.9 | 1.000 |
| Decane | 1.07 | 0.2 | 0.999 |
| Dodecane | 0.05 | 2.3 | 0.949 |
| Dimethyl disulphide (DMDS) | 1.04 | 1.5 | 0.999 |
| Heptane | 0.97 | 4.0 | 0.999 |
| Hexane | 1.03 | 2.3 | 0.999 |
| Isooctane | 1.09 | 1.2 | 1.000 |
| Methyl ethyl ketone (MEK) | 1.01 | 4.3 | 0.999 |
| 2-methylpentane | 1.01 | 1.4 | 0.994 |
| Pentane | 1.20 | 8.9 | 0.998 |
| Toluene | 0.93 | 2.0 | 0.999 |
| Theoretical Concentration (ppm) | RSD% | RPD% |
|---|---|---|
| 5 | 15% | 8% |
| 15 | 13% | 7% |
| 37 | 16% | 8% |
| 51 | 16% | 8% |
| 77 | 5% | 3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartore, L.; Polvara, E.; Invernizzi, M.; Sironi, S. Determination of Air Pollutants: Application of a Low-Cost Method for Preparation of VOC Mixtures at Known Concentration. Sustainability 2022, 14, 9149. https://doi.org/10.3390/su14159149
Sartore L, Polvara E, Invernizzi M, Sironi S. Determination of Air Pollutants: Application of a Low-Cost Method for Preparation of VOC Mixtures at Known Concentration. Sustainability. 2022; 14(15):9149. https://doi.org/10.3390/su14159149
Chicago/Turabian StyleSartore, Lorenzo, Elisa Polvara, Marzio Invernizzi, and Selena Sironi. 2022. "Determination of Air Pollutants: Application of a Low-Cost Method for Preparation of VOC Mixtures at Known Concentration" Sustainability 14, no. 15: 9149. https://doi.org/10.3390/su14159149
APA StyleSartore, L., Polvara, E., Invernizzi, M., & Sironi, S. (2022). Determination of Air Pollutants: Application of a Low-Cost Method for Preparation of VOC Mixtures at Known Concentration. Sustainability, 14(15), 9149. https://doi.org/10.3390/su14159149