Preparation of Special Wettability Quartz Sand Filter Media and Its Synchronous Oil/Water Mixture Separation and Dye Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Quartz Sand Filter Media with Special Wettability
2.3. Characterization of Filter Media
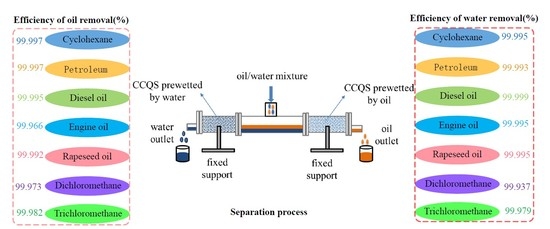
2.4. Separation of Oil/Water Mixture
2.5. Wetting Stability
2.6. Dye Adsorption
2.7. Synchronous Separation of Oil/Water Mixture and Adsorption Dyes
3. Result and Discussion
3.1. Surface Wettability
3.2. Wetting Stability
3.3. Characterization of Filter Media
3.3.1. FTIR Analysis
3.3.2. SEM Analysis
3.3.3. XPS Analysis
3.4. Separation of Oil/Water Mixture
3.5. Dye Adsorption
3.5.1. Effect of Adsorbent Dosage
3.5.2. Effect of Adsorption Time
3.5.3. Effect of Initial Dye Concentration
3.6. Synchronous Separation of Oil/Water Mixture and Adsorption Dye
3.7. Modified Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- He, X.; Liu, Q.; Xu, Z. Treatment of oily wastewaters using magnetic Janus nanoparticles of asymmetric surface wettability. J. Colloid Interface Sci. 2020, 568, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Matchinski, E.J.; Chen, B.; Ye, X.; Jing, L.; Lee, K. Marine Oil Spills—Oil Pollution, Sources and Effects, World Seas: An Environmental Evaluation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 391–406. [Google Scholar]
- Nguyen, T.T.; Phung, T.K.; Bui, X.-T.; Doan, V.-D.; van Tran, T.; Lim, K.T.; Nguyen, T.D. Removal of cationic dye using polyvinyl alcohol membrane functionalized by D-glucose and agar. J. Water Process Eng. 2021, 40, 101982. [Google Scholar] [CrossRef]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Z. Novel and cutting-edge applications for a solvent-responsive superoleophobic–superhydrophilic surface: Water-infused omniphobic surface and separating organic liquid mixtures. Chem. Eng. J. 2020, 381, 122629. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Xie, A.; Dai, X.; Yan, Y.; Dai, J. Facile surface coating of metal-tannin complex onto PVDF membrane with underwater Superoleophobicity for oil-water emulsion separation. Surf. Coat. Technol. 2020, 389, 125630. [Google Scholar] [CrossRef]
- Yu, T.; Halouane, F.; Mathias, D.; Barras, A.; Wang, Z.; Lv, A.; Lu, S.; Xu, W.; Meziane, D.; Tiercelin, N.; et al. Preparation of magnetic, superhydrophobic/superoleophilic polyurethane sponge: Separation of oil/water mixture and demulsification. Chem. Eng. J. 2020, 384, 123339. [Google Scholar] [CrossRef]
- Zou, L.; Phule, A.D.; Sun, Y.; Zhu, T.Y.; Wen, S.; Zhang, Z.X. Superhydrophobic and superoleophilic polyethylene aerogel coated natural rubber latex foam for oil-water separation application. Polym. Test. 2020, 85, 106451. [Google Scholar] [CrossRef]
- Nine, M.J.; Kabiri, S.; Sumona, A.K.; Tung, T.T.; Moussa, M.M.; Losic, D. Superhydrophobic/superoleophilic natural fibres for continuous oil-water separation and interfacial dye-adsorption. Sep. Purif. Technol. 2020, 233, 116062. [Google Scholar] [CrossRef]
- Wang, G.; Liang, W.; Wang, B.; Zhang, Y.; Li, J.; Shi, L.; Guo, Z. Conductive and transparent superhydrophobic films on various substrates by in situ deposition. Appl. Phys. Lett. 2013, 102, 203703. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Srijanto, B.R.; Nguyen, T.D.; Vega, C.; Fuentes-Cabrera, M.; Collier, C.P. Dynamic defrosting on nanostructured superhydrophobic surfaces. Langmuir 2013, 29, 9516–9524. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Ganguly, R.; Schutzius, T.M.; Megaridis, C.M. Wettability patterning for high-rate, pumpless fluid transport on open, non-planar microfluidic platforms. Lab A Chip 2014, 14, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Lartey, P.O.; Li, D.; Li, J.; Qin, W.; Guo, K.; Ma, J. Fluoropolymer-based Hybrid Superhydrophobic Nanocomposite Coating with Antifouling and Self-Cleaning Properties for Efficient Oil/Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129504. [Google Scholar] [CrossRef]
- Yin, X.; Yu, S.; Wang, L.; Li, H.; Xiong, W. Design and preparation of superhydrophobic NiS nanorods on Ni mesh for oil-water separation. Sep. Purif. Technol. 2020, 234, 116126. [Google Scholar] [CrossRef]
- Yue, X.; Li, Z.; Zhang, T.; Yang, D.; Qiu, F. Design and fabrication of superwetting fiber-based membranes for oil/water separation applications. Chem. Eng. J. 2019, 364, 292–309. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef]
- Deng, Y.; Peng, C.; Dai, M.; Lin, D.; Ali, I.; Alhewairini, S.S.; Zheng, X.; Chen, G.; Li, J.; Naz, I. Recent development of super-wettable materials and their applications in oil-water separation. J. Clean. Prod. 2020, 266, 121624. [Google Scholar] [CrossRef]
- Tan, L.; Han, N.; Qian, Y.; Zhang, H.; Gao, H.; Zhang, L.; Zhang, X. Superhydrophilic and underwater superoleophobic poly (acrylonitrile-co-methyl acrylate) membrane for extremely efficient separation of oil-in-water emulsions. J. Membr. Sci. 2018, 564, 712–721. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, G.; Bai, R.; Shen, S.; Zhou, X.; Wyman, I. Fabrication of superhydrophilic and underwater superoleophobic membranes via an in situ crosslinking blend strategy for extremely efficient oil/water emulsion separation. J. Membr. Sci. 2019, 569, 60–70. [Google Scholar] [CrossRef]
- Lai, H.; Yu, X.; Liu, M.; Cheng, Z. One-step solution immersion process for the fabrication of low adhesive underwater superoleophobic copper mesh film toward high-flux oil/water separation. Appl. Surf. Sci. 2018, 448, 241–247. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, C.; Raza, A.; Song, R.; Zhang, T.-J.; Pehkonen, S.O.; Liang, B. Nanostructured TiO2/CuO dual-coated copper meshes with superhydrophilic, underwater superoleophobic and self-cleaning properties for extremely efficient oil/water separation. Chem. Eng. J. 2017, 328, 497–510. [Google Scholar] [CrossRef]
- Pi, P.; Hou, K.; Zhou, C.; Wen, X.; Xu, S.; Cheng, J.; Wang, S. A novel superhydrophilic-underwater superoleophobic Cu2S coated copper mesh for efficient oil-water separation. Mater. Lett. 2016, 182, 68–71. [Google Scholar] [CrossRef]
- Bigui, W.; Cheng, Y.; Jianlin, L.; Gang, W.; Liang, D.; Xiaosan, S.; Fuping, W.; Hua, L.; Qing, C. Fabrication of superhydrophilic and underwater superoleophobic quartz sand filter for oil/water separation. Sep. Purif. Technol. 2019, 229, 115808. [Google Scholar] [CrossRef]
- Tong, H.; Chen, H.; Zhao, Y.; Liu, M.; Cheng, Y.; Lu, J.; Tao, Y.; Du, J.; Wang, H. Robust PDMS-based porous sponge with enhanced recyclability for selective separation of oil-water mixture. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129228. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Li, D.; Tang, X.; Feng, H.; Qi, W.; Wang, Q. Multifunctional walnut shell layer used for oil/water mixtures separation and dyes adsorption. Appl. Surf. Sci. 2017, 419, 869–874. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, H.; Sun, Y.; Zhang, L.; Xu, L.; Hao, L.; Yang, H. Covalent layer-by-layer grafting (LBLG) functionalized superhydrophobic stainless steel mesh for oil/water separation. Appl. Surf. Sci. 2017, 406, 150–160. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Li, B.; Zhang, J.; Wang, A. Magnetic, durable, and superhydrophobic polyurethane@ Fe3O4@ SiO2@ fluoropolymer sponges for selective oil absorption and oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 4936–4946. [Google Scholar] [CrossRef]
- Zhang, N.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A review on oil/water mixture separation material. Ind. Eng. Chem. Res. 2020, 59, 14546–14568. [Google Scholar] [CrossRef]
- Xue, Z.; Cao, Y.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Fang, P.; Huang, L.; Pan, W.; Wu, S.; Feng, X.; Song, J.; Xing, Y. Facile preparation of durable superhydrophobic-superoleophilic mesh using simple chemical oxidation for oil-water separation under harsh conditions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126777. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Capillarity-driven both light and heavy oil/water separation via combined system of opposite superwetting meshes. Sep. Purif. Technol. 2019, 215, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Yang, Y.; Li, J.; Zha, F.; Lei, Z. A prewetting induced underwater superoleophobic or underoil (super) hydrophobic waste potato residue-coated mesh for selective efficient oil/water separation. Green Chem. 2016, 18, 541–549. [Google Scholar] [CrossRef]
- Milionis, A.; Loth, E.; Bayer, I.S. Recent advances in the mechanical durability of superhydrophobic materials. Adv. Colloid Interface Sci. 2016, 229, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, J.; Zhang, Z.; Lu, C. Study on structure and thermal stability properties of cellulose fibers from rice straw. Carbohydr. Polym. 2011, 85, 245–250. [Google Scholar] [CrossRef]
- Xu, F.; Liu, C.; Geng, Z.; Sun, J.; Sun, R.; Hei, B.; Lin, L.; Wu, S.; Je, J. Characterisation of degraded organosolv hemicelluloses from wheat straw. Polym. Degrad. Stab. 2006, 91, 1880–1886. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, N.; Zhou, Q.; Wang, Z.; Duan, C.; Cai, C.; Zhang, X.; Xu, J. Facile preparation of hollow amino-functionalized organosilica microspheres by a template-free method. J. Mater. Chem. 2012, 22, 18010–18017. [Google Scholar] [CrossRef]
- Sorarù, G.D.; D’Andrea, G.; Glisenti, A. XPS characterization of gel-derived silicon oxycarbide glasses. Mater. Lett. 1996, 27, 1–5. [Google Scholar] [CrossRef]
- Wei, B.; Luo, X.; Song, X.; Guo, H.; Dai, L.; Zhang, H.; Wang, G. Quartz Sand Filter Media with Special Wettability for Continuous and Efficient Oil/Water Separation and Dye Adsorption. Processes 2020, 8, 1083. [Google Scholar] [CrossRef]
- Hongwei, Z.; Junye, Q.; Yinglong, C.; Shide, M.; Jianlin, L.; Bigui, W. Continuous and efficient oil/water separation by special wettability granular filter media. J. Water Reuse Desalination 2022, 12, 242–259. [Google Scholar] [CrossRef]
- Gondal, M.A.; Sadullah, M.S.; Dastageer, M.A.; McKinley, G.H.; Panchanathan, D.; Varanasi, K.K. Study of factors governing oil–water separation process using TiO2 films prepared by spray deposition of nanoparticle dispersions. ACS Appl. Mater. Interfaces 2014, 6, 13422–13429. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, X.; Tian, Y.; Wu, Y.; Wang, X.; Zhai, J.; Jiang, L. Photo-induced water–oil separation based on switchable superhydrophobicity–superhydrophilicity and underwater superoleophobicity of the aligned ZnO nanorod array-coated mesh films. J. Mater. Chem. 2012, 22, 19652–19657. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, Y.; Tang, F.; Liu, S. Mesoporous/microporous silica materials: Preparation from natural sands and extremely efficient fixed-bed adsorption of methylene blue in wastewater. Microporous Mesoporous Mater. 2018, 257, 9–18. [Google Scholar] [CrossRef]
- Belhajjia, C.; Abid, A.; Msaad, A.; Labaali, Z.; Zouhri, A. Synthesis, characterization and adsorption of Malachite green dye using novel materiel produced from opuntia ficus indica. Mater. Today Proc. 2021, 37, 4001–4006. [Google Scholar] [CrossRef]
- Sobolčiak, P.; Popelka, A.; Tanvir, A.; Al-Maadeed, M.A.; Adham, S.; Krupa, I. Some Theoretical Aspects of Tertiary Treatment of Water/Oil Emulsions by Adsorption and Coalescence Mechanisms: A Review. Water 2021, 13, 652. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, J.; Wu, Z.; Zhu, S.; Gao, Y.; Shi, C. The effect of surface modification on chemical and crystalline structure of the cellulose III nanocrystals. Carbohydr. Polym. 2020, 235, 115962. [Google Scholar] [CrossRef]
- Ye, D.; Farriol, X. Improving accessibility and reactivity of celluloses of annual plants for the synthesis of methylcellulose. Cellulose 2005, 12, 507–515. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Cao, X.; Lu, D.; Luo, F.; Shao, W. Preparation and evaluation of orange peel cellulose adsorbents for effective removal of cadmium, zinc, cobalt and nickel. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 512–521. [Google Scholar] [CrossRef]
- Heinze, T. Cellulose: Structure and properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer: Cham, Switzerland, 2015; pp. 1–52. [Google Scholar]
- Liu, J.; Zhu, X.; Zhang, H.; Wu, F.; Wei, B.; Chang, Q. Superhydrophobic coating on quartz sand filter media for oily wastewater filtration. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 509–514. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yinglong, C.; Hanyue, G.; Shide, M.; Tingting, Z.; Bigui, W. Preparation of Special Wettability Quartz Sand Filter Media and Its Synchronous Oil/Water Mixture Separation and Dye Adsorption. Sustainability 2022, 14, 9860. https://doi.org/10.3390/su14169860
Yinglong C, Hanyue G, Shide M, Tingting Z, Bigui W. Preparation of Special Wettability Quartz Sand Filter Media and Its Synchronous Oil/Water Mixture Separation and Dye Adsorption. Sustainability. 2022; 14(16):9860. https://doi.org/10.3390/su14169860
Chicago/Turabian StyleYinglong, Che, Guo Hanyue, Man Shide, Zhang Tingting, and Wei Bigui. 2022. "Preparation of Special Wettability Quartz Sand Filter Media and Its Synchronous Oil/Water Mixture Separation and Dye Adsorption" Sustainability 14, no. 16: 9860. https://doi.org/10.3390/su14169860
APA StyleYinglong, C., Hanyue, G., Shide, M., Tingting, Z., & Bigui, W. (2022). Preparation of Special Wettability Quartz Sand Filter Media and Its Synchronous Oil/Water Mixture Separation and Dye Adsorption. Sustainability, 14(16), 9860. https://doi.org/10.3390/su14169860