Innovative Resource Recovery from Industrial Sites: A Critical Review
Abstract
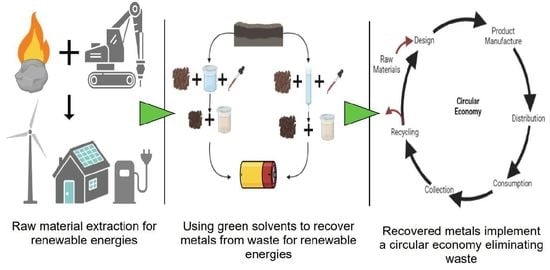
:1. Introduction
2. Contaminated Sites from Past Metallurgical Activity
2.1. Industrial Sites
2.2. Disused Mines
2.3. Landfills
| Type of Landfill | Recovery Option | Limitations | Reference |
|---|---|---|---|
| Soils | Soil Removal | Limited to small working areas High initial costs Extensive machinery required | [40] |
| Leachate | Coagulation/flocculation Adsorption Membrane process | High operating costs Pollutant transfer between phases Low pollutant removal efficiency Low process performance | [41,42] |
| Waste Electrical and Electronic Equipment | Incineration Acid leaching Hydraulic shaking bed separation | Secondary pollutants production Non-metal materials cannot be recycled High operating costs Hard to recover metals except for copper Toxic to the environment | [43] |
| Construction Waste | Landfill | High cost to recycle Lack of enthusiasm to recover resources Minimal communication between contractors | [44] |
| Wastewater | Membrane filtration Chemical precipitation Biosorption | High operational costs Not selective Toxic sludge generated Disposal limits application (biosorption) | [45,46] |
| Hazardous Waste | Pyrolysis | Inefficient metal recovery Energy-intensive High operation costs Toxic dioxins and furans produced | [47] |
3. Remediation of Contaminated Sites
4. Management of Contaminated Soil
4.1. Physical Remediation Approaches
4.2. Phytoremediation Approaches
4.3. Chemical Remediation Approaches
5. Remediation vs. Recovery
6. Emerging Extraction Techniques
7. Green Solvent Leaching
8. Development Challenges and Growing Demand
8.1. Recovery of Metals to Meet Sustainable Development Goals
8.2. Political Perceptions
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watari, T.; Nansai, K.; Nakajima, K. Major metals demand, supply, and environmental impacts to 2100: A critical review. Resour. Conserv. Recycl. 2021, 164, 105107. [Google Scholar] [CrossRef]
- Grandell, L.; Lehtilä, A.; Kivinen, M.; Koljonen, T.; Kihlman, S.; Lauri, L.S. Role of critical metals in the future markets of clean energy technologies. Renew. Energy 2016, 95, 53–62. [Google Scholar] [CrossRef]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling Chain for Spent Lithium-Ion Batteries. Metals 2020, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Moreau, V.; Dos Reis, P.C.; Vuille, F. Enough Metals? Resource Constraints to Supply a Fully Renewable Energy System. Resources 2019, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Timperley, J. Explainer: These Six Metals Are Key to a Low-Carbon Future. Carbon Brief Website. 2018. Available online: https://www.carbonbrief.org/explainer-these-six-metals-are-key-to-a-low-carbon-future/ (accessed on 13 March 2022).
- Jones, P.T.; Geysen, D.; Tielemans, Y.; Van Passel, S.; Pontikes, Y.; Blanpain, B.; Quaghebeur, M.; Hoekstra, N. Enhanced Landfill Mining in view of multiple resource recovery: A critical review. J. Clean. Prod. 2012, 55, 45–55. [Google Scholar] [CrossRef]
- International Renewable Energy Agency. Global Energy Transformation: A Roadmap to 2050 (2019 Edition); International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019. [Google Scholar]
- USGS National Minerals Information Center. 2013 Minerals Yearbook—Copper; USGS National Minerals Information Center: Reston, VA, USA, 2015. [Google Scholar]
- Brinded, A. Metal Madness? Mater. World 2022, 30, 6. [Google Scholar]
- Kavlak, G.; McNerney, J.; Jaffe, R.L.; Trancik, J.E. Metal production requirements for rapid photovoltaics deployment. Energy Environ. Sci. 2015, 8, 1651–1659. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, S.; Toledano, P.; Rietbergen, J.; Villiers-Piaget, D. Mining and the SDGS: A 2020 Status Update; Responsible Mining Foundation: Geneva, Switzerland, 2020. [Google Scholar]
- Lèbre, É.; Corder, G.; Golev, A. The Role of the Mining Industry in a Circular Economy: A Framework for Resource Management at the Mine Site Level. J. Ind. Ecol. 2017, 21, 662–672. [Google Scholar] [CrossRef]
- Schipper, B.W.; Lin, H.-C.; Meloni, M.A.; Wansleeben, K.; Heijungs, R.; van der Voet, E. Estimating global copper demand until 2100 with regression and stock dynamics. Resour. Conserv. Recycl. 2018, 132, 28–36. [Google Scholar] [CrossRef]
- Jadhao, P.R.; Mishra, S.; Pandey, A.; Pant, K.K.; Nigam, K.D.P. Biohydrometallurgy: A Sustainable Approach for Urban Mining of Metals and Metal Refining. In Catalysis for Clean Energy and Environmental Sustainability; Pant, K.K., Gupta, S.K., Ahmad, E., Eds.; Springer: Cham, Switzerland, 2021; pp. 865–892. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M. Deep eutectic solvents. The new generation of green solvents in analytical chemistry. TrAC Trends Anal. Chem. 2021, 134, 116108. [Google Scholar] [CrossRef]
- Harris, B. Toward a Sustainable Metals Extraction Technology. In Mine Wastes and Water, Ecological Engineering and Metals Extraction; Kalin-Seidenfaden, M., Wheeler, W.N., Eds.; Springer: Cham, Switzerland, 2022; pp. 17–28. [Google Scholar] [CrossRef]
- Jaffe, S. Balancing Li battery DEMAND with Materials Supply. 2018. Available online: https://www.vaneck.com/uploadfiles/conference/reports/cairn.pdf (accessed on 4 April 2022).
- Katwala, A. The spiralling environmental cost of our lithium battery addiction. WIRED, 5 September 2018. [Google Scholar]
- British Lithium. Battery Grade Lithium Produced from Cornish Granite 2020. Available online: https://britishlithium.co.uk/ (accessed on 21 February 2021).
- UK Trade & Investment. Land Remediation: Bringing Brownfield Sites Back to Use. 2015. Available online: https://www.gov.uk/government/publications/land-remediation-bringing-brownfield-sites-back-to-use/land-remediation-bringing-brownfield-sites-back-to-use (accessed on 17 March 2022).
- Sinnett, D. Going to waste? The potential impacts on nature conservation and cultural heritage from resource recovery on former mineral extraction sites in England and Wales. J. Environ. Plan. Manag. 2019, 62, 1227–1248. [Google Scholar] [CrossRef] [Green Version]
- Riley, A.L.; MacDonald, J.M.; Burke, I.T.; Renforth, P.; Jarvis, A.P.; Hudson-Edwards, K.A.; McKie, J.; Mayes, W.M. Legacy iron and steel wastes in the UK: Extent, resource potential, and management futures. J. Geochem. Explor. 2020, 219, 106630. [Google Scholar] [CrossRef]
- Renforth, P.; Washbourne, C.-L.; Taylder, J.; Manning, D.A.C. Silicate Production and Availability for Mineral Carbonation. Environ. Sci. Technol. 2011, 45, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and Recycling of By-Products in the Steel Sector: Recent Achievements Paving the Way to Circular Economy and Industrial Symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Tunsu, C.; Menard, Y.; Eriksen, D.; Ekberg, C.; Petranikova, M. Recovery of critical materials from mine tailings: A comparative study of the solvent extraction of rare earths using acidic, solvating and mixed extractant systems. J. Clean. Prod. 2019, 218, 425–437. [Google Scholar] [CrossRef]
- Seredkin, M.; Zabolotsky, A.; Jeffress, G. In situ recovery, an alternative to conventional methods of mining: Exploration, resource estimation, environmental issues, project evaluation and economics. Ore Geol. Rev. 2016, 79, 500–514. [Google Scholar] [CrossRef]
- Mayes, W.; Johnston, D.; Potter, H.; Jarvis, A. A national strategy for identification, prioritisation and management of pollution from abandoned non-coal mine sites in England and Wales. I.: Methodology development and initial results. Sci. Total. Environ. 2009, 407, 5435–5447. [Google Scholar] [CrossRef]
- Potter, H.; Johnston, D. Inventory of Closed Mining Waste Facilities; UK Environment Agency: Bristol, UK, 2014. [Google Scholar]
- Hudson-Edwards, K.A.; Dold, B. Mine Waste Characterization, Management and Remediation. Minerals 2015, 5, 82–85. [Google Scholar] [CrossRef] [Green Version]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Krook, J.; Svensson, N.; Eklund, M. Landfill mining: A critical review of two decades of research. Waste Manag. 2012, 32, 513–520. [Google Scholar] [CrossRef]
- Prechthai, T.; Padmasri, M.; Visvanathan, C. Quality assessment of mined MSW from an open dumpsite for recycling potential. Resour. Conserv. Recycl. 2008, 53, 70–78. [Google Scholar] [CrossRef]
- van der Zee, D.; Achterkamp, M.; de Visser, B. Assessing the market opportunities of landfill mining. Waste Manag. 2004, 24, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Hogland, W.; Hogland, M.; Marques, M. Enhanced landfill mining: Material recovery, energy utilization and economics in the EU (Directive) perspective. In Proceedings of the International Academic Symposium on Enhanced Landfill Mining, Houthalen-Helchteren, Belgium, January 2011; pp. 209–222. [Google Scholar]
- Wagner, T.P.; Raymond, T. Landfill mining: Case study of a successful metals recovery project. Waste Manag. 2015, 45, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Jagodzińska, K.; Zaini, I.N.; Svanberg, R.; Yang, W.; Jönsson, P.G. Pyrolysis of excavated waste from landfill mining: Characterisation of the process products. J. Clean. Prod. 2020, 279, 123541. [Google Scholar] [CrossRef]
- Hogland, W.; Marques, M.; Nimmermark, S. Landfill mining and waste characterization: A strategy for remediation of contaminated areas. J. Mater. Cycles Waste Manag. 2004, 6, 119–124. [Google Scholar] [CrossRef]
- Blengini, G.; Mathieux, F.; Mancini, L.; Nyberg, M.; Viegas, H. Recovery of Critical and Other Raw Materials from Mining Waste and Landfills: State of Play on Existing Practices; European Commission: Kirchberg, Luxembourg, 2019; Available online: https://core.ac.uk/download/pdf/199318323.pdf (accessed on 20 May 2022).
- Bučinskas, A.; Kriipsalu, M.; Denafas, G. Proposal for Feasibility Assessment Model for Landfill Mining and Its Implementation for Energy Generation Scenarios. Sustainability 2018, 10, 2882. [Google Scholar] [CrossRef] [Green Version]
- Griffin, G.; Gaustad, G.; Badami, K. A framework for firm-level critical material supply management and mitigation. Resour. Policy 2019, 60, 262–276. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, Z. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Pennington, D.; Tzimas, E.; Baranzelli, C.; Dewulf, J.; Manfredi, S.; Nuss, P.; Grohol, M.; Van Maercke, A.; Kayam, Y.; Solar, S.; et al. Assessment of the Methodology for Establishing the EU List of Critical Raw Materials; European Commission: Kirchberg, Luxembourg, 2017. [Google Scholar]
- Siddi, M. The European Green Deal: Assesing Its Current State and Future Implementation; Finnish Institute of International Affairs: Helsinki, Finland, 2020. [Google Scholar]
- British Geological Survey. Minerals Produced in the United Kingdom in 2017; British Geological Survey: Nottinghamshire, UK, 2017. [Google Scholar]
- Wang, H.; Ren, Z.J. Bioelectrochemical metal recovery from wastewater: A review. Water Res. 2014, 66, 219–232. [Google Scholar] [CrossRef]
- European Commission. The Raw Materials Initiative—Meeting Our Critical Needs for Growth and Jobs in Europe; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Velenturf, A.P.; Jopson, J.S. Making the business case for resource recovery. Sci. Total Environ. 2018, 648, 1031–1041. [Google Scholar] [CrossRef]
- Caliman, F.A.; Robu, B.M.; Smaranda, C.; Pavel, V.L.; Gavrilescu, M. Soil and groundwater cleanup: Benefits and limits of emerging technologies. Clean Technol. Environ. Policy 2010, 13, 241–268. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 2018, 8, 216. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Dumat, C.; Naidu, R.; Khalid, S.; Rahman, M.M.; Bibi, I. A meta-analysis of the distribution, sources and health risks of arsenic-contaminated groundwater in Pakistan. Environ. Pollut. 2018, 242, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on Remediation Technologies of Soil Contaminated by Heavy Metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, P.P. A field demonstration of the simulation optimisation approach for remediation system design. Groundwater 2002, 40, 258–266. [Google Scholar] [CrossRef]
- Jankaite, A.; Vasarevičius, S. Remediation technologies for soils contaminated with heavy metals. J. Environ. Eng. Landsc. Manag. 2005, 13, 109–113. [Google Scholar] [CrossRef]
- Dellisanti, F.; Rossi, P.L.; Valdrè, G. In-field remediation of tons of heavy metal-rich waste by Joule heating vitrification. Int. J. Miner. Process. 2009, 93, 239–245. [Google Scholar] [CrossRef]
- Guo, X.; Wei, Z.; Wu, Q.; Li, C.; Qian, T.; Zheng, W. Effect of soil washing with only chelators or combining with ferric chloride on soil heavy metal removal and phytoavailability: Field experiments. Chemosphere 2016, 147, 412–419. [Google Scholar] [CrossRef]
- Li, C.-T.; Lee, W.-J.; Huang, K.-L.; Fu, S.-F.; Lai, Y.-C. Vitrification of Chromium Electroplating Sludge. Environ. Sci. Technol. 2007, 41, 2950–2956. [Google Scholar] [CrossRef]
- Navarro, A.; Cardellach, E.; Cañadas, I.; Rodríguez, J. Solar thermal vitrification of mining contaminated soils. Int. J. Miner. Process. 2013, 119, 65–74. [Google Scholar] [CrossRef]
- Song, Y.; Ammami, M.; Benamar, A.; Mezazigh, S.; Wang, H. Effect of EDTA, EDDS, NTA and citric acid on electrokinetic remediation of As, Cd, Cr, Cu, Ni, Pb and Zn contaminated dredged marine sediment. Environ. Sci. Pollut. Res. 2016, 23, 10577–10586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Ma, J.; Fan, X.; Luo, Q. Research progress on enhancement of in situ remediation of heavy metal by electrokinetics. Environ. Sci. 2007, 16, 223–227. [Google Scholar]
- Ghosh, M.; Singh, S. A review on phytoremediation of heavy metals and utilization of it’s by products. Asian J. Energy Environ. 2005, 6, 214–231. [Google Scholar]
- Khalid, H.; Zia-Ur-Rehman, M.; Naeem, A.; Khalid, M.U.; Rizwan, M.; Ali, S.; Umair, M.; Sohail, M.I. Solanum nigrum L.: A Novel Hyperaccumulator for the Phyto-Management of Cadmium Contaminated Soils. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Vara Prasad, M.N., Fujita, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 451–477. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Sylvain, B.; Mikael, M.-H.; Florie, M.; Emmanuel, J.; Marilyne, S.; Sylvain, B.; Domenico, M. Phytostabilization of As, Sb and Pb by two willow species (S. viminalis and S. purpurea) on former mine technosols. Catena 2016, 136, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Bhalerao, S. Arbuscular mycorrhizal fungi: A potential biotechnology tool for phytoremediation of heavy metal contaminated soils. Int. J. Sci. Nat. 2013, 4, 1–15. [Google Scholar]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilisation: A green approach to contaminant containment. Adv. Agron. 2011, 112, 145–204. [Google Scholar] [CrossRef]
- Sakakibara, M.; Watanabe, A.; Inoue, M.; Sano, S.; Kaise, T. Phytoextraction And Phytovolatilization Of Arsenic From As-Contaminated Soils By Pteris vittata. Proc. Annu. Int. Conf. Soils Sediments Water Energy 2010, 12, 26. [Google Scholar]
- Nikolić, M.; Stevović, S. Family Asteraceae as a sustainable planning tool in phytoremediation and its relevance in urban areas. Urban For. Urban Green. 2015, 14, 782–789. [Google Scholar] [CrossRef]
- Udovic, M.; Lestan, D. Fractionation and bioavailability of Cu in soil remediated by EDTA leaching and processed by earthworms (Lumbricus terrestris L.). Environ. Sci. Pollut. Res. 2009, 17, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Takano, H.; Kamiya, T.; Itou, T.; Sekiya, N.; Inahara, M.; Sakurai, Y. Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: Cd extraction mechanism and bench-scale verification. Chemosphere 2008, 70, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chen, J.; Wang, X. Removal of arsenic and cadmium with sequential soil washing techniques using Na 2 EDTA, oxalic and phosphoric acid: Optimization conditions, removal effectiveness and ecological risks. Chemosphere 2016, 156, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, Q.; Huang, R.; Wu, K.; Li, Z. Contrasting impacts of mobilisation and immobilisation amendments on soil health and heavy metal transfer to food chain. Ecotoxicol. Environ. Saf. 2020, 209, 111836. [Google Scholar] [CrossRef]
- Vollprecht, D.; Machiels, L.; Jones, P. The EU Training Network for Resource Recovery through Enhanced Landfill Mining—A Review. Processes 2021, 9, 394. [Google Scholar] [CrossRef]
- Winterstetter, A.; Laner, D.; Rechberger, H.; Fellner, J. Framework for the evaluation of anthropogenic resources: A landfill mining case study—Resource or reserve? Resour. Conserv. Recycl. 2015, 96, 19–30. [Google Scholar] [CrossRef]
- Mönkäre, T.J.; Palmroth, M.R.; Rintala, J.A. Characterization of fine fraction mined from two Finnish landfills. Waste Manag. 2016, 47, 34–39. [Google Scholar] [CrossRef]
- Silva, R.; de Brito, J.; Dhir, R. Comparative analysis of existing prediction models on the creep behaviour of recycled aggregate concrete. Eng. Struct. 2015, 100, 31–42. [Google Scholar] [CrossRef]
- Makarichi, L.; Jutidamrongphan, W.; Techato, K.-A. The evolution of waste-to-energy incineration: A review. Renew. Sustain. Energy Rev. 2018, 91, 812–821. [Google Scholar] [CrossRef]
- Graedel, T.E.; Allwood, J.; Birat, J.-P.; Buchert, M.; Hagelüken, C.; Reck, B.; Sibley, S.F.; Sonnemann, G. What Do We Know About Metal Recycling Rates? J. Ind. Ecol. 2011, 15, 355–366. [Google Scholar] [CrossRef]
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Obaid, S.S.; Gaikwad, D.; Sayyed, M.; Al-Rashdi, K.; Pawar, P. Heavy metal ions removal from waste water by the natural zeolites. Mater. Today Proc. 2018, 5, 17930–17934. [Google Scholar] [CrossRef]
- Bosso, S.; Enzweiler, J. Evaluation of heavy metal removal from aqueous solution onto scolecite. Water Res. 2002, 36, 4795–4800. [Google Scholar] [CrossRef]
- Inglezakis, V.J. The concept of “capacity” in zeolite ion-exchange systems. J. Colloid Interface Sci. 2005, 281, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.T.; Quintelas, C.; Figueiredo, H.; Neves, I.C. Comparative Study between Natural and Artificial Zeolites as Supports for Biosorption Systems. Mater. Sci. Forum 2006, 514–516, 1294–1298. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.; Figueiredo, H.; Quintelas, C.; Neves, I.C.; Tavares, T. Zeolites as supports for the biorecovery of hexavalent and trivalent chromium. Microporous Mesoporous Mater. 2008, 116, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Vaca Mier, M.; López Callejas, R.; Gehr, R.; Jiménez Cisneros, B.E.; Alvarez, P.J.J. Heavy metal removal with Mexican clinoptilolite: Multi-component ionic exchange. Water Res. 2001, 35, 373–378. [Google Scholar] [CrossRef]
- Zou, W.; Feng, X.; Wei, W.; Zhou, Y.; Wang, R.; Zheng, R. Converting Spent LiFePO4 Battery into Zeolitic Phosphate for Highly Efficient Heavy Metal Adsorption. Inorg. Chem. 2021, 60, 9496–9503. [Google Scholar] [CrossRef]
- Shuya, L.; Yang, C.; Xuefeng, C.; Wei, S.; Yaqing, W.; Yue, Y. Separation of lithium and transition metals from leachate of spent lithium-ion batteries by solvent extraction method with Versatic 10. Sep. Purif. Technol. 2020, 250, 117258. [Google Scholar] [CrossRef]
- Prior, T.; Wäger, P.A.; Stamp, A.; Widmer, R.; Giurco, D. Sustainable governance of scarce metals: The case of lithium. Sci. Total Environ. 2013, 461–462, 785–791. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Yakhmi, J.V. Microbial fuel cells to recover heavy metals. Environ. Chem. Lett. 2014, 12, 483–494. [Google Scholar] [CrossRef]
- Chouler, J.; Padgett, G.A.; Cameron, P.J.; Preuss, K.; Titirici, M.-M.; Ieropoulos, I.; Di Lorenzo, M. Towards effective small scale microbial fuel cells for energy generation from urine. Electrochim. Acta 2016, 192, 89–98. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Sharma, V.N. Bioelectricity production from various wastewaters through microbial fuel cell technology. J. Biochem. Technol. 2010, 2, 133–137. [Google Scholar]
- Wu, H.; Zuo, J.; Zillante, G.; Wang, J.; Yuan, H. Status quo and future directions of construction and demolition waste research: A critical review. J. Clean. Prod. 2019, 240, 118163. [Google Scholar] [CrossRef]
- Ezziat, L.; Elabed, A.; Ibnsouda, S.; El Abed, S. Challenges of Microbial Fuel Cell Architecture on Heavy Metal Recovery and Removal From Wastewater. Front. Energy Res. 2019, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Nancharaiah, Y.; Venkata Mohan, S.; Lens, P.N.L. Recent advances in nutrient removal and recovery in biological and bioelectrochemical systems. Bioresour. Technol. 2016, 215, 173–185. [Google Scholar] [CrossRef]
- Choi, C.; Hu, N.; Lim, B. Cadmium recovery by coupling double microbial fuel cells. Bioresour. Technol. 2014, 170, 361–369. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.; Wu, D.; Huang, L.; Zhou, P.; Quan, X.; Chen, G. Dependency of simultaneous Cr(VI), Cu(II) and Cd(II) reduction on the cathodes of microbial electrolysis cells self-driven by microbial fuel cells. J. Power Sources 2015, 273, 1103–1113. [Google Scholar] [CrossRef]
- Xu, Y.-S.; Zheng, T.; Yong, X.-Y.; Zhai, D.-D.; Si, R.-W.; Li, B.; Yu, Y.-Y.; Yong, Y.-C. Trace heavy metal ions promoted extracellular electron transfer and power generation by Shewanella in microbial fuel cells. Bioresour. Technol. 2016, 211, 542–547. [Google Scholar] [CrossRef]
- Abourached, C.; Catal, T.; Liu, H. Efficacy of single-chamber microbial fuel cells for removal of cadmium and zinc with simultaneous electricity production. Water Res. 2014, 51, 228–233. [Google Scholar] [CrossRef]
- Pan, J.; Hassas, B.V.; Rezaee, M.; Zhou, C.; Pisupati, S.V. Recovery of rare earth elements from coal fly ash through sequential chemical roasting, water leaching, and acid leaching processes. J. Clean. Prod. 2020, 284, 124725. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Challenges and opportunities in the recovery of gold from electronic waste. RSC Adv. 2020, 10, 4300–4309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yesil, H.; Tugtas, A. Removal of heavy metals from leaching effluents of sewage sludge via supported liquid membranes. Sci. Total Environ. 2019, 693, 133608. [Google Scholar] [CrossRef]
- Doble, M.; Kruthiventi, A. Alternate solvents. In Green Chemistry and Engineering; Academic Press: Cambridge, MA, USA, 2007; pp. 93–104. [Google Scholar]
- Annoni, R.; Souza, P.S.; Petrániková, M.; Miskufova, A.; Havlík, T.; Mansur, M.B. Submerged-arc welding slags: Characterization and leaching strategies for the removal of aluminum and titanium. J. Hazard. Mater. 2012, 244–245, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Funari, V.; Mäkinen, J.; Salminen, J.; Braga, R.; Dinelli, E.; Revitzer, H. Metal removal from Municipal Solid Waste Incineration fly ash: A comparison between chemical leaching and bioleaching. Waste Manag. 2017, 60, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.; Park, K.-H.; Parhi, P.; Park, J. Direct leaching of molybdenum and cobalt from spent hydrodesulphurization catalyst with sulphuric acid. Hydrometallurgy 2012, 111–112, 46–51. [Google Scholar] [CrossRef]
- Matjie, R.; Scurrell, M.; Bunt, J. The selective dissolution of alumina, cobalt and platinum from a calcined spent catalyst using different lixiviants. Miner. Eng. 2005, 18, 801–810. [Google Scholar] [CrossRef]
- Hocheng, H.; Su, C.; Jadhav, U.U. Bioleaching of metals from steel slag by Acidithiobacillus thiooxidans culture supernatant. Chemosphere 2014, 117, 652–657. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, Y.; Yan, L.; Yang, R. Recovery of nickel, copper and cobalt from low-grade Ni–Cu sulfide tailings. Hydrometallurgy 2005, 80, 54–58. [Google Scholar] [CrossRef]
- Buzatu, T.; Popescu, G.; Birloaga, I.; Săceanu, S. Study concerning the recovery of zinc and manganese from spent batteries by hydrometallurgical processes. Waste Manag. 2013, 33, 699–705. [Google Scholar] [CrossRef]
- Özel, N.; Elibol, M. A review on the potential uses of deep eutectic solvents in chitin and chitosan related processes. Carbohydr. Polym. 2021, 262, 117942. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, L.; Ferrantelli, P.; Medici, F. Heavy metals extraction from contaminated soil: Recovery of the flushing solution. J. Environ. Manag. 2005, 77, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ramón, D.J.; Guillena, G. Deep Eutectic Solvents: Synthesis, Properties, and Applications; Wiley-VCH: Weinheim, Germany, 2020. [Google Scholar]
- Bangde, P.S.; Jain, R.; Dandekar, P. Alternative Approach to Synthesize Methylated Chitosan Using Deep Eutectic Solvents, Biocatalyst and “Green” Methylating Agents. ACS Sustain. Chem. Eng. 2016, 4, 3552–3557. [Google Scholar] [CrossRef]
- Dwamena, A. Recent Advances in Hydrophobic Deep Eutectic Solvents for Extraction. Separations 2019, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- van Osch, D.J.G.P.; Zubeir, L.F.; van den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, S.; Poletti, F.; Zanardi, C.; Pigani, L.; Zanfrognini, B.; Corsi, E.; Dossi, N.; Salomäki, M.; Kivelä, H.; Lukkari, J.; et al. Chemical and electrochemical properties of a hydrophobic deep eutectic solvent. Electrochim. Acta 2018, 295, 124–129. [Google Scholar] [CrossRef]
- Zaib, Q.; Eckelman, M.J.; Yang, Y.; Kyung, D. Are deep eutectic solvents really green?: A life-cycle perspective. Green Chem. 2022, 24, 7924–7930. [Google Scholar] [CrossRef]
- Dang, C.-C.; Xie, G.-J.; Liu, B.-F.; Xing, D.-F.; Ding, J.; Ren, N.-Q. Heavy metal reduction coupled to methane oxidation: Mechanisms, recent advances and future perspectives. J. Hazard. Mater. 2020, 405, 124076. [Google Scholar] [CrossRef]
- El Achkar, T.; Fourmentin, S.; Greige-Gerges, H. Deep eutectic solvents: An overview on their interactions with water and biochemical compounds. J. Mol. Liq. 2019, 288, 111028. [Google Scholar] [CrossRef]
- Pachauri, K.; Reisinger, A. IPCC Fourth Assessment Report; International Panel on Climate Change: Geneva, Switzerland, 2007. [Google Scholar]
- Brett, C.M.A. Deep eutectic solvents and applications in electrochemical sensing. Curr. Opin. Electrochem. 2018, 10, 143–148. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Marchel, M.; Cieśliński, H.; Boczkaj, G. Thermal Instability of Choline Chloride-Based Deep Eutectic Solvents and Its Influence on Their Toxicity—Important Limitations of DESs as Sustainable Materials. Ind. Eng. Chem. Res. 2022, 61, 11288–11300. [Google Scholar] [CrossRef]
- Grathwohl, P.; Susset, B. Comparison of percolation to batch and sequential leaching tests: Theory and data. Waste Manag. 2009, 29, 2681–2688. [Google Scholar] [CrossRef]
- Dash, S.; Mohanty, S. Mathematical Modeling Aspect in Solvent Extraction of Metals. Sep. Purif. Rev. 2019, 50, 74–95. [Google Scholar] [CrossRef]
- Kalbe, U.; Berger, W.; Simon, F.-G.; Eckardt, J.; Christoph, G. Results of interlaboratory comparisons of column percolation tests. J. Hazard. Mater. 2007, 148, 714–720. [Google Scholar] [CrossRef]
- Torkaman, R.; Torab, M.; Safdari, J.; Moosavian, M.; Asadollahzadeh, M. Mass transfer coefficients in pulsed column for separation of Samarium and Gadolinium. Iran. J. Chem. Eng. 2017, 36, 146. [Google Scholar]
- Smol, M.; Marcinek, P.; Duda, J.; Szołdrowska, D. Importance of Sustainable Mineral Resource Management in Implementing the Circular Economy (CE) Model and the European Green Deal Strategy. Resources 2020, 9, 55. [Google Scholar] [CrossRef]
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- European Commission. Communication No. 614, 2015. Closing the Loop—An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Galos, K.; Nieć, M.; Saługa, P.W.; Uberman, R. The basic problems of mineral resources valuation methodologies within the framework of System of Integrated Environmental and Economic Accounts. Gospod. Surowcami Miner. 2015, 31, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Department for Environment, Food and Rural Affairs. Circular Economy Package Policy Statement. 2020. Available online: https://www.gov.uk/government/publications/circular-economy-package-policy-statement/circular-economy-package-policy-statement (accessed on 13 May 2022).
- Stretton, A.; Harris, L. Access to Critical Materials; UK Parliament: London, UK, 2019. [Google Scholar]
- Monier, V.; Hestin, M.; Cavé, J.; Laureysens, I.; Watkins, E.; Reisinger, H. Development of Guidance on Extended Producer Responsibility (EPR) Final Report; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Jain, P.; Townsend, T.G.; Johnson, P. Case study of landfill reclamation at a Florida landfill site. Waste Manag. 2013, 33, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Galvão, N.; de Souza, J.B.; de Sousa Vidal, C.M. Landfill leachate treatment by electrocoagulation: Effects of current density and electrolysis time. J. Environ. Chem. Eng. 2020, 8, 104368. [Google Scholar] [CrossRef]
- Asaithambi, P.; Beyene, D.; Abdul Aziz, A.R.; Alemayehu, E. Removal of pollutants with determination of power consumption from landfill leachate wastewater using an electrocoagulation process: Optimization using response surface methodology (RSM). Appl. Water Sci. 2018, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y. Minimization management of construction waste. In Proceedings of the 2011 International Symposium on Water Resource and Environmental Protection, Xi’an, China, 20–22 May 2011; Volume 4, pp. 2769–2772. [Google Scholar]
- Gaur, N.; Flora, G.; Yadav, M.; Tiwari, A. A review with recent advancements on bioremediation-based abolition of heavy metals. Environ. Sci. Process. Impacts 2014, 16, 180–193. [Google Scholar] [CrossRef] [PubMed]


| Metal | Extraction Process | Source of Waste | Recovery Yield (%) | Reference |
|---|---|---|---|---|
| Al | Chemical Leaching (NaOH, HCl, H2SO4) | Welding Slags | 80.00 | [104] |
| Chemical Leaching (H2SO4) | Spent hydrodesulphurisation catalyst | 11.03 | [107] | |
| Chemical Leaching (NaOH) | Calcined spent catalyst | 89.00 | [106] | |
| Chemical Leaching (H2SO4) | Municipal solid waste | >85.00 | [105] | |
| Cu | Chemical Leaching (H2SO4) | Municipal solid waste | >85.00 | [105] |
| Bioleaching | Steel slag | 27.00 | [106] | |
| Chelating Agent (EDTA) | Artificially contaminated soil | 93.90 | [108] | |
| Chemical Leaching (HNO3) | Sulphide tailing | 85.00 | [109] | |
| Co | Chemical Leaching (H2SO4) | Spent hydrodesulphurisation catalyst | 96.25 | [107] |
| Chemical Leaching (HNO3) | Sulphide tailing | 54.60 | [109] | |
| Mn | Chemical Leaching (NaOH) | Spent batteries | 96.00 | [110] |
| Chemical Leaching (H2SO4) | Municipal solid waste | >85.00 | [105] | |
| Zn | Chemical Leaching (NaOH) | Spent batteries | 82.00 | [110] |
| Chemical Leaching (H2SO4) | Municipal solid waste | >85.00 | [105] | |
| Bioleaching | Steel slag | 60.00 | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huntington, V.E.; Coulon, F.; Wagland, S.T. Innovative Resource Recovery from Industrial Sites: A Critical Review. Sustainability 2023, 15, 489. https://doi.org/10.3390/su15010489
Huntington VE, Coulon F, Wagland ST. Innovative Resource Recovery from Industrial Sites: A Critical Review. Sustainability. 2023; 15(1):489. https://doi.org/10.3390/su15010489
Chicago/Turabian StyleHuntington, Victoria E., Frédéric Coulon, and Stuart T. Wagland. 2023. "Innovative Resource Recovery from Industrial Sites: A Critical Review" Sustainability 15, no. 1: 489. https://doi.org/10.3390/su15010489
APA StyleHuntington, V. E., Coulon, F., & Wagland, S. T. (2023). Innovative Resource Recovery from Industrial Sites: A Critical Review. Sustainability, 15(1), 489. https://doi.org/10.3390/su15010489