1. Introduction
The ongoing progress of the economy and technology, along with the increasing standard of life, has resulted in significant emissions of pollutants that have had adverse effects on soil safety. Metal(loid)s are introduced into the soil by many anthropogenic activities, including mining operations, chemical manufacturing processes, agricultural practices, medicinal applications, and the utilization of sewage for irrigation purposes. The presence of excessive metal(loid)s within the soil has the potential to diminish its overall quality [
1]. Furthermore, the consumption of food crops cultivated in areas contaminated with metal(loid)s can have severe implications for human health, including detrimental effects on vital organs such as the liver, as well as an increased risk of developing cancer. Regional centralized rehabilitation systems have been used in China for decades to address soil metal(loid) contamination. The nation has also introduced ecological restoration and improved pollution prevention and control. Additionally, soil metal(loid) pollution is managed collaboratively.
Soil contains several minerals, organic compounds, and other components. The system is a complicated mix of solids, liquids, gases, and three-phase substances [
2]. When dealing with metal(loid)-contaminated soil, anti-penetration [
3], membrane filtration [
4], ion exchange [
5], and other methods are difficult. The transportation of metal(loid) ions from soil to crops through plant roots poses a potential threat to human health along the food chain, with only the free-ionized forms of these metals being capable of such movement. The metal(loid) ion then undergoes solidification in the sedimentation phase or is immobilized by the adsorbent, hence impeding the process of metal(loid) accumulation in crops. Instead of implementing total isolation of metal(loid)s from the soil environment, scientists frequently employ the technique of metal(loid) localization. This approach aims to mitigate the risks associated with metal(loid)s in soil by means of processes such as adsorption, mineralization, oxidation, or sedimentation of metal(loid) ions. Consequently, the prioritization of developing environmentally acceptable site repair materials that can establish a stable mineralization structure has emerged, with a focus on large-scale removal and effective selectivity [
6,
7].
Layered double hydroxides (LDHs) are increasingly acknowledged for their potential to resolve issues of inefficiency and high energy consumption in traditional soil treatment methods due to their exceptional tunability. This article synthesizes current knowledge on LDHs, detailing their structural intricacies, physicochemical attributes, and the methodologies employed in their synthesis and subsequent modification. We delve into the mechanisms underpinning LDHs’ action and their prospective applications in the remediation of metal(loid)-laden soils. Furthermore, the paper forecasts the future trajectory and innovative developments of LDHs in environmental remediation. This outlook includes rigorous validation of their adsorption mechanisms, precise structural control, and practical industrial applications, highlighting ongoing interest and research momentum in this field and paving the way for future exploration and challenges.
2. Structure and Properties of LDHs
2.1. Structure of LDHs
LDHs exhibit intercalation properties and may be described by their chemical composition, which is represented as
, and its fundamental layered structure is depicted in
Figure 1. The term M
2+ refers to divalent cations, specifically including Mg
2+, Zn
2+, Ca
2+, Ni
2+, among others. On the other hand, M
3+ represents trivalent anions, such as Al
3+, Cr
3+, Co
3+, and so forth. Additionally, the variable ‘m’ signifies the quantity of water molecules present in the crystalline structure of the molecule. The central position within the ortho-octahedron is occupied by the primary metal ions of the lamellar plate. These metal ions form ligand interactions with OH
− ions situated at the six vertices of the ortho-octahedron, resulting in the creation of the anodic layer. This anodic layer, in turn, produces an indefinitely extended lamellar compound through co-prisms. The positive layer and the negative layer, composed of anions and water of crystallization, are interconnected through Coulomb force attraction, resulting in the formation of a perpetually revolving lamellar structure. The exceptional stability of LDHs can be attributed to the formation of hydrogen bonds between the hydroxide groups in the lamellae and the electronegative atoms of the intercalated anions.
2.2. Characterization of LDHs
2.2.1. Acid-Base Bifunctionality
LDHs typically have slightly alkaline properties. The lamellae contain hydroxyl groups that function as electron-rich bases capable of interacting with adsorbed Lewis acids, such as metal atoms, metal ions, and hydrogen ions, among others, at basic locations. The relative basicity of various types of LDHs is comparable to that of hydroxides of divalent metals. This basicity is influenced by the types and characteristics of the ionic bonds they establish with oxygen. Additionally, the anions present in the interlayer of LDHs can contribute to their acidity. However, the acidity provided by simple anions consisting of single atoms is generally weaker compared to that of homo-poly and hetero-polyacidic anions [
8].
2.2.2. Structural Tunability
The lamellar anions and cations present in LDHs can be modified by the manipulation of the preparation process.
In the context of laminar cations, it is possible to substitute both M2+ and M3+ ions in the laminar composition of LDHs with other metal ions that possess equivalent valence and comparable radii. This substitution process results in the formation of novel LDH structures. The adjustment of the ratio between divalent and trivalent metal cations in the lamellae allows for the regulation of the charge density of the lamellae and, therefore, the quantity of interlayer anions. This adjustment can be employed to selectively synthesize LDH-like structures with varying properties.
To enhance the interaction between the lamellae and the interlayer anions, it is possible to substitute the existing anions with anions that exhibit stronger forces such as Coulombic forces and hydrogen bonding. The exchange capacity of anions is directly influenced by their charge and radius, with a stronger exchange capacity observed for anions with higher charge and smaller radius. By manipulating the arrangement of interlayer anions in LDHs, it is possible to modify the original distance between layers. This process also results in the introduction of novel chemical characteristics to the LDHs and has an impact on the distribution of electrical charge within the material. The degree of interlayer anion exchange is influenced by the amount of anion charges participating in the exchange as well as their chemical characteristics. This process becomes more facile as the anion charge and electronegativity increase, while the anion radius decreases. The common anion selectivity order is as follows: carbonate (CO
32−) has the highest selectivity, followed by sulfate (SO
42−), hydroxide (OH
−), fluoride (F
−), chloride (Cl
−), bromide (Br
−), nitrate (NO
3−), and iodide (I
−) [
9,
10,
11,
12].
2.2.3. Thermal Stability
LDHs have notable thermal stability, maintaining their structural integrity and functional properties even at temperatures as high as 600 °C. The material exhibits a high degree of thermal stability. At temperatures below 200 °C, the material undergoes a process of dehydration, namely losing interlayer water molecules. However, upon cessation of heating and subsequent replenishment of water molecules, the material can recover its original state. Once the temperature reaches approximately 400 °C, the decomposition of interlayer anions commences, leading to the conversion of LDHs into their respective metal oxides or monomers. Within the temperature range of 400 °C to 550 °C, LDHs undergo a series of transformations, involving the removal of physically adsorbed water and interlayer water molecules of crystallization, the disruption of the ordered structure, and the decomposition of lamellar hydroxyls and interlayer anions. Ultimately, LDHs evolve into layered double oxides (LDOs) characterized by high stability and significant specific surface areas. Importantly, this entire process is reversible [
13]. At temperatures exceeding 600 °C, the LDOs undergo a process known as sintering, resulting in the formation of spinel. This transformation leads to a decrease in specific surface area and pore space, rendering the structure irrecoverable.
2.2.4. Memory Effect
The memory effect of LDHs pertains to their capacity to re-adsorb anions and water molecules from solutions containing specific anions after calcination, allowing the layered double oxides (LDOs) to reconstruct their layered structure. This technique enables the incorporation of anions that are challenging to produce directly into LDHs, resulting in the formation of diverse intercalated structures [
14].
Figure 2 illustrates the comprehensive procedure of the memory effect.
3. Preparation and Modification of LDHs
3.1. Preparation of LDHs
Scholarly research has extensively explored a variety of techniques for synthesizing LDHs and their composites, highlighting each method’s unique characteristics and implications. Co-precipitation, a cost-effective method, demands meticulous pH control throughout the process, impacting the uniformity and quality of the final product. In contrast, hydrothermal synthesis, despite its high-temperature requirements, ensures greater uniformity and stability of the produced LDHs. Furthermore, the mechanochemical synthesis method, though less commonly used, shows promise for generating a significant number of defect sites, presenting an intriguing direction for future research. This section aims to summarize and contrast the various synthesis procedures, as elaborated in the previous sections, elucidating their distinct advantages and limitations in LDH production.
3.1.1. Co-Precipitation
The co-precipitation method, also referred to as the salt–alkali preparation method, is widely employed for the synthesis of LDHs. This method involves the addition of specific amounts of metal cations to an alkaline solution, resulting in the desired pH level during the reaction. Subsequently, co-precipitation takes place, leading to the formation of a homogeneous precipitate consisting of the corresponding cations after a period of aging. The alkali solution typically consists of compounds such as NaOH, Na2CO3, K2CO3, and aqueous solutions of urea, among others. In the co-precipitation synthesis of LDHs, stringent pH control is pivotal, governed by thermodynamic and kinetic principles. This approach necessitates maintaining the pH slightly above the solubility peak of the involved metal hydroxides, ensuring uniform and complete precipitation of metal ions. Precise pH regulation serves dual purposes: it prevents rapid formation of non-uniform mixed-metal hydroxides, thus maintaining the structural integrity of LDHs, and it stabilizes the thermodynamic properties of the precipitate. This promotes the formation of crystalline, well-ordered LDHs rather than amorphous phases. Dynamic pH adjustment throughout the synthesis process is essential for consistently yielding LDHs with uniform layered structures and optimal properties.
The simplicity and ease of use of the co-precipitation method has become its great advantage in preparation. Wang et al. [
15] employed a co-precipitation hydrothermal approach to synthesize Mg/Al-LDHs using a Mg/Al-rich leachate derived from high titanium blast furnace slag. A one-factor test was conducted to improve the preparation conditions, aiming to achieve Mg/Al-LDHs with enhanced relative crystallinity and crystal integrity.
3.1.2. Hydrothermal Synthesis
The hydrothermal synthesis method, along with co-precipitation, is frequently employed in various applications. Hydrothermal synthesis involves a gradual and controlled addition of mixed salt solution and mixed alkali solution, followed by vigorous mixing. The resulting solution is promptly transferred to an autoclave for aging, filtration, washing, and subsequent drying at a specific temperature [
16]. The aforementioned technique has the capability to transform oxides or hydroxides that were previously insoluble and non-crystalline into soluble compounds. This transformation facilitates the growth of highly crystalline crystals, resulting in a more uniform crystalline structure [
17]. Consequently, this method allows for the synthesis of a greater quantity of LDHs. However, it is important to note that the process necessitates more rigorous conditions, typically exceeding 100 °C.
Hydrothermal synthesis significantly benefits from the application of elevated pressure and temperature conditions [
18]. These conditions facilitate the reaction between solid phases in a way that exceeds the capacities of co-precipitation methods. Under these high-pressure environments, the solubility of reactants in water increases markedly, allowing for reactions that are otherwise challenging at lower temperatures. This heightened solubility under pressure not only accelerates reaction rates but also contributes to the formation of more uniform and highly crystalline LDH structures. Unlike co-precipitation, which operates under milder conditions and might result in less uniformity in crystal size and shape, hydrothermal synthesis excels in producing LDHs with a higher degree of homogeneity and crystallinity. This method is particularly effective for reactions involving reactants that are less stable or soluble under normal conditions, thus expanding the scope of possible LDH formulations. Cui et al. [
19] conducted hydrothermal synthesis to produce LDHs-H, a hexagonal hollow hydrotalcite with an increased number of adsorption sites. Through competitive ionization experiments, it was demonstrated that LDHs-H exhibited preferential selectivity in the adsorption of metal(loid) ions. Furthermore, LDHs-H displayed enhanced removal efficiency for metal(loid) ions within the pH range of 7 to 10.
3.1.3. Mechanochemical Synthesis Method
The mechanochemical synthesis method involves the application of mechanical force to initiate chemical reactions or create alterations in the internal structure and properties of compounds. This approach aims to facilitate material modification or the synthesis of novel materials [
20]. The ball mill employs high-speed vibration and rotation to facilitate the forceful impact, grinding, and agitation of hydroxides or oxides by hard balls. This process induces plastic deformation in the powder particles and generates a significant number of defects within them. Consequently, the diffusion activation energy of the elements is reduced, leading to the initiation of a chemical reaction that enables the synthesis of LDHs compounds [
21]. The aforementioned approach possesses several benefits, including a straightforward procedure, a substantial output, and the absence of any byproduct formation.
Wang et al. [
22] employed Mg(OH)
2 and Al(OH)
3 as primary constituents to generate precursors by the process of mechanical ball milling. The present study aimed to examine the impact of ball milling time and stirring time in water on the synthesis of Mg/Al-LDH. The experimental conditions included a controlled addition of raw materials with a molar ratio of Mg(OH)
2:Al(OH)
3 = 3:1, a ball-to-feed ratio of 35:1, and a grinding speed of 700 rpm.
3.2. Modification of LDHs
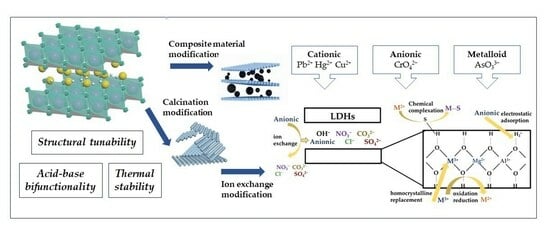
The aforementioned techniques of synthesizing LDHs exhibit specific remediation effects on metal(loid)s remediation in sewage. However, their remediation mechanisms mostly rely on ion exchange and electrostatic attraction, resulting in limited specific remediation of pollutants. Hence, the utilization of the memory effect and structural tunability of LDHs can be employed to modify these materials, thereby enhancing their stability and selectivity. This, in turn, can lead to improved remediation efficacy in various complex practical applications.
3.2.1. Calcination Modification
Upon calcination of LDHs at temperatures exceeding 400 °C, the well-organized structure of the lamellae undergoes disruption, resulting in an increase in disorder. This leads to a more uniform distribution of constituents, an enlargement and homogenization of pore radius, an augmentation in the quantity of micropores, and an elevation in specific surface area. Consequently, these alterations contribute to an enhanced remediation effect.
Zhang [
23] synthesized MgAl and CaAl-LDH by urine and co-deposition methods and used brass-burning modified water slides to repair Cd and BPA pollution in soil by changing solidifiers, soil pollutant concentration, pH, etc.
3.2.2. Ion Exchange Modification
Anionic intercalation materials with diverse structures and functionalities can be achieved through the deliberate design and assembly of interlayer anions, facilitated by the memory effect of LDHs and the interchangeability of interlayer particles. Furthermore, in situations where metal ions exhibit instability in alkaline environments or when the anion An- lacks soluble M2+ and M3+ salts, the ion-exchange method can be employed as an alternative approach for the synthesis of LDHs when the co-precipitation method is not feasible.
In cases where the introduction of large inorganic anionic groups (such as PO
43−, etc.) into the interlayer of LDHs proves challenging through direct ion exchange methods, a common approach involves initially propping up the interlayer with bulky organic anions. This results in the preparation of LDH materials supported by organic anionic columns, which creates a more favorable environment for the subsequent insertion of inorganic anions [
24].
He et al. [
25] examined the remediation characteristics and mechanism of arsenic (As) and cadmium (Cd) in water using fulvic acid insertion-modified FeMnNi-LDH composites. These composites demonstrated the ability to effectively remove both As
3+ and Cd
2+ from aquatic environments. The researchers observed that the composites adsorbed As
3+ well and reduced its toxicity. Furthermore, the composites enhanced Cd
2+ adsorption. Consequently, these findings suggest that the fulvic acid insertion-modified FeMnNi-LDH composites hold an ideal potential for environmental restoration purposes.
Shen et al. [
26] synthesized Mg-Al-LDHs intercalated with tartaric acid. The researchers achieved this by employing the ion exchange approach, using carbonate intercalated Mg-Al-LDHs as a precursor. The resulting intercalated Mg-Al-LDHs exhibited a well-defined crystalline phase. Notably, these materials had a high adsorption capacity for Ni
2+ ions, with the adsorption rate remaining over 60% even at a concentration of 100 mg/L Ni
2+.
3.2.3. Composite Material Modification
The aggregation and deposition of single LDH materials in actual remediation processes is a common issue, mostly attributed to their high ionic density. This phenomenon leads to a reduction in the overall remediation effectiveness [
27]. Composite materials including biochar, activated carbon, zero-valent iron, and magnetic compounds exhibit laminar LDH distribution. When comparing LDHs to hybrid adsorbents comprising LDHs, it can be seen that the latter exhibit improved remediation properties and higher stability. This can be attributed to the increase in specific surface area. In contrast, the altered materials exhibit enhanced selectivity towards various metal(loid) ions, demonstrate less toxicity when combined with magnetic particles, and may be conveniently separated and reused [
28].
The coalescence of biochar and LDHs enables the amalgamation of their respective physicochemical characteristics. The utilization of porous biochar as a carrier matrix for LDHs has been found to be an efficient method for the decoration of LDHs. This approach results in an increased reaction area and prevents the aggregation and sinking of LDHs. Furthermore, the incorporation of biochar into LDHs has been seen to boost the remediation capacity of biochar-LDH composites for a diverse array of pollutants. Zubair et al. [
29] produced composites of calcined banana straw biochar and LDHs using a hydrothermal process at 110 °C for 4 h and a 500 °C process for 4 h. Biochar and LDH composites with hexagonal and rhombic 3R structures were produced. These composites showed remarkable structural stability during phosphate adsorption and desorption.
Composites including layers of LDHs can integrate activated carbon/graphene components as well. Just like biochar, the activated carbon materials exhibit active spots on their surface that facilitate the growth of LDHs upon activation. Through modification, LDHs materials with favorable electrical conductivity and a large specific surface area can be synthesized. Fan [
30] et al. conducted an experiment with basin planting to investigate the impact of a bio-carbon modified LDHs on the development of spinach, accumulation of Cd (cadmium), bio-enrichment coefficient, and the effective status of Cd in the soil. The substance exhibits a notable drop in the active state of soil Cd content, resulting in a reduction in Cd content in spinach. Furthermore, the substance diminishes the enrichment capacity while demonstrating promising potential for remediating soil contaminated with Cd.
The chemical precipitation method may also synthesize magnetic LDHs by combining the magnetic substrate with the LDH substance [
31]. A magnetic field helps magnetic LDHs disperse uniformly in the reaction fluid. This avoids high-specific-surface-area microparticle agglomeration. In the meantime, the applied magnetic field rotates, moving the magnetic catalyst into the system and enhancing its stirring function. He [
32] modified LDH with sodium mercury hydroxide to create zero-iron-containing LDH (ZVI-LDH) and repaired six-value merger-polluted soil. XRD shows that modified ZVI-LDH differs from LDH in crystal structure and form but retains LDH’s layered structure and crystalline structure. By studying its response and mechanisms it can be concluded that: Fe
2+ from NaBH
4 and the Fe-Al-LDH layer plate restores oxidation restoration reaction to zero iron and zero price iron, restoring Cr(VI) to Cr(III). VI-ZLDH removes and fixes hatch-priced manganese characteristics throughout pH ranges.
4. Mechanism and Application of Metal(Loid) Remediation by LDHs
4.1. Mechanism of Metal(Loid) Adsorption by LDHs
The process of LDHs fixation on metal(loid) ions in the soil may be categorized into three distinct stages [
33]. In the first stage, the primary mechanism of metal(loid) adsorption onto LDHs involves static attraction and hydrogen bonding, primarily occurring on the outer surface of LDHs. As the external surface adsorption reaches saturation, the adsorbent molecule initiates a secondary stage wherein the surface properties of LDHs transition from being waterproof to hydrophobic. Consequently, the metal(loid) ions are transferred to the internal cavity of the LDH microspheres, with a portion of the ions being adsorbed into the layered structure. Certain ionic metal(loid)s undergo ion exchange with the original ion present in the layered double hydroxide (LDH) material, while other ionic ions become immobilized inside the LDH structure by crystal replacement [
34]. This process leads to the formation of M-LDH with much reduced solubility, hence facilitating long-term mineralization. Furthermore, it should be noted that the interlayer material situated between the layers of LDHs is susceptible to oxidation by metal(loid) ions, leading to oxidation or clustering reactions. This process effectively mitigates the harmful effects of these ions. The third aspect pertains to the final equilibrium stage, wherein the adsorbent nears saturation and a significant portion of the plaster ions undergo reaction. Consequently, this phase exhibits a relatively slower rate due to increased adsorbance resistance. The adsorption process is primarily governed by external surface and intra-particle propagation mechanisms. These mechanisms involve the adsorptive and crystal substitution effects, which are responsible for immobilizing and adsorbing metal(loid)s in the soil. Additionally, the adhesive material can contribute to the adsorption process through jointing, oxidation, and restoration effects [
35]. These factors collectively enhance the selectivity and adhesion performance of the adsorption process. The aforementioned four methods will be presented individually.
4.1.1. Adsorption on the Outer Surface
Zang et al. [
36] have classified the adsorption of metal(loid) ions on LDHs into two main categories: interlayer ion-exchange adsorption and outer surface adsorption. The outer surface adsorption can be categorized into two main aspects: chemical bonding and electrostatic action. Chemical bonding involves the formation of inner complexes between metal(loid) ions and surface functional groups, which is also referred to as special adsorption. On the other hand, electrostatic action refers to the distribution of metal(loid) ions at a specific distance from the solid particle surface, resulting in the formation of outer complexes, also known as non-specialized adsorption. The extent of non-specialized adsorption is significantly influenced by the pH level.
The LDH samples display a positive charge as a result of the abundance of OH
2+ ions. Hence, the association between groups in a solution and CrO
42− can be achieved through electrostatic attraction. The electrostatic attraction of the CrO
42− anion is rather feeble in relation to the processes of anion exchange and adsorption-coupled reduction [
37]. The figure presented in
Figure 3 illustrates the probable adsorption mechanism of CrO
42− on LDHs materials.
4.1.2. Chemical Complexation
The utilization of chemical complexation in water environment treatment has been extensively employed since its discovery. This process involves the interaction between metal ions and the surface functional groups of materials through a coordination reaction, resulting in the formation of attached or chelated precipitates. This approach effectively achieves the adsorption of metal ions [
38]. The utilization of chemical complexation in LDHs also results in enhanced stability and selectivity for their adsorption capabilities.
Jawad et al. [
39] synthesized Fe-MoS
4/LDH by incorporating the MoS
42− anion into the layered structure of FeMgAl-NO
3. This integration resulted in the chemical bonding of (-S-)
2− groups, which offered safeguarding against solubility and direct exposure to oxygen. The material exhibited favorable adsorption capacity and effective selectivity towards Hg
2+ and Pb
2+ ions, among others. Kinetic and isothermal investigations indicated that the adsorption mechanism is associated with the formation of complexes between the functional groups present in the material and the metal ions.
4.1.3. Adsorption–Reduction/Oxidation
The memory effect of LDHs allows for the addition of ions possessing oxidizing or reducing capabilities. These ions undergo a redox process upon interaction with the adsorbed metal(loid) ions, resulting in their conversion into less dangerous valence states and so improving the adsorption effectiveness of LDHs.
When the CrO
42− ion is exposed to certain organic substances or reducing agents, particularly in acidic conditions, it undergoes facile reduction to Cr
3+. This reduction process is attributed to the high redox potential value of CrO
42− ions, which typically exceeds +1.35 V under ordinary conditions [
40]. When exposed to the adsorbent’s electron-donating hydroxyl group, CrO
42− spontaneously reduces to Cr
3+. LDHs would adsorb reduced Cr
3+ from CrO
42−, allowing isomeric substitution and complexation to remove it.
Chen et al. [
41] conducted a study in which they synthesized porous carbon sourced from biomass and modified LDHs for the purpose of removing CrO
42− ions from aqueous solutions. The X-ray photoelectron spectroscopy (XPS) analysis of the adsorbed LDHs provided evidence for the presence of both CrO
42− and Cr
3+ species on the surface and interlayer areas of the LDHs. This suggests that a portion of the Cr(VI) anions underwent reduction to Cr
3+. The reduction process was facilitated by the hydroxyl groups present on the surface of porous carbon and Ni/Al-LDH, which acted as electron donors.
4.1.4. Homocrystalline Replacement
The identification of an appropriate mineralizer for facilitating the formation of low-solubility mineralization of metal(loid) ions is of utmost significance due to the substantial decrease in the bioefficiency of metal(loid)s while in a sedimentary condition [
42]. This can be achieved by reducing solubility and the use of long-term management strategies to enhance efficacy. Research findings indicate that the presence of metal(loid) ions within carbonates has been observed to diminish the biological efficacy of metal(loid)s. The solubility accumulation constant (Ksp) of LDHs is significantly lower than that of the corresponding metal’s carbonate or hydroxide due to their unique structure [
43]. This characteristic allows for the implementation of a location mineralization strategy, wherein metal(loid) ions are immobilized within the LDH crystal to form an M-LDH (M: metal(loid) element) with extremely low solubility. This approach effectively hinders the solubility and mobility of metal(loid) pollutants, facilitating long-term mineralization of metal(loid)lic ions. The mineralization process primarily relies on the distribution of unsaturated sites, resulting in enhanced resistance to interference and increased selectivity.
Kong et al. [
44] CaAl-LDH as a repair agent, rapidly mineralizing Cd
2+ in solution (
t < 2 min) through synchronous replacement and dissolving/reconstruction. The maximum elimination rate was 592 mg/g at 480 mg/L of Cd
2+, surpassing most current materials. In the soil system, 28 days of site repair using the CaAl-LDH repair agent reduced Cd active state concentration from 0.473 mg/kg to 0.015 mg/kg, a 96.8% reduction and outstanding mineralizing characteristics.
4.1.5. Soil Remediation Mechanisms
The prevailing strategies for soil decontamination from metal(load)s encompass a range of chemical methods such as leaching, stabilization, electrokinetic treatments with permeable barriers, and redox reactions. LDHs address the contamination predominantly through chemical stabilization. They adsorb metal(loid)s onto their surfaces and engage in ion exchange processes, substituting hazardous soil ions with benign ones within their layers. This not only immobilizes the metal(loid)s but also reduces their bioavailability and toxicity. Furthermore, LDHs can be modified to catalyze oxidation/reduction reactions, thereby transforming the valence states of metals and further mitigating their hazardous nature. The intercalation of metal(loid)s within the LDH layers sequesters the contaminants away from the soil matrix, significantly restricting their mobility and potential ecological and health impacts. This multifaceted approach showcases the capacity of LDHs to serve as a versatile and efficient medium for soil remediation, tackling metal(loid) pollutants in a comprehensive manner.
The application of LDHs not only diminishes the uptake of toxic metal(loid)s by plants, crucial for agricultural safety and preventing entry into the human food chain, but also decreases the risk of groundwater contamination through leaching. Additionally, incorporating LDHs into the soil can enhance soil quality by improving soil structure and nutrient retention, fostering better plant growth. This dual effect of contaminant immobilization and soil quality enhancement underscores the potential of LDHs in sustainable agricultural practices and land management strategies, contributing to the overarching goals of ecological restoration.
Shao et al. [
45] conducted experiments to explore the adsorption properties of biochar-supported layered double hydroxide composites (LB) on Cu(II) and As(V) in water. Characterization techniques like XRD, FT-IR, BET, and SEM confirmed the successful loading of layered double hydroxide structures onto biochar surfaces. Further investigations revealed that LB application enhanced soil pH and electrical conductivity; urease and sucrase enzyme activities increased by 93.78%–374.35% and 84.35%–520.04%, respectively, peaking at a 1% LB addition. The effective copper content of the soil decreased by 35.54%–63.00%, while available arsenic significantly reduced by 8.39%–29.04%. LB treatment induced transformation of acid-soluble copper to other states and soluble arsenic to a more stable form. The remediation mechanism primarily involved pH elevation (for Cu(II) only) and complexation of arsenic with metal oxides. Seed germination tests and high-throughput sequencing assessed LB’s impact on plant growth and soil microbial communities. Optimal plant growth conditions were observed at 1% LB addition. Although LB enhanced microbial community richness, a 2% addition suppressed microbial diversity and richness.
4.2. Application of LDHs to Metal(Loid) Remediation
In our study, we employ a comparative analysis framework to delve into the diverse applications of layered double hydroxides (LDHs) in environmental remediation, focusing on their structural adjustability which offers a multitude of remediation pathways. This framework accentuates the intrinsic flexibility of LDHs’ layered structure, which not only enhances surface area and pore volume but also allows for extensive modifications to tailor their properties for specific remediation challenges. Our analysis encompasses a wide range of LDH applications in both soil and aquatic ecosystems, examining the effectiveness of various modification strategies in enhancing ion exchange and adsorption capabilities. The synthesis of these findings is systematically presented in
Table 1. This table comprehensively details the effectiveness of LDHs against different types of metal(loid) contaminations, highlighting the modification methods employed and the resultant adsorption efficiencies, thereby offering a nuanced and comparative analytical perspective on the adaptability and application efficiency of LDHs in environmental remediation.
LDHs demonstrate high-efficiency adsorption of metal(loid)s in soils, and their application in aquatic systems further showcases their comprehensive remediation capabilities. Several studies have supplemented LDHs with additional adaptable materials based on a single layer structure, such as bio-carbon [
45,
47,
49,
53,
54,
55], ZVI [
32], etc., to achieve high adsorption efficiency by improving the process of fixing metal(loid)s during the adsorptive phase. Chicken fecal modification LDHs exhibit enhanced adsorption activity for Pb
2+ fixation processes [
48], with a fixing rate of 95%. Kong [
44] conducted a study on the mineralization properties of LDH in soil and successfully recovered materials during the treatment of Cd
2+. The adsorption capacity of LDH can be significantly enhanced by treating it with ion exchange methods such as PAS and amino acids, or by using composite materials like biocarbon and magnetic material that have been treated with ionized metal(loid)s including Ni
2+ [
49], Cu
2+ [
50], Hg
2+ [
50], and UO
22+ [
51]. Enhancing the uptake of CrO
42− and SbO
6H
6− plasma-ionized metals by utilizing the interlayer ion exchange characteristics of LDHs. For instance, Xin [
32], Xu [
52], and others introduce iron components into the LDH layer plate. Subsequently, the LDH adsorption triggers an oxidation restoration process, resulting in an ideal adsorptive action. Zhu [
55] conducted a study on the influence of pre-treatment and polarization shields on electrokinetic permeable reactive barriers (EK-PRB) in uranium-polluted soils repaired with Fe/Mn/C-LDH, putting up novel concepts for the restoration of soil sites.
The comparative analysis of LDHs in soil and water remediation, as presented in our data, underscores several key insights. Firstly, the efficiency of LDHs in adsorbing specific pollutants is highly dependent on the chosen modification approach, suggesting the need for tailor-made solutions based on the nature of the contaminant. This variability in adsorption efficiency highlights the importance of selecting highly selective modification strategies for specific pollutants. Secondly, the versatility of LDHs is evident in their ability to incorporate a wide range of modification materials, including everyday substances like chicken manure, which significantly enhance adsorption capabilities. This adaptability points to the broad applicability of LDHs in various remediation contexts. Finally, while LDHs demonstrate promising potential in metal(loid) adsorption, there is a noticeable gap in research regarding their overall ecological impact in soil restoration. Future studies should focus on understanding how LDHs interact with soil ecosystems, including effects on microbial communities and soil chemistry, to ensure environmentally sustainable applications.
5. Conclusions
The adsorption technique has been noted for its cost-effectiveness and efficacy in removing metal(loid)s from soils. However, its lack of specificity for diverse pollutants can be a drawback. Layered double hydroxides (LDHs) emerge as a robust alternative, thanks to their unique layered structure that facilitates the intercalation and exchange of metal ions, allowing for a more targeted remediation strategy. This structural flexibility not only bolsters the adsorption efficiency of LDHs but also provides specificity, addressing the broad-spectrum limitations of conventional adsorption methods and offering a more customized solution for soil decontamination challenges.
The upcoming research phase should focus on two primary areas: technological innovations in LDH modifications and the environmental impact of LDH applications. Enhancing the stability of LDHs during modification is crucial, particularly when integrating multiple adsorption mechanisms. Ensuring the coexistence of these mechanisms without interference and avoiding degradation of the original structure are key challenges. Development of new, low-cost modification methods that bypass high-temperature requirements is also essential. Furthermore, a deeper understanding of the adsorption mechanisms of LDHs is needed, emphasizing comprehensive characterization to monitor changes during adsorption and the exploration of ion exchange processes and electron transfer within the metal framework. Additionally, the environmental impact of LDHs, including their interaction with soil microbial communities and their influence on soil pH, should be thoroughly investigated. This approach aims not only to mitigate soil contamination through adsorption but also to enhance the soil’s self-purification capacity, thus minimizing human intervention in natural processes.
Advancing from laboratory-based research to field application poses considerable challenges for the practical use of LDHs in soil decontamination. Predominantly, research efforts have been confined to laboratory conditions, applying LDHs to soil samples that have undergone specific treatments, with limited exploration in real-world remediation scenarios. To effectively address this, it is imperative to conduct comprehensive field studies that take into account the intricate dynamics of various environmental factors such as hydrology, climate, geology, and microbiology. Additionally, the economic feasibility of scaling up LDH production remains an area of uncertainty, which is critical for their adoption in widespread environmental applications. Innovating cost-reduction and scalability strategies is essential for the commercialization of LDHs. Moreover, the integration of LDHs with other soil remediation technologies merits exploration. While LDHs demonstrate remarkable efficiency in selective adsorption, a sole reliance on this method might not sufficiently remediate all types of metal(loid) contamination in soils. The long-term effects of adsorbent residues in soil also necessitate careful consideration. Therefore, examining collaborative approaches, such as the combination of LDHs with permeable reactive barriers and other remediation techniques, offers promising pathways for enhancing the scope and effectiveness of soil decontamination strategies.
In summary, LDHs, with their remarkable attributes, have made substantial strides in disciplines such as environmental science, materials science, and chemical engineering. This paper’s thorough analysis aims to further the application of LDHs in soil remediation, broadening both understanding and use of these materials for soil contamination challenges. Additionally, we envisage that integrating diverse scientific perspectives through multidisciplinary research will enhance strategies for mitigating metal(loid) soil contamination. The synergy of various scientific fields is likely to reveal new methods and insights, thereby improving soil decontamination processes and leading the way for ground-breaking environmental management solutions.