Impact of Vertical Canopy Position on Leaf Spectral Properties and Traits across Multiple Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Description
2.2. Experimental Setup
2.3. Leaf Spectral Measurement
Leaf Traits Measurement
2.4. Statistical Analysis
2.4.1. Impact of Canopy Vertical Position on Leaf Spectral Properties and Traits
2.4.2. Discriminating Leaf Samples into Respective Canopy Positions Groups
3. Results
3.1. Characteristics of Leaf Traits
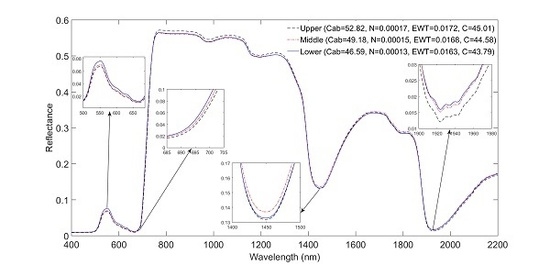
3.2. Impact of Canopy Position on Leaf Spectral Properties
3.3. Variation in Leaf Functional Trait Content across the Vertical Canopy Profile
3.4. Discriminating Leaf Samples into Respective Canopy Positions Groups
4. Discussion
4.1. Effect of Canopy Position on Leaf Spectral Properties and Leaf Traits
4.2. Implication of This Study to Remote Sensing of Plant Traits
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.C.; Jalili, A.; Montserrat-Martí, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Fitzgerald, D.B.; Bower, L.M.; Pianka, E.R. Functional traits, convergent evolution, and periodic tables of niches. Ecol. Lett. 2015, 18, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Scheiter, S.; Langan, L.; Higgins, S.I. Next-generation dynamic global vegetation models: Learning from community ecology. New Phytol. 2013, 198, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, A.K.; Pettorelli, N.; Coops, N.C.; Geller, G.N.; Hansen, M.; Lucas, R.; Mücher, C.A.; O’Connor, B.; Paganini, M.; Pereira, H.M.; et al. Environmental science: Agree on biodiversity metrics to track from space. Nature 2015, 523, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.M.; Ferrier, S.; Walters, M.; Geller, G.N.; Jongman, R.H.G.; Scholes, R.J.; Bruford, M.W.; Brummitt, N.; Butchart, S.H.M.; Cardoso, A.C.; et al. Essential biodiversity variables. Science 2013, 339, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferwerda, J.G.; Skidmore, A.K. Can nutrient status of four woody plant species be predicted using field spectrometry? ISPRS J. Photogramm. Remote Sens. 2007, 62, 406–414. [Google Scholar] [CrossRef]
- Kokaly, R.F. Investigating a physical basis for spectroscopic estimates of leaf nitrogen concentration. Remote Sens. Environ. 2001, 75, 153–161. [Google Scholar] [CrossRef]
- Dusseux, P.; Hubert-Moy, L.; Corpetti, T.; Vertès, F. Evaluation of SPOT imagery for the estimation of grassland biomass. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 72–77. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C. Inversion of a radiative transfer model for estimating vegetation LAI and chlorophyll in a heterogeneous grassland. Remote Sens. Environ. 2008, 112, 2592–2604. [Google Scholar] [CrossRef]
- Schmidt, K.S.; Skidmore, A.K. Spectral discrimination of vegetation types in a coastal wetland. Remote Sens. Environ. 2003, 85, 92–108. [Google Scholar] [CrossRef]
- Mariey, L.; Signolle, J.P.; Amiel, C.; Travert, J. Discrimination, classification, identification of microorganisms using FTIR spectroscopy and chemometrics. Vib. Spectrosc. 2001, 26, 151–159. [Google Scholar] [CrossRef]
- Rapaport, T.; Hochberg, U.; Shoshany, M.; Karnieli, A.; Rachmilevitch, S. Combining leaf physiology, hyperspectral imaging and partial least squares-regression (PLS-R) for grapevine water status assessment. ISPRS J. Photogramm. Remote Sens. 2015, 109, 88–97. [Google Scholar] [CrossRef]
- Verrelst, J.; Camps-Valls, G.; Muñoz-Marí, J.; Rivera, J.P.; Veroustraete, F.; Clevers, J.G.P.W.; Moreno, J. Optical remote sensing and the retrieval of terrestrial vegetation bio-geophysical properties—A review. ISPRS J. Photogramm. Remote Sens. 2015, 108, 273–290. [Google Scholar] [CrossRef]
- Homolová, L.; Malenovský, Z.; Clevers, J.G.P.W.; García-Santos, G.; Schaepman, M.E. Review of optical-based remote sensing for plant trait mapping. Ecol. Complex. 2013, 15, 1–16. [Google Scholar] [CrossRef]
- Thomas, V.; Treitz, P.; McCaughey, J.H.; Noland, T.; Rich, L. Canopy chlorophyll concentration estimation using hyperspectral and LiDAR data for a boreal mixedwood forest in northern Ontario, Canada. Int. J. Remote Sens. 2008, 29, 1029–1052. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Huang, W.; Yang, G. Non-uniform vertical nitrogen distribution within plant canopy and its estimation by remote sensing: A review. Field Crops Res. 2013, 142, 75–84. [Google Scholar] [CrossRef]
- Coble, A.P.; VanderWall, B.; Mau, A.; Cavaleri, M.A. How vertical patterns in leaf traits shift seasonally and the implications for modeling canopy photosynthesis in a temperate deciduous forest. Tree Physiol. 2016, 36, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Werger, M.J.A. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 1987, 72, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Reynolds, J.; Harley, P.; Tenhunen, J. Coordination theory of leaf nitrogen distribution in a canopy. Oecologia 1993, 93, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K. Leaf canopy as a dynamic system: Ecophysiology and optimality in leaf turnover. Ann. Bot. 2005, 95, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Weerasinghe, L.K.; Creek, D.; Crous, K.Y.; Xiang, S.; Liddell, M.J.; Turnbull, M.H.; Atkin, O.K. Canopy position affects the relationships between leaf respiration and associated traits in a tropical rainforest in far north Queensland. Tree Physiol. 2014, 34, 564–584. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, H.-Y.; Zhang, Y.-S.; Song, X.; Feng, W.; Kang, G.-Z.; Wang, C.-Y.; Guo, T.-C. Estimating canopy leaf nitrogen concentration in winter wheat based on multi-angular hyperspectral remote sensing. Eur. J. Agron. 2016, 73, 170–185. [Google Scholar] [CrossRef]
- Khavaninzadeh, A.R.; Veroustraete, F.; Van Wittenberghe, S.; Verrelst, J.; Samson, R. Leaf reflectance variation along a vertical crown gradient of two deciduous tree species in a Belgian industrial habitat. Environ. Pollut. 2015, 204, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Peng, Y.; Du, W.; Le, Y.; Li, L. Remote estimation of leaf and canopy water content in winter wheat with different vertical distribution of water-related properties. Remote Sens. 2015, 7, 4626–4650. [Google Scholar] [CrossRef]
- Van Wittenberghe, S.; Alonso, L.; Verrelst, J.; Hermans, I.; Valcke, R.; Veroustraete, F.; Moreno, J.; Samson, R. A field study on solar-induced chlorophyll fluorescence and pigment parameters along a vertical canopy gradient of four tree species in an urban environment. Sci. Total Environ. 2014, 466–467, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Upadhyaya, M.K. Effects of leaf position on reflectance, transmittance and absorption of red and far-red light in tomato, chenopodium album and amaranthus retroflexus leaves. Weed Res. 2018, 58, 17–24. [Google Scholar] [CrossRef]
- Li, P.; Wang, Q. Developing and validating novel hyperspectral indices for leaf area index estimation: Effect of canopy vertical heterogeneity. Ecol. Indic. 2013, 32, 123–130. [Google Scholar] [CrossRef]
- Yu, K.-Q.; Zhao, Y.-R.; Li, X.-L.; Shao, Y.-N.; Liu, F.; He, Y. Hyperspectral imaging for mapping of total nitrogen spatial distribution in pepper plant. PLoS ONE 2015, 9, e116205. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Wang, J.; Yang, G.; Zhang, D.; Li, H.; Fu, Y.; Li, Z. Comparison of spectral indices and wavelet transform for estimating chlorophyll content of maize from hyperspectral reflectance. APPRES 2013, 7, 0735751–07357511. [Google Scholar] [CrossRef]
- Wang, Q.; Li, P. Canopy vertical heterogeneity plays a critical role in reflectance simulation. Agric. Meteorol. 2013, 169, 111–121. [Google Scholar] [CrossRef]
- Sprintsin, M.; Chen, J.M.; Desai, A.; Gough, C.M. Evaluation of leaf-to-canopy upscaling methodologies against carbon flux data in North America. J. Geophys. Res. Biogeosci. 2012, 117, 1–17. [Google Scholar] [CrossRef]
- Wilkes, P.; Jones, S.D.; Suarez, L.; Haywood, A.; Mellor, A.; Woodgate, W.; Soto-Berelov, M.; Skidmore, A.K. Using discrete-return airborne laser scanning to quantify number of canopy strata across diverse forest types. Methods Ecol. Evolut. 2016, 7, 700–712. [Google Scholar] [CrossRef]
- Whitehurst, A.; Swatantran, A.; Blair, J.; Hofton, M.; Dubayah, R. Characterization of canopy layering in forested ecosystems using full waveform LiDAR. Remote Sens. 2013, 5, 2014–2036. [Google Scholar] [CrossRef]
- ASD Inc. Integrating Sphere User Manual; Analytical Spectral Devices, Inc. (ASD): Boulder, CO, USA, 2008. [Google Scholar]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Opti-Sciences, Inc. CCM-300 Chlorophyll Content Meter; Opti-Sciences, Inc.: Hudson, NY, USA, 2011. [Google Scholar]
- ADC-BioScientific Ltd. Am350 Portable Leaf Area Meter; ADC BioScientific Ltd.: Hoddesdon, UK, 2013. [Google Scholar]
- Perkin-Elmer, Inc. 2400 Series II CHNS/O Elemental Analysis; Perkin Elmer, Inc.: Waltham, MA, USA, 2005. [Google Scholar]
- Meerdink, S.K.; Roberts, D.A.; King, J.Y.; Roth, K.L.; Dennison, P.E.; Amaral, C.H.; Hook, S.J. Linking seasonal foliar traits to VSWIR-TIR spectroscopy across California ecosystems. Remote Sens. Environ. 2016, 186, 322–338. [Google Scholar] [CrossRef]
- Wang, Z.; Skidmore, A.K.; Darvishzadeh, R.; Heiden, U.; Heurich, M.; Wang, T. Leaf nitrogen content indirectly estimated by leaf traits derived from the prospect model. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 3172–3180. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Carvalho, S.; Schlerf, M.; van der Putten, W.H.; Skidmore, A.K. Hyperspectral reflectance of leaves and flowers of an outbreak species discriminates season and successional stage of vegetation. Int. J. Appl. Earth Obs. Geoinf. 2013, 24, 32–41. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Makvandi, S.; Ghasemzadeh-Barvarz, M.; Beaudoin, G.; Grunsky, E.C.; McClenaghan, M.B.; Duchesne, C.; Boutroy, E. Partial least squares-discriminant analysis of trace element compositions of magnetite from various VMS deposit subtypes: Application to mineral exploration. Ore Geol. Rev. 2016, 78, 388–408. [Google Scholar] [CrossRef]
- Pereira, H.V.; Amador, V.S.; Sena, M.M.; Augusti, R.; Piccin, E. Paper spray mass spectrometry and PLS-DA improved by variable selection for the forensic discrimination of beers. Anal. Chim. Acta 2016, 940, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 26. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Feng, G.; Yuan, F.; Yue, S.; Gao, X.; Liu, Y.; Liu, B.; Ustin, S.L.; Chen, X. Improving estimation of summer maize nitrogen status with red edge-based spectral vegetation indices. Field Crops Res. 2014, 157, 111–123. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Gitelson, A.A. Remote estimation of crop and grass chlorophyll and nitrogen content using red-edge bands on Sentinel-2 and -3. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Red edge shift and biochemical content in grass canopies. ISPRS J. Photogramm Remote Sens. 2007, 62, 34–42. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Ackerly, D.D. Canopy-level photosynthetic compensation after defoliation in a tropical understorey palm. Funct. Ecol. 2001, 15, 252–262. [Google Scholar]
- Chazdon, R.L. Light variation and carbon gain in rain forest understorey palms. J. Ecol. 1986, 74, 995–1012. [Google Scholar] [CrossRef]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Ali, A.M.; Darvishzadeh, R.; Skidmore, A.K.; Duren, I.V.; Heiden, U.; Heurich, M. Estimating leaf functional traits by inversion of prospect: Assessing leaf dry matter content and specific leaf area in mixed mountainous forest. Int. J. Appl. Earth Obs. Geoinf. 2016, 45 Pt A, 66–76. [Google Scholar] [CrossRef]
- Tissue, D.T.; Lewis, J.D.; Wullschleger, S.D.; Amthor, J.S.; Griffin, K.L.; Anderson, O.R. Leaf respiration at different canopy positions in sweetgum (Liquidambar styraciflua) grown in ambient and elevated concentrations of carbon dioxide in the field. Tree Physiol. 2002, 22, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Photosynthesis and resource distribution through plant canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K.; Hirose, T. Photosynthetic nitrogen-use efficiency in evergreen broad-leaved woody species coexisting in a warm-temperate forest. Tree Physiol. 2000, 20, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Kull, O.; Kruijt, B. Acclimation of photosynthesis to light: A mechanistic approach. Funct. Ecol. 1999, 13, 24–36. [Google Scholar] [CrossRef]
- He, Y.; Mui, A. Scaling up semi-arid grassland biochemical content from the leaf to the canopy level: Challenges and opportunities. Sensors 2010, 10, 11072–11087. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.L.; Aber, J.D.; Matson, P.A.; Card, D.H.; Swanberg, N.; Wessman, C.; Spanner, M. Remote sensing of forest canopy and leaf biochemical contents. Remote Sens. Environ. 1988, 24, 85–108. [Google Scholar] [CrossRef]
- Luo, J.; Ma, R.; Feng, H.; Li, X. Estimating the total nitrogen concentration of reed canopy with hyperspectral measurements considering a non-uniform vertical nitrogen distribution. Remote Sens. 2016, 8, 789. [Google Scholar] [CrossRef]
- Wang, Z.; Skidmore, A.K.; Wang, T.; Darvishzadeh, R.; Heiden, U.; Heurich, M.; Latifi, H.; Hearne, J. Canopy foliar nitrogen retrieved from airborne hyperspectral imagery by correcting for canopy structure effects. Int. J. Appl. Earth Obs. Geoinf. 2017, 54, 84–94. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, T.; Skidmore, A.K.; Darvishzadeh, R.; Niemann, K.O.; Liu, J. Canopy leaf water content estimated using terrestrial LiDAR. Agric. For. Meteorol. 2017, 232, 152–162. [Google Scholar] [CrossRef]







| Leaf Trait | Upper (n = 40) | Middle (n = 40) | Lower (n = 40) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | |
| N (μg/cm2) | 7.31 × 10−5 | 2.94 × 10−4 | 1.68 × 10−4 | 6.29 × 10−5 | 3.15 × 10−5 | 4.04 × 10−4 | 1.46 × 10−4 | 6.7 × 10−5 | 5.24 × 10−5 | 2.36 × 10−4 | 1.31 × 10−4 | 5.07 × 10−5 |
| Cab (μg/cm2) | 30.96 | 72.85 | 52.82 | 14.44 | 27.03 | 69.54 | 49.19 | 13.77 | 24.86 | 68.41 | 46.6 | 15.7 |
| SLA (cm2/g) | 53.67 | 380.4 | 201.94 | 105.42 | 57.43 | 512.21 | 220.16 | 140.87 | 54.3 | 530.21 | 234.08 | 144.92 |
| C (%) | 37.96 | 47.55 | 45.01 | 1.63 | 41.33 | 46.69 | 44.58 | 1.14 | 38.94 | 46.4 | 43.79 | 1.61 |
| EWT (cm) | 6.28 × 10−3 | 2.56 × 10−2 | 0.0172 | 6.77 × 10−3 | 5.98 × 10−3 | 2.53 × 10−2 | 0.0162 | 6.28 × 10−3 | 5.46 × 10−3 | 2.76 × 10−2 | 0.0163 | 6.57 × 10−3 |
| Canopy Position Combination | ||||
|---|---|---|---|---|
| Species | Trait | Middle vs. Lower | Upper vs. Lower | Middle vs. Upper |
| F. benjamina | N | 0.219 | 0.000 *** | 0.04 ** |
| Cab | 0.68 | 0.025 ** | 0.14 | |
| SLA | 0.79 | 0.025 ** | 0.10 * | |
| C | 0.12 | 0.002 *** | 0.18 | |
| EWT | 0.11 | 0.623 | 0.49 | |
| C. japonica | N | 0.92 | 0.002 *** | 0.000 *** |
| Cab | 0.81 | 0.12 | 0.029 ** | |
| SLA | 0.72 | 0.912 | 0.467 | |
| C | 0.96 | 0.933 | 0.99 | |
| EWT | 0.18 | 0.984 | 0.23 | |
| C. elegans | N | 0.89 | 0.000 *** | 0.000 *** |
| Cab | 0.28 | 0.002 *** | 0.06 * | |
| SLA | 0.66 | 0.000 *** | 0.000 *** | |
| C | 0.34 | 0.05 ** | 0.56 | |
| EWT | 0.06 * | 0.013 ** | 0.77 | |
| F. lizei | N | 0.11 | 0.6 | 0.51 |
| Cab | 0.000 *** | 0.000 *** | 0.94 | |
| SLA | 0.05 ** | 0.67 | 0.007 *** | |
| C | 0.08 * | 0.07 * | 0.99 | |
| EWT | 0.14 | 0.006 *** | 0.35 | |
| Pooled | N | 0.516 | 0.02 ** | 0.24 |
| Cab | 0.71 | 0.051 * | 0.51 | |
| SLA | 0.493 | 0.075 * | 0.63 | |
| C | 0.048 ** | 0.0009 *** | 0.39 | |
| EWT | 0.93 | 0.083 * | 0.035 ** | |
| Model | Internal Validation | External Validation | |||
|---|---|---|---|---|---|
| N | Ncal | Nval | nlv | Accuracy (%) (S.D) | Accuracy (%) |
| 120 | 84 | 36 | 9 | 72 (8.3) | 64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gara, T.W.; Darvishzadeh, R.; Skidmore, A.K.; Wang, T. Impact of Vertical Canopy Position on Leaf Spectral Properties and Traits across Multiple Species. Remote Sens. 2018, 10, 346. https://doi.org/10.3390/rs10020346
Gara TW, Darvishzadeh R, Skidmore AK, Wang T. Impact of Vertical Canopy Position on Leaf Spectral Properties and Traits across Multiple Species. Remote Sensing. 2018; 10(2):346. https://doi.org/10.3390/rs10020346
Chicago/Turabian StyleGara, Tawanda W., Roshanak Darvishzadeh, Andrew K. Skidmore, and Tiejun Wang. 2018. "Impact of Vertical Canopy Position on Leaf Spectral Properties and Traits across Multiple Species" Remote Sensing 10, no. 2: 346. https://doi.org/10.3390/rs10020346
APA StyleGara, T. W., Darvishzadeh, R., Skidmore, A. K., & Wang, T. (2018). Impact of Vertical Canopy Position on Leaf Spectral Properties and Traits across Multiple Species. Remote Sensing, 10(2), 346. https://doi.org/10.3390/rs10020346