A Spectral Mapping Signature for the Rapid Ohia Death (ROD) Pathogen in Hawaiian Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leaf-Level Methods
2.1.1. Leaf Chemistry and Pathology
2.1.2. Leaf Spectroscopy
2.2. Canopy-Level Methods
3. Results
3.1. Leaf-Level Results
3.1.1. Leaf Chemistry and Pathology
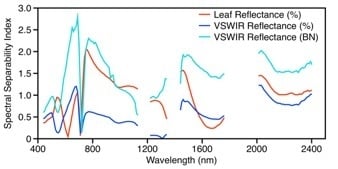
3.1.2. Leaf Spectroscopy
3.1.3. Leaf Spectral–Chemical Relationships
3.2. Canopy-Level Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghelardini, L.; Pepori, A.L.; Luchi, N.; Capretti, P.; Santini, A. Drivers of emerging fungal diseases of forest trees. For. Ecol. Manag. 2016, 381, 235–246. [Google Scholar] [CrossRef]
- Freer-Smith, P.H.; Webber, J.F. Tree pests and diseases: The threat to biodiversity and the delivery of ecosystem services. Biodivers. Conserv. 2017, 26, 3167–3181. [Google Scholar] [CrossRef]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The Consequence of Tree Pests and Diseases for Ecosystem Services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, M.J.; Brockerhoff, E.G.; Wingfield, B.D.; Slippers, B. Planted forest health: The need for a global strategy. Science 2015, 349, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Hubbes, M. The American elm and Dutch elm disease. For. Chron. 1999, 75, 265–273. [Google Scholar] [CrossRef]
- Brasier, C.M.; Buck, K.W. Rapid Evolutionary Changes in a Globally Invading Fungal Pathogen (Dutch Elm Disease). Biol. Invasions 2001, 3, 223–233. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; John Wiley and Sons, Inc.: New York, NY, USA, 1974. [Google Scholar]
- Hodges, C.S.; Adee, K.T.; Stein, J.D.; Wood, H.B.; Doty, R.D. Decline of ohia (Metrosideros polymorpha) in Hawaii: A review. Gen. Tech. Rep. 1986, 1–22. [Google Scholar] [CrossRef]
- Barbosa, J.M.; Asner, G.P. Effects of long-term rainfall decline on the structure and functioning of Hawaiian forests. Environ. Res. Lett. 2017, 12, 94002. [Google Scholar] [CrossRef]
- Keith, L.M.; Hughes, R.F.; Sugiyama, L.S.; Heller, W.P.; Bushe, B.C.; Friday, J.B. First Report of Ceratocystis Wilt on ʻŌhiʻa (Metrosideros polymorpha). Plant Dis. 2015, 99, 1276. [Google Scholar] [CrossRef]
- Barnes, I.; Fourie, A.; Wingfield, M.J.; Harrington, T.C.; McNew, D.L.; Sugiyama, L.S.; Luiz, B.C.; Heller, W.P.; Keith, L.M. New Ceratocystis species associated with rapid death of Metrosideros polymorpha in Hawaiʻi. Persoonia 2018, in press. [Google Scholar]
- RAPID ʻŌHIʻA DEATH, Part 1: Strategic Response Plan. Available online: https://cms.ctahr.hawaii.edu/Portals/222/Ohia Documents/ROD Strategic Response Plan Final 112216 (1).pdf?ver=2016-12-19-092946-040 (accessed on 16 January 2018).
- Asner, G.; Martin, R. Spectral and chemical analysis of tropical forests: Scaling from leaf to canopy levels. Remote Sens. Environ. 2008, 112, 3958–3970. [Google Scholar] [CrossRef]
- Asner, G.P.; Knapp, D.E.; Boardman, J.; Green, R.O.; Kennedy-Bowdoin, T.; Eastwood, M.; Martin, R.E.; Anderson, C.; Field, C.B. Carnegie Airborne Observatory-2: Increasing science data dimensionality via high-fidelity multi-sensor fusion. Remote Sens. Environ. 2012, 124, 454–465. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Knapp, D.E.; Tupayachi, R.; Anderson, C.B.; Sinca, F.; Vaughn, N.R.; Llactayo, W. Airborne laser-guided imaging spectroscopy to map forest trait diversity and guide conservation. Science 2017, 355, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, N.R.; Asner, G.P.; Brodrick, P.G.; Martin, R.E.; Heckler, J.W.; Knapp, D.E.; Hughes, R.F. An Approach for High-resolution Mapping of Hawaiian Metrosideros Forest Mortality Using Laser-guided Imaging Spectroscopy. Remote Sens. 2018, in press. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin Coicalteau reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Toth, G.B.; Pavia, H. Removal of dissolved brown algal phlorotannins using insoluble Polyvinylpyrrolidone (PVPP). J. Chem. Ecol. 2001, 27, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Heller, W.; Keith, L. Real-time PCR assays to detect and distinguish the rapid ohia death pathogens. Phytopathology 2018. in review. [Google Scholar]
- Moller, W.J.; DeVay, J.E. Carrot as a species-selective isolation medium for Ceratocystis fimbriata. Phytopathology 1968, 58, 123–124. [Google Scholar]
- Asner, G.P.; Martin, R.E.; Knapp, D.E.; Tupayachi, R.; Anderson, C.; Carranza, L.; Martinez, P.; Houcheime, M.; Sinca, F.; Weiss, P. Spectroscopy of canopy chemicals in humid tropical forests. Remote Sens. Environ. 2011, 115, 3587–3598. [Google Scholar] [CrossRef]
- Haaland, D.M.; Thomas, E.V. Partial least-squares methods for spectral Analyses. 1. Relation to other quantitative calibration methods and the extraction of qualitative information. Anal. Chem. 1988, 60, 1193–1202. [Google Scholar] [CrossRef]
- Chen, S.; Hong, X.; Harris, C.J.; Sharkey, P.M. Sparse modeling using orthogonal forest regression with PRESS statistic and regularization. IEEE Trans. Syst. Man Cybern. 2004, 34, 898–911. [Google Scholar] [CrossRef]
- Somers, B.; Zortea, M.; Plaza, A.; Asner, G.P. Automated Extraction of Image-Based Endmember Bundles for Improved Spectral Unmixing. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 396–408. [Google Scholar] [CrossRef]
- Feilhauer, H.; Asner, G.P.; Martin, R.E.; Schmidtlein, S. Brightness-normalized Partial Least Squares Regression for hyperspectral data. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1947–1957. [Google Scholar] [CrossRef]
- Asner, G.P.; Anderson, C.B.; Martin, R.E.; Tupayachi, R.; Knapp, D.E.; Sinca, F. Landscape biogeochemistry reflected in shifting distributions of chemical traits in the Amazon forest canopy. Nat. Geosci. 2015, 8, 567–573. [Google Scholar] [CrossRef]
- Balzotti, C.S.; Asner, G.P.; Taylor, P.G.; Cleveland, C.C.; Cole, R.; Martin, R.E.; Nasto, M.; Osborne, B.B.; Porder, S.; Townsend, A.R. Environmental controls on canopy foliar nitrogen distributions in a Neotropical lowland forest. Ecol. Appl. 2016, 26, 2451–2464. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, L.A.; Flint Hughes, R.; Friday, J.B.; Keith, L.M.; Barbosa, J.M.; Friday, N.J.; Liu, Z.; Sowards, T.G. Assessing spatial distribution, stand impacts and rate of Ceratocystis fimbriata induced ʻōhiʻa (Metrosideros polymorpha) mortality in a tropical wet forest, Hawaiʻi Island, USA. For. Ecol. Manag. 2016, 377, 83–92. [Google Scholar] [CrossRef]
- Hwang, S.; Ko, W. Quantitative studies of Phytophthora cinnamomi in decline and healthy Ohia forests. Trans. Br. Mycol. Soc. 1978, 70. [Google Scholar] [CrossRef]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Anderson, C.B.; Knapp, D.E. Quantifying forest canopy traits: Imaging spectroscopy versus field survey. Remote Sens. Environ. 2015, 158, 15–27. [Google Scholar] [CrossRef]
- Martin, R.; Chadwick, K.; Brodrick, P.; Carranza-Jimenez, L.; Vaughn, N.; Asner, G. An Approach for Foliar Trait Retrieval from Airborne Imaging Spectroscopy of Tropical Forests. Remote Sens. 2018, 10, 199. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M. Global convergence in the vulnerability of forests to drought. Nature 2012. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Stenlid, J.; Martínez-Vilalta, J. The effect of fungal pathogens on the water and carbon economy of trees: Implications for drought-induced mortality. New Phytol. 2014, 203, 1028–1035. [Google Scholar] [CrossRef] [PubMed]







| Trait | Green Foliage (n = 22) | Brown Foliage (n = 21) | ||
|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |
| LMA (g m−2) * | 315.6 (125.3) | 169.8–622.4 | 172.9 (46.0) | 105.7–319.9 |
| N (%) | 0.72 (0.18) | 0.45–1.05 | 0.86 (0.22) | 0.35–1.36 |
| Chl-ab (mg g−1) * | 1.68 (0.78) | 0.81–4.01 | 0.21 (0.23) | 0.01–0.98 |
| Phenols (mg g−1) * | 90.7 (14.7) | 80.3–101.1 | 25.7 (14.7) | 5.1–57.0 |
| Tannins (mg g−1) * | 40.6 (5.4) | 36.7–44.4 | 19.2 (8.9) | 5.1–32.7 |
| C (%) | 50.6 (1.2) | 49.0–54.2 | 50.9 (2.5) | 48.7–60.2 |
| NSC (%) * | 47.9 (6.3) | 37.7–67.3 | 37.0 (6.7) | 20.5–46.1 |
| Cellulose (%) * | 13.7 (5.1) | 4.0–21.4 | 22.8 (3.7) | 16.8–30.5 |
| Lignin (%) | 29.3 (7.7) | 17.1–44.7 | 31.3 (5.9) | 20.6–41.5 |
| Water (%) * | 47.1 (6.8) | 37.3–57.9 | 15.9 (4.1) | 10.3–24.1 |
| qPCR Result | Num. Trees | % of Tested |
|---|---|---|
| Ceratocystis sp. A | 13 | 59 |
| Ceratocystis sp. B | 4 | 18 |
| Negative | 5 | 23 |
| Total | 22 | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asner, G.P.; Martin, R.E.; Keith, L.M.; Heller, W.P.; Hughes, M.A.; Vaughn, N.R.; Hughes, R.F.; Balzotti, C. A Spectral Mapping Signature for the Rapid Ohia Death (ROD) Pathogen in Hawaiian Forests. Remote Sens. 2018, 10, 404. https://doi.org/10.3390/rs10030404
Asner GP, Martin RE, Keith LM, Heller WP, Hughes MA, Vaughn NR, Hughes RF, Balzotti C. A Spectral Mapping Signature for the Rapid Ohia Death (ROD) Pathogen in Hawaiian Forests. Remote Sensing. 2018; 10(3):404. https://doi.org/10.3390/rs10030404
Chicago/Turabian StyleAsner, Gregory P., Roberta E. Martin, Lisa M. Keith, Wade P. Heller, Marc A. Hughes, Nicholas R. Vaughn, R. Flint Hughes, and Christopher Balzotti. 2018. "A Spectral Mapping Signature for the Rapid Ohia Death (ROD) Pathogen in Hawaiian Forests" Remote Sensing 10, no. 3: 404. https://doi.org/10.3390/rs10030404
APA StyleAsner, G. P., Martin, R. E., Keith, L. M., Heller, W. P., Hughes, M. A., Vaughn, N. R., Hughes, R. F., & Balzotti, C. (2018). A Spectral Mapping Signature for the Rapid Ohia Death (ROD) Pathogen in Hawaiian Forests. Remote Sensing, 10(3), 404. https://doi.org/10.3390/rs10030404