Normalized Difference Vegetation Vigour Index: A New Remote Sensing Approach to Biodiversity Monitoring in Oil Polluted Regions
Abstract
:1. Introduction
- Are vascular plants susceptible to oil pollution and does this affect their spectral signatures?
- Is there any relationship between plant spectral signatures and vascular plant species diversity?
- Can this relationship be modelled to estimate the diversity of vascular plants on oil-polluted transects?
2. Materials and Methods
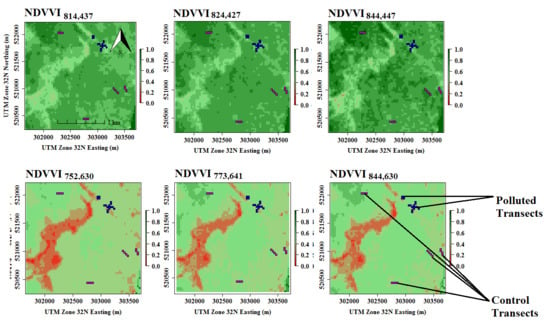
2.1. Study Area
2.2. Establishment of Study Transects
2.3. Field Survey of Vascular Plant Species
2.4. Soil Sampling and Analysis
2.5. Hyperspectral Image Description and Preparation
2.6. Processing Landsat 8 and Sentinel 2A Images
2.7. Vegetation Indices
- Chlorophyll Content: used to monitor changes in green biomass, chlorophyll content and leaf structure. High values indicate increased chlorophyll content, green biomass and vegetation vigour and
- Primary Productivity: measure changes in the photosynthetic light use efficiency of plants. High values indicate reduced light use efficiency, hence reduced productivity.
2.8. Derivation of Stress-Sensitive Wavelengths by Sensitivity Analysis
- Rn is reflectance of non-stressed vegetation
- Ru is reflectance of stressed vegetation
- R∆ is reflectance difference
- Rs is reflectance sensitivity
2.9. Calculation of the Normalized Difference Vegetation Vigour Index (NDVVI)
- Ri = reflectance at least sensitive wavelength
- Rj = reflectance at most sensitive wavelength
2.10. Statistical Analyses
3. Results
3.1. Sorenson’s Similarity and Diversity Indices of Transects
3.2. Vegetation Data Analysis
3.3. Sensitivity Analysis and Comparison of Hyperion Wavelengths
3.4. Modelling the Vascular Plant Species Diversity of the Investigated Transects
3.4.1. Model Calibration Using Training Data
3.4.2. Model Validation Using Test Data
3.5. Model Implementation and Evaluation Using Random Pixels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Global Issues: Why Is Biodiversity Important? Who Cares? Available online: http://www.globalissues.org/article/170/why-is-biodiversity-important-who-cares (accessed on 24 January 2015).
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.K.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Matulich, K.L.; Hooper, D.U.; Byrnes, J.E.; Duffy, E.; Gamfeldt, L.; Balvanera, P.; O’Connor, M.I.; Gonzalez, A. The functional role of producer diversity in ecosystems. Am. J. Bot. 2011, 98, 572–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vihervaara, P.; Mononen, L.; Auvinen, A.; Virkkala, R.; Lu, Y.; Pippuri, I.; Packalen, P.; Valbuena, R.; Valkama, J. How to Integrate Remotely Sensed Data and Biodiversity for Ecosystem Assessments at Landscape Scale. Landsc. Ecol. 2014, 30, 501–516. [Google Scholar] [CrossRef]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and Ecosytem Services: A Multi-Layered Relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Norris, K. Biodiversity in the Context of Ecosystem Services: The Applied Need for Systems Approaches. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Waide, R.B.; Willig, M.R.; Steiner, C.F.; Mittelbach, G.; Gough, L.; Dodson, S.I.; Juday, G.P.; Parmenter, R. The Relationship between Productivity and Species Richness. Annu. Rev. Ecol. Syst. 1999, 30, 257–300. [Google Scholar] [CrossRef]
- Chapin, F.S.; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Osvaldo, E.S.; Hobbie, S.E.; et al. Consequences of Changing Biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Pereira, H.M.; Krug, C.; Leadley, P.W.; Visconti, P.; Januchowski-Hartley, S.R.; Krug, R.M.; Alkemade, R.; Bellard, C.; Cheung, W.W.L.; et al. A framework to identify enabling and urgent actions for the 2020 Aichi Targets. Basic Appl. Ecol. 2014, 15, 633–638. [Google Scholar] [CrossRef]
- Strategic Plan for Biodiversity 2011–2020. Available online: https://www.cbd.int/sp/default.shtml (accessed on 20 February 2018).
- Johnson, D.D.P.; Hay, S.I.; Rogers, D.J. Contemporary Environmental Correlates of Endemic Bird Areas Derived from Meteorological Satellite Sensors. Proc. Biol. Sci. 1998, 265, 951–959. [Google Scholar] [CrossRef]
- Nagendra, H. Using Remote Sensing to Assess Biodiversity. Int. J. Remote Sens. 2001, 22, 2377–2400. [Google Scholar] [CrossRef]
- Carlson, K.M.; Asner, G.P.; Hughes, F.R.; Ostertag, R.; Martin, R.E. Hyperspectral Remote Sensing of Canopy Biodiversity in Hawaiian Lowland Rainforests. Ecosystems 2007, 10, 536–549. [Google Scholar] [CrossRef] [Green Version]
- Wilfong, B.N.; Gorchov, D.L.; Henry, M.C. Detecting an Invasive Shrub in Deciduous Forest Understories Using Remote Sensing. Weed Sci. 2009, 57, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Foody, G.M.; Cutler, M.E.J. Tree biodiversity in protected and logged Bornean tropical rain forests and its measurement by satellite remote sensing. J. Biogeogr. 2003, 30, 1053–1066. [Google Scholar] [CrossRef]
- Yoccoz, N.G.; Nichols, J.D.; Boulinier, T. Monitoring of Biological Diversity in Space and Time. Trends Ecol. Evol. 2001, 16, 446–453. [Google Scholar] [CrossRef]
- Kerr, J.T.; Ostrovsky, M. From space to species: Ecological applications for remote sensing. Trends Ecol. Evol. 2003, 18, 299–305. [Google Scholar] [CrossRef]
- Muchoney, D.M. Earth Observations for Terrestrial Biodiversity and Ecosystems. Remote Sens. Environ. 2008, 112, 1909–1911. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Likens, G.E. The Science and Application of Ecological Monitoring. Biol. Conserv. 2010, 143, 1317–1328. [Google Scholar] [CrossRef]
- Han, X.; Smyth, R.L.; Young, B.E.; Brooks, T.M.; Sánchez de Lozada, A.; Bubb, P.; Butchart, S.H.M.; Larsen, F.W.; Hamilton, H.; Hansen, M.C.; et al. A Biodiversity Indicators Dashboard: Addressing Challenges to Monitoring Progress towards the Aichi Biodiversity Targets Using Disaggregated Global Data. PLoS ONE 2014, 9, e112046. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.M.; Ferrier, S.; Walters, M.; Geller, G.N.; Jongman, R.H.G.; Scholes, R.J.; Bruford, M.W.; Brummitt, N.; Butchart, S.H.M.; Cardoso, A.C.; et al. Essential Biodiversity Variables. Science 2013, 339, 277–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend Peterson, A.; Soberón, J. Essential biodiversity variables are not global. Biodivers. Conserv. 2018, 27, 1277–1288. [Google Scholar] [CrossRef]
- Group on Earth Observations Biodiversity Observation Network (GEO BON). Adequacy of Biodiversity Observation Systems to Support the CBD 2020 Targets; GEO BON: Leipzig, Germany, 2011; pp. 72–78. [Google Scholar]
- Schmeller, D.S.; Weatherdon, L.V.; Loyau, A.; Bondeau, A.; Brotons, L.; Brummitt, N.; Geijzendorffer, I.R.; Haase, P.; Kuemmerlen, M.; Martin, C.S.; et al. A suite of essential biodiversity variables for detecting critical biodiversity change. Biol. Rev. 2018, 93, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Global Biodiversity Information Facility (GBIF). Available online: http://www.gbif.org/ (accessed on 26 March 2016).
- Scholes, R.J.; Walters, M.; Turak, E.; Saarenmaa, H.; Heip, C.H.; Tuama, É.Ó.; Faith, D.P.; Mooney, H.A.; Ferrier, S.; Jongman, R.H.; et al. Building a Global Observing System for Biodiversity. Curr. Opin. Environ. Sustain. 2012, 4, 139–146. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; De Jong, S.M.; Epema, G.F.; Van Der Meer, F.D.; Bakker, W.H.; Skidmore, A.K.; Scholte, K.H. Derivation of the red edge index using the MERIS standard band setting. Int. J. Remote Sens. 2002, 23, 3169–3184. [Google Scholar] [CrossRef]
- Andrew, M.E.; Wulder, M.A.; Nelson, T.A. Potential Contributions of Remote Sensing to Ecosystem Service Assessments. Prog. Phys. Geogr. 2014, 38, 328–353. [Google Scholar] [CrossRef]
- Guyon, D.; Guillot, M.; Vitasse, Y.; Cardot, H.; Hagolle, O.; Delzon, S.; Wigneron, J. Monitoring elevation variations in leaf phenology of deciduous broadleaf forests from SPOT/VEGETATION time-series. Remote Sens. Environ. 2011, 115, 615–627. [Google Scholar] [CrossRef]
- Asner, G.P.; Jones, M.O.; Martin, R.E.; Knapp, D.E.; Hughes, R.F. Remote sensing of native and invasive species in Hawaiian forests. Remote Sens. Environ. 2008, 112, 1912–1926. [Google Scholar] [CrossRef]
- Gould, W. Remote sensing of vegetation, plant species richness, and regional biodiversity hotspots. Ecol. Appl. 2000, 10, 1861–1870. [Google Scholar] [CrossRef]
- Rocchini, D.; Hernández-Stefanoni, J.L.; He, K.S. Advancing species diversity estimate by remotely sensed proxies: A conceptual review. Ecol. Inform. 2015, 25, 22–28. [Google Scholar] [CrossRef]
- Aneece, I.P.; Epstein, H.; Lerdau, M. Correlating species and spectral diversities using hyperspectral remote sensing in early-successional fields. Ecol. Evol. 2017, 7, 3475–3488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foody, G.M.; Mathur, A. A relative evaluation of multiclass image classification by support vector machines. IEEE Trans. Geosci. Remote Sens. 2004, 42, 1335–1343. [Google Scholar] [CrossRef] [Green Version]
- Féret, J.; Asner, G.P. Mapping tropical forest canopy diversity using high-fidelity imaging spectroscopy. Ecol. Appl. 2014, 24, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Asner, G.P.; Martin, R.E. Canopy phylogenetic, chemical and spectral assembly in a lowland Amazonian forest. New Phytol. 2011, 189, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Asner, G.P.; Martin, R.E.; Ford, A.J.; Metcalfe, D.J.; Liddell, M.J. Leaf chemical and spectral diversity in Australian tropical forests. Ecol. Appl. 2009, 19, 236–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B. Hyperspectral remote sensing of vegetation growing condition and regional environment. In Proceedings of the 2010 2nd Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing, Reykjavik, Iceland, 14–16 June 2010; pp. 1–4. [Google Scholar]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Ustin, S.L.; Verdebout, J.; Schmuck, G.; Andreoli, G.; Hosgood, B. Estimating leaf biochemistry using the PROSPECT leaf optical properties model. Remote Sens. Environ. 1996, 56, 194–202. [Google Scholar] [CrossRef]
- Thenkabail, P. Hyperspectral Remote Sensing of Vegetation; Taylor & Francis: Didcot, UK, 2011. [Google Scholar]
- Kuenzer, C.; van Bejima, S.; Gessner, U.; Dech, S. Land Surface Dynamics and Environmental Challenges of the Niger Delta, Africa: Remote Sensing- Based Analyses Spanning Three Decades (1986–2013). Appl. Geogr. 2014, 53, 354–368. [Google Scholar] [CrossRef]
- Biodiversity. Available online: http://www.merriam-webster.com/dictionary/biodiversity (accessed on 11 April 2014).
- Reynolds, S.C.; Marston, C.G.; Hassani, H.; King, G.C.P.; Bennett, M.R. Environmental hydro-refugia demonstrated by vegetation vigour in the Okavango Delta, Botswana. Sci. Rep. 2016, 6, 35951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melillo, J.M.; McGuire, A.D.; Kicklighter, D.W.; Moore, B.; Vorosmarty, C.J.; Schloss, A.L. Global climate change and terrestrial net primary production. Nature 1993, 363, 234–240. [Google Scholar] [CrossRef]
- Vrieling, A. Satellite remote sensing for water erosion assessment: A review. CATENA 2006, 65, 2–18. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Margules, C.R.; Botkin, D.B. Indicators of Biodiversity for Ecologically Susteainable Forest Management. Conserv. Biol. 2000, 14, 941–950. [Google Scholar] [CrossRef]
- United States Environment Protection Agency (USEPA). Ecological Effects Test Guidelines OCSPP 850.4150: Vegetative Vigor; USEPA: Washington, DC, USA, 2012. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). Test No. 227: Terrestrial Plant Test: Vegetative Vigour Test; OECD: Paris, France, 2006. [Google Scholar]
- Xu, C.; Li, Y.; Hu, J.; Yang, X.; Sheng, S.; Liu, M. Evaluating the difference between the normalized difference vegetation index and net primary productivity as the indicators of vegetation vigor assessment at landscape scale. Environ. Monit. Assess 2012, 184, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, J.; Carman, S.; Segal, C.; Jarecke, P.; Clancy, P.; Browne, W. Overview of the Hyperion Imaging Spectrometer for the NASA EO-1 mission. In Proceedings of the IEEE 2001 International Geoscience and Remote Sensing Symposium, Sydney, NSW, Australia, 9–13 July 2001; Volume 7, p. 3038. [Google Scholar]
- Cadotte, M.W.; Cardinale, B.J.; Oakley, T.H. Evolutionary history and the effect of biodiversity on plant productivity. Proc. Natl. Acad. Sci. USA 2008, 105, 17012–17017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilman, D. Biodiversity: Population Versus Ecosystem Stability. Ecology 1996, 77, 350–363. [Google Scholar] [CrossRef]
- Naeem, S.; Thompson, L.J.; Lawler, S.P.; Lawton, J.H.; Woodfin, R.M. Declining biodiversity can alter the performance of ecosystems. Nature 1994, 368, 734–737. [Google Scholar] [CrossRef]
- Hector, A.; Schmid, B.; Beierkuhnlein, C.; Caldeira, M.C.; Diemer, M.; Dimitrakopoulos, P.G.; Finn, J.A.; Freitas, H.; Giller, P.S.; Good, J.; et al. Plant Diversity and Productivity Experiments in European Grasslands. Science 1999, 286, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef] [Green Version]
- Hector, A. The Effect of Diversity on Productivity: Detecting the Role of Species Complementarity. Oikos 1998, 82, 597–599. [Google Scholar] [CrossRef]
- Maselli, F. Monitoring forest conditions in a protected Mediterranean coastal area by the analysis of multiyear NDVI data. Remote Sens. Environ. 2004, 89, 423–433. [Google Scholar] [CrossRef]
- Salinas-Zavala, C.A.; Douglas, A.V.; Diaz, H.F. Interannual variability of NDVI in northwest Mexico. Associated climatic mechanisms and ecological implications. Remote Sens. Environ. 2002, 82, 417–430. [Google Scholar] [CrossRef]
- Karthikeyan, N.; Shashikkumar, M.C.; Ramanamurthy, J. A study on vegetation vigour as affected by soil properties using remote sensing approach. In Proceedings of the RSTSCC-2010, Chennai, India, 13–15 November 2010; pp. 107–110. [Google Scholar]
- Munyati, C.; Ratshibvumo, T. Characterising vegetation cover in relation to land use in the Inkomati catchment, South Africa, using Landsat imagery. Area 2011, 43, 189–201. [Google Scholar] [CrossRef]
- Wiesmair, M.; Otte, A.; Waldhardt, R. Relationships between plant diversity, vegetation cover, and site conditions: Implications for grassland conservation in the Greater Caucasus. Biodivers. Conserv. 2017, 26, 273–291. [Google Scholar] [CrossRef]
- Wulder, M. Optical Remote-Sensing Techniques for the Assessment of Forest Inventory and Biophysical Parameters. Progress Phys. Geogr. 1998, 22, 449–476. [Google Scholar] [CrossRef]
- Rocchini, D.; Boyd, D.S.; Féret, J.; Foody, G.M.; He, K.S.; Lausch, A.; Nagendra, H.; Wegmann, M.; Pettorelli, N. Satellite remote sensing to monitor species diversity: Potential and pitfalls. Remote Sens. Ecol. Conserv. 2016, 2, 25–36. [Google Scholar] [CrossRef]
- Boyd, D.S.; Danson, F.M. Satellite Remote Sensing of Forest Resources: Three Decades of Research Development. Prog. Phys. Geogr. 2005, 29, 1–26. [Google Scholar] [CrossRef]
- Warren, S.D.; Alt, M.; Olson, K.D.; Irl, S.D.H.; Steinbauer, M.J.; Jentsch, A. The Relationship between the Spectral Diversity of Satellite Imagery, Habitat Heterogeneity, and Plant Species Richness. Ecol. Inform. 2014, 24, 160–168. [Google Scholar] [CrossRef]
- Galidaki, G.; Gitas, I. Mediterranean Forest Species Mapping Using Classification of Hyperion Imagery. Geocarto Int. 2015, 30, 48–61. [Google Scholar] [CrossRef]
- Lucas, R.; Blonda, P.; Bunting, P.; Jones, G.; Inglada, J.; Arias, M.; Kosmidou, V.; Petrou, Z.I.; Manakos, I.; Adamo, M.; et al. The Earth Observation Data for Habitat Monitoring (EODHaM) system. Int. J. Appl. Earth Obs. Geoinf. 2015, 37, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Eigemeier, E.; Heiskanen, J.; Rautiainen, M.; Vesanto, V.; Majasalmi, T.; Stenberg, P. Narrowband Vegetation Indices for Estimating Boreal Forest Leaf Area Index. Remote Sens. Appl. 2012. [Google Scholar] [CrossRef] [Green Version]
- United Nations Environmental Programme. Environmental Assessment of Ogoniland Report; United Nations Environmental Programme: Nairobi, Kenya, 2011. [Google Scholar]
- Agbagwa, I.O.; Ndukwu, B.C. Oil and Gas Pipeline Construction-Induced Forest Fragmentation and Biodiversity Loss in the Niger Delta, Nigeria. Nat.Resour. 2014, 5, 698–718. [Google Scholar] [CrossRef]
- Omo-Irabor, O.O.; Olobaniyi, S.B.; Akunna, J.; Venus, V.; Maina, J.M.; Paradzayi, C. Mangrove Vulnerability Modelling in Parts of Western Niger Delta, Nigeria Using Satellite Images, GIS Techniques and Spatial Multi-Criteria Analysis (SMCA). Environ. Monit. Assess 2011, 178, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Elenwo, E.; Akankali, J. Impact of Climate Change on Aquatic Fauna of Economic Importance in Niger Delta, Nigeria. Atmos. Clim. Sci. 2014, 4, 710–720. [Google Scholar] [CrossRef]
- Ugochukwu, C.N.C.; Ertel, J. Negative Impacts of Oil Exploration on Biodiversity Management in the Niger Delta Area of Nigeria. Impact Assess. Proj. Apprais. 2008, 26, 139–147. [Google Scholar]
- World Bank. Defining an Environmental Development Strategy for the Niger Delta; World Bank: Washington, DC, USA, 1995; Volume 1. [Google Scholar]
- Ebigwa, J.K.; Akomaye, F. Species Diversity and Regeneration Potential of Some Mixed Mangrove Forests in Escravos Communities Delta State Nigeria. Res. J. For. 2014, 8, 34–47. [Google Scholar] [CrossRef]
- Adegbehin, J.O.; Nwaigbo, L.C. Mangrove resources in Nigeria: Use and Management Perspectives. Nat. Resour. 1990, 26, 13–25. [Google Scholar]
- Osuji, L.C.; Ezebuiro, P.E. Hydrocarbon contamination of a typical mangrove floor in Niger Delta, Nigeria. Int. J. Environ. Sci. Technol. 2006, 3, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Nigerian Oil Spill Monitor. Available online: https://oilspillmonitor.ng/ (accessed on 5 October 2015).
- Buckland, S.T.; Borchers, D.L.; Johnston, A.; Henrys, P.A.; Marques, T.A. Line Transect Methods for Plant Surveys. Biometrics 2007, 63, 989–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kercher, S.M.; Frieswyk, C.B.; Zedler, J.B. Effects of sampling teams and estimation methods on the assessment of plant cover. J. Veg. Sci. 2003, 14, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Cain, S.A.; Castro, G.M. Manual of Vegetation Analysis; Harper: New York, NY, USA, 1959. [Google Scholar]
- Ubom, R.M. Ethnobotany and Biodiversity Conservation in the Niger Delta, Nigeria. Int. J. Bot. 2010, 6, 310–322. [Google Scholar]
- Agbagwa, I.O.; Ekeke, C. Structure and Phytodiversity of Freshwater Swamp Forest in Oil-rich Bonny, Rivers State, Nigeria. Res. J. For. 2011, 5, 66–77. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis Palaeontol. Palaentol. Electron. 2001, 4, 4–9. [Google Scholar]
- Sattout, E.; Caligari, P.D.S. Forest Biodiversity Assessment in Relic Ecosystem: Monitoring and Management Practice Implications. Diversity 2011, 3, 531–546. [Google Scholar] [CrossRef]
- Petrovic, A.; Jurisic, A.; Rajkovic, D. Seasonal Distribution and Species Association among Spider Mites (Acari: Tetranychidae) and Predatory Mites (Acari: Phytoseiidae and Acari: Stigmaeidae) in Serbian Apple Orchards. Int. J. Acarol. 2010, 36, 519–526. [Google Scholar] [CrossRef]
- Hurlbert, S.H. The Nonconcept of Species Diversity: A Critique and Alternative Parameters. Ecology 1971, 52, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, A.; Chiu, C. Species Richness Estimation and Comparison. In Wiley StatsRef: Statistics Reference Online, 1st ed.; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Hu, X.-S. Hubbell’s fundamental biodiversity parameter and the Simpson diversity index. Ecol. Lett. 2005, 8, 386–390. [Google Scholar] [CrossRef]
- United States Environment Protection Agency (USEPA). U. S. EPA Method 8015D (SW-846): Nonhalogenated Organics Using GC/FID; USEPA: Washington, DC, USA, 2003.
- Peng, Y.; Fan, M.; Song, J.; Cui, T.; Li, R. Assessment of plant species diversity based on hyperspectral indices at a fine scale. Sci. Rep. 2018, 8, 4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thenkabail, P.S.; Mariotto, I.; Gumma, M.K.; Middleton, E.M.; Landis, D.R.; Huemmrich, K.F. Selection of Hyperspectral Narrowbands (HNBs) and Composition of Hyperspectral Twoband Vegetation Indices (HVIs) for Biophysical Characterisation and Discrimination of Crop Types Using Field Reflectance and Hyperion/EO-1 Data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 6, 427–439. [Google Scholar] [CrossRef]
- Datt, B.; McVicar, T.R.; Van Niel, T.G.; Jupp, D.L.B.; Pearlman, J.S. Preprocessing EO-1 Hyperion hyperspectral data to support the application of agricultural indexes. TGRS 2003, 41, 1246–1259. [Google Scholar] [CrossRef]
- Scheffler, D.; Karrasch, P. Preprocessing of hyperspectral images—A comparative study of destriping algorithms for eo-1 hyperion. In Proceedings of the SPIE Remote Sensing Conference, Dresden, Germany, 23–26 September 2013. [Google Scholar]
- Adler-Golden, S.; Richtsmeier, S.; Conforti, P.; Bernstein, L. Spectral image destriping using a low-dimensional model. In SPIE Defense, Security, and Sensing; Shen, S.S., Lewis, P.E., Eds.; SPIE: Baltimore, MD, USA, 2013; p. 8. [Google Scholar]
- Felde, G.W.; Anderson, G.P.; Cooley, T.W.; Matthew, M.W.; Adler-Golden, S.M.; Berk, A.; Lee, J. Analysis of Hyperion Data with the FLAASH Atmospheric Correction Algorithm. In Proceedings of the 2003 IEEE International Geoscience and Remote Sensing Symposium, Toulouse, France, 21–25 July 2003; Volume 1, p. 92. [Google Scholar]
- Dadon, A.; Ben-Dor, E.; Karnieli, A. Use of Derivative Calculations and Minimum Noise Fraction Transform for Detecting and Correcting the Spectral Curvature Effect (Smile) in Hyperion Images. TGRS 2010, 48, 2603–2612. [Google Scholar] [CrossRef]
- Gersman, R.; Ben-Dor, E.; Beyth, M.; Avigad, D.; Abraha, M.; Kibreab, A. Mapping of hydrothermally altered rocks by the EO-1 Hyperion sensor, Northern Danakil Depression, Eritrea. Int. J. Remote Sens. 2008, 29, 3911–3936. [Google Scholar] [CrossRef]
- Green, A.A.; Berman, M.; Switzer, P.; Craig, M.D. A transformation for ordering multispectral data in terms of image quality with implications for noise removal. IEEE Trans. Geosci. Remote Sens. 1988, 26, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Chen, G.; Tian, L.; Qin, K.; Qian, S. Minimum Noise Fraction versus Principal Component Analysis as a Preprocessing Step for Hyperspectral Imagery Denoising. Can. J. Remote Sens. 2016, 42, 106–116. [Google Scholar] [CrossRef]
- STEP: Science Toolbox Exploitation Platform. Available online: http://step.esa.int/main/toolboxes/snap/ (accessed on 18 January 2018).
- MuellerWilm, U.; Devignot, O.; Pessiot, L. Sen2Cor Configuration and User Manual. Available online: http://step.esa.int/thirdparties/sen2cor/2.4.0/Sen2Cor_240_Documenation_PDF/S2-PDGS-MPC-L2A-SUM-V2.4.0.pdf (accessed on 18 January 2018).
- European Commission. European Space Agency Sentinel-2 User Handbook; European Commission: Brussels, Belgium, 2015; pp. 34–64. [Google Scholar]
- Landsat Science Products. Available online: https://landsat.usgs.gov/landsat-science-data-products (accessed on 23 February 2016).
- Bhandari, A.K.; Kumar, A.; Singh, G.K. Feature Extraction using Normalized Difference Vegetation Index (NDVI): A Case Study of Jabalpur City. Procedia Technol. 2012, 6, 612–621. [Google Scholar] [CrossRef]
- Barati, S.; Rayegani, B.; Saati, M.; Sharifi, A.; Nasri, M. Comparison the accuracies of different spectral indices for estimation of vegetation cover fraction in sparse vegetated areas. Egypt. J. Remote Sens. Space Sci. 2011, 14, 49–56. [Google Scholar] [CrossRef]
- Pu, R.; Bell, S.; English, D. Developing Hyperspectral Vegetation Indices for Identifying Seagrass Species and Cover Classes. J. Coast. Res. 2015, 31, 595–615. [Google Scholar] [CrossRef]
- Bargain, A.; Robin, M.; Méléder, V.; Rosa, P.; Le Menn, E.; Harin, N.; Barillé, L. Seasonal spectral variation of Zostera noltii and its influence on pigment-based Vegetation Indices. J. Exp. Mar. Biol. Ecol. 2013, 446, 86–94. [Google Scholar] [CrossRef]
- Gong, P.; Pu, R.; Biging, G.S.; Larrieu, M.R. Estimation of Forest Leaf Area Index Using Vegetation Indices Derived from Hyperion Hyperspectral Data. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1355–1362. [Google Scholar] [CrossRef]
- Arellano, P.; Tansey, K.; Balzter, H.; Boyd, D.S. Detecting the effects of hydrocarbon pollution in the Amazon forest using hyperspectral satellite images. Environ. Pollut. 2015, 205, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Galvao, L.S.; Roberts, D.A.; Formaggio, A.R.; Numata, I.; Breunig, F.M. View angle effects on the discrimination of soybean varieties and on the relationships between vegetation indices and yield using off-nadir Hyperion data. Remote Sens. Environ. 2009, 113, 846–856. [Google Scholar] [CrossRef]
- Hestir, E.L.; Khanna, S.; Andrew, M.E.; Santos, M.J.; Viers, J.H.; Greenberg, J.A.; Rajapakse, S.S.; Ustin, S.L. Identification of Invasive Vegetation Using Hyperspectral Remote Sensing in The California Delta Ecosystem. Remote Sens. Environ. 2008, 112, 4034–4047. [Google Scholar] [CrossRef]
- Vaiphasa, C.; Skidmore, A.K.; de Boer, W.F.; Vaiphasa, T. A hyperspectral band selector for plant species discrimination. ISPRS J. Photogramm. Remote Sens. 2007, 62, 225–235. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Narrow band vegetation indices overcome the saturation problem in biomass estimation. Int. J. Remote Sens. 2004, 25, 3999–4014. [Google Scholar] [CrossRef]
- Pearson, R.L.; Miller, L.D. Remote mapping of standing crop biomass for estimation of the productivity of the short-grass Prairie. In Proceedings of the 8th International Symposium on Remote Sensing of Environment, Ann Arbor, MI, USA, 2–6 October 1972; pp. 1357–1381. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N. Signature analysis of leaf reflectance spectra: Algorithm development for remote sensing of chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Datt, B. A New Reflectance Index for Remote Sensing of Chlorophyll Content in Higher Plants: Tests using Eucalyptus Leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Vogelmann, J.E.; Rock, B.N.; Moss, D.M. Red edge spectral measurements from sugar maple leaves. Int. J. Remote Sens. 1993, 14, 1563–1575. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Filella, I.; Gamon, J.A. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Abedi, V.; Bassaganya-Riera, J.; Wendelsdorf, K.; Bisset, K.; Deng, X.; Eubank, S.; Hontecillas, R.; Hoops, S.; Marathe, M. Chapter 6—Agent-Based Modeling and High Performance Computing. In Computational Immunology; Bassaganya-Riera, J., Ed.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 79–111. [Google Scholar]
- Cacuci, D.G.; Ionescu-Bujor, M.; Navon, I.M. Sensitivity and Uncertainty Analysis, 1st ed.; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 11–21. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Li, L.; Ustin, S.L.; Lay, M. Application of AVIRIS data in detection of oil-induced vegetation stress and cover change at Jornada, New Mexico. Remote Sens. Environ. 2005, 94, 1–16. [Google Scholar] [CrossRef]
- Mishra, D.R.; Cho, H.J.; Ghosh, S.; Fox, A.; Downs, C.; Merani, P.B.T.; Kirui, P.; Jackson, N.; Mishra, S. Post-spill state of the marsh: Remote estimation of the ecological impact of the Gulf of Mexico oil spill on Louisiana Salt Marshes. Remote Sens. Environ. 2012, 118, 176–185. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Fischer, J.; Cunningham, R. Vegetation cover thresholds and species responses. Biol. Conserv. 2005, 124, 311–316. [Google Scholar] [CrossRef]
- Carter, G.A.; Miller, R.L. Early detection of plant stress by digital imaging within narrow stress-sensitive wavebands. Remote Sens. Environ. 1994, 50, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K. Vegetation Stress: An Introduction to the Stress Concept in Plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Bawa, K.; Rose, J.; Ganeshaiah, K.N.; Barve, N.; Kiran, M.C.; Umashaanker, R. Assessing Biodiversity from Space. Conserv. Ecol. 2002, 6, 7. [Google Scholar] [CrossRef]
- Mohammadi, J.; Shataee, S. Possibility investigation of tree diversity mapping using Landsat ETM+ data in the Hyrcanian forests of Iran. Remote Sens. Environ. 2010, 114, 1504–1512. [Google Scholar] [CrossRef]
- Mapfumo, R.B.; Murwira, A.; Masocha, M.; Andriani, R. The relationship between satellite-derived indices and species diversity across African savanna ecosystems. Int. J. Appl. Earth Observ. Geoinf. 2016, 52, 306–317. [Google Scholar] [CrossRef]
- Heumann, B.W.; Hackett, R.A.; Monfils, A.K. Testing the spectral diversity hypothesis using spectroscopy data in a simulated wetland community. Ecol. Inform. 2015, 25, 29–34. [Google Scholar] [CrossRef]
- Albek, E. Estimation of Point and Diffuse Contaminant Loads to Streams by Non-Parametric Regression Analysis of Monitoring Data. Water Air Soil Pollut. 2003, 147, 229–243. [Google Scholar] [CrossRef]
- Hayfield, T.; Racine, J.S. Nonparametric Econometrics: The np Package. J. Stat. Softw. 2008, 27, 1–32. [Google Scholar] [CrossRef]
- Hurvich, C.M.; Simonoff, J.S.; Tsai, C. Smoothing parameter selection in nonparametric regression using an improved Akaike information criterion. J. R. Stat. Soc. Ser. B 1998, 60, 271–293. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, W.; Xia, T.; Yu, Q.; Yang, P.; Li, Z.; Song, Q. Exploring the Use of Google Earth Imagery and Object-Based Methods in Land Use/Cover Mapping. Remote Sens. 2013, 5, 6026–6042. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Gong, P. Google Earth as a virtual globe tool for Earth science applications at the global scale: Progress and perspectives. Int. J. Remote Sens. 2012, 33, 3966–3986. [Google Scholar] [CrossRef]
- Kaimaris, D.; Georgoula, O.; Patias, P.; Stylianidis, E. Comparative analysis on the archaeological content of imagery from Google Earth. J. Cult. Heritage 2011, 12, 263–269. [Google Scholar] [CrossRef]
- Noomen, M.F.; Skidmore, A.K. The Effects of High Soil CO2 Concentrations on Leaf Reflectance of Maize Plants. Int. J. Remote Sens. 2009, 30, 481–497. [Google Scholar] [CrossRef]
- Clark, R.N.; Swayze, G.A.; Leifer, I.; Livo, K.E.; Kokaly, R.; Hoefen, T.; Lundeen, S.; Eastwood, M.; Green, R.O.; Pearson, N.; et al. A Method for Quantitative Mapping of Thick Oil Spills Using Imaging Spectroscopy; U.S. Geological Survey Open-File Report; USGS: Reston, VA, USA, 2010.
- Kokaly, R.F.; Couvillion, B.R.; Holloway, J.M.; Roberts, D.A.; Ustin, S.L.; Peterson, S.H.; Khanna, S.; Piazza, S.C. Spectroscopic remote sensing of the distribution and persistence of oil from the Deepwater Horizon spill in Barataria Bay marshes. Remote Sens. Environ. 2013, 129, 210–230. [Google Scholar] [CrossRef]
- Kühn, F.; Oppermann, K.; Hörig, B. Hydrocarbon Index—An algorithm for hyperspectral detection of hydrocarbons. Int. J. Remote Sens. 2004, 25, 2467–2473. [Google Scholar] [CrossRef]
- Levin, N.; Shmida, A.; Levanoni, O.; Tamari, H.; Kark, S. Predicting Mountain Plant Richness and Rarity from Space Using Satellite-Derived Vegetation Indices. Divers. Distrib. 2007, 13, 692–703. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. The Chlorophyll Fluorescence Ratio F735/F700 as an Accurate Measure of the Chlorophyll Content in Plants. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Adamu, B.; Tansey, K.; Ogutu, B. Using Vegetation Spectral Indices to Detect Oil Pollution in the Niger Delta. Remote Sens. Lett. 2015, 6, 145–154. [Google Scholar] [CrossRef]
- Scholten, M.C.T.; Leendertse, P.C. The impact of oil pollution on salt marsh vegetation. In Ecological Responses to Environmental Stresses; Rozema, J., Verkleij, J.A.C., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 184–190. [Google Scholar]
- Al-Hawas, G.H.S.; Shukry, W.M.; Azzoz, M.M.; Al-Moaik, R.M.S. The effect of sublethal concentrations of crude oil on the metabolism of Jojoba (Simmodsia chinensis) seedlings. Int. Res. J. Plant Sci. 2012, 3, 54–62. [Google Scholar]
- Baruah, P.; Saikia, R.; Baruah, P.; Deka, S. Effect of Crude Oil Contamination on the Chlorophyll Content and Morpho-Anatomy of Cyperus brevifolius (Rottb.) Hassk. Environ. Sci. Pollut. Res. 2014, 21, 12530–12538. [Google Scholar] [CrossRef] [PubMed]
- Omosun, G.; Markson, A.A.; Mbanasor, O. Growth and anatomy of amaranthus hybridus as affected by different crude oil concentrations. Eurasian J. Sci. Res. 2008, 3, 70–74. [Google Scholar]
- Lopes, A.; da Rosa-Osman, S.M.; Piedade, M.T.F. Effects of crude oil on survival, morphology, and anatomy of two aquatic macrophytes from the Amazon floodplains. Hydrobiologia 2009, 636, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Curran, P.J.; Dungan, J.L.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll content in slash pine. Tree Physiol. 1990, 7, 33–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noomen, M.F.; van der Werff, H.M.A.; van der Meer, F.D. Spectral and spatial indicators of botanical changes caused by long-term hydrocarbon seepage. Ecol. Inform. 2012, 8, 55–64. [Google Scholar] [CrossRef]
- Robson, D.B.; Knight, J.D.; Farrell, R.E.; Germida, J.J. Natural revegetation of hydrocarbon-contaminated soil in semi-arid grasslands. Can. J. Bot. 2004, 82, 22–30. [Google Scholar] [CrossRef]
- Arellano, P.; Tansey, K.; Balzter, H.; Tellkamp, M. Plant Family-Specific Impacts of Petroleum Pollution on Biodiversity and Leaf Chlorophyll Content in the Amazon Rainforest of Ecuador. PLoS ONE 2017, 12, e0169867. [Google Scholar] [CrossRef] [PubMed]
- Behl, S.; Donval, A.; Stibor, H. The relative importance of species diversity and functional group diversity on carbon uptake in phytoplankton communities. Limnol. Oceanogr. 2011, 56, 683–694. [Google Scholar] [CrossRef] [Green Version]
- Clark, M.L.; Roberts, D.A. Species-Level Differences in Hyperspectral Metrics among Tropical Rainforest Trees as Determined by a Tree-Based Classifier. Remote Sens. 2012, 4, 1820–1855. [Google Scholar] [CrossRef]
- Rapport, D.J.; Regier, H.A.; Hutchinson, T.C. Ecosystem Behavior Under Stress. Am. Nat. 1985, 125, 617–640. [Google Scholar] [CrossRef]
- Ogbo, M.E.; Zibigha, M.; Odogu, G. The Effect of Crude Oil on Growth of the Weed (Paspalum scrobiculatum L.) -Phytoremediation Potential of the Plant. Afr. J. Environ. Sci. Technol. 2009, 3, 229–233. [Google Scholar]
- Chima, U.D.; Vure, G. Implications of crude oil pollution on natural regeneration of plant species in an oil-producing community in the Niger Delta Region of Nigeria. J. For. Res. 2014, 25, 915–921. [Google Scholar] [CrossRef]
- Lin, Q.; Mendelssohn, I.A. Impacts and Recovery of the Deepwater Horizon Oil Spill on Vegetation Structure and Function of Coastal Salt Marshes in the Northern Gulf of Mexico. Environ. Sci. Technol. 2012, 46, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Kinako, P.D.S. Short-term effects of oil pollution on species numbers and productivity of a simple terrestrial ecosystem. Environ. Pollut. Ser. A Ecol. Biol. 1981, 26, 87–91. [Google Scholar] [CrossRef]
- Baruah, D.; Sarma, S.K. Impact of crude oil pollution on species number and live standing herbaceous crop biomass. Environmentalist 1996, 16, 291–295. [Google Scholar] [CrossRef]
- Tanee, F.B.G.; Albert, E. Reconnaissance Assessment of Long-Term Effects of Crude Oil Spill on Soil Chemical Properties and Plant Composition at Kwawa, Ogoni, Nigeria. J. Environ. Sci. Technol. 2015, 8, 320–329. [Google Scholar] [CrossRef]
- Orji, F. Bioremediation of petroleum hydrocarbon-polluted mangrove swamps using nutrient formula produced from water hyacint (eicchornia crassipes). Am. J. Environ. Sci. 2013, 9, 348–366. [Google Scholar] [CrossRef]
- Hejda, M.; PyÅ¡ek, P.; JaroÅ¡Ãk, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef] [Green Version]
- COP 6 Decision VI/26: Strategic Plans for the CBD. Available online: http://www.cbd.int/2010-target/about.shtml (accessed on 14 October 2014).













| Procedure | Purpose | Module/Tool | Software |
|---|---|---|---|
| Image subset | Focus on study area and reduce processing time | Region of Interest (ROI) | ENVI 5.3 |
| Atmospheric correction | Removal of atmospheric interference to retrieve surface reflectance. | Fast Line-of-sight Atmospheric Analysis of Hypercubes (FLAASH) | ENVI 5.3 [100] |
| Removal of smile effect | Enhance retrieval of surface reflectance | Cross-Track Illumination Correction (CTIC) | ENVI 5.3 [101,102] |
| Noise reduction and Destripping | Maximize signal to noise ratio (SNR) and minimise data dimensionality | Minimum Noise Fraction Transformation (MNFT) | ENVI 5.3 [103,104] |
| Feature | Landsat 8-OLI | Sentinel 2A |
|---|---|---|
| Product ID | LC08_L1TP_188057_20160104_20170404_ 01_T1_LC81880572016004LGN02 | S2A_OPER_MSI_L1C_TL_SGS__ 20151222T100120_20151222T151903_ A002607_T32NKL_N02_01_01 |
| Bands | 9 | 13 |
| Spectral | 0.435–1.384 | 0.44–2.22 |
| Spatial | 30 m | 10 m, 20 m, 60 m |
| Radiometric | 16 bits | 12 Bits |
| Temporal | 16 days | 10 days |
| Sensor | Operational Land Imager (OLI) | Multispectral Instrument (MSI) |
| Type | Multispectral | Multispectral |
| Satellite | Landsat 8 | Sentinel-2A |
| Mission | Landsat Program | Sentinel |
| Operator | U.S. Geological Survey (USGS) | European Space Agency (ESA) |
| Index | Formula | Reference |
|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | NIR − Red/NIR + Red | [119] |
| Red-Edge NDVI (RENDVI) | (R750 − R705)/(R750 + R705) | [120] |
| Modified Red-Edge NDVI (MRENDVI) | (R750 − R705)/(R750 + R705 − 2 x R445) | [121] |
| Modified Red-Edge Simple Ratio Index (MRESRI) | (R750 − R445)/(R705 + R445) | [122] |
| Vogelmann Red Edge Index 1 (VREI1) | R740/R720 | [123] |
| Photochemical Reflectance Index (PRI) | (R531 − R570)/(R531 + R570) | [124] |
| Structure Insensitive Pigment Index (SIPI) | (R800 − R445)/(R800 + R445) | [125] |
| Model ID | Regression Method | Predictors |
|---|---|---|
| 1A | Partial Least Squares (PLS) | NDVVIs |
| 1B | ,, | NBVIs |
| 2A | Non-Parametric Multivariate Regression (NPM) | NDVVIs |
| 2B | ,, | NBVIs |
| Transect A | Transect B | Transect C | Transect D | P2 | Transect C1 | Transect C2 | Transect C3 | |
|---|---|---|---|---|---|---|---|---|
| Transect B | 0.62 | |||||||
| Transect C | 0.53 | 0.68 | ||||||
| Transect D | 0.55 | 0.64 | 0.72 | |||||
| P2 | 0.18 | 0.31 | 0.25 | 0.37 | ||||
| Transect C1 | 0.57 | 0.55 | 0.58 | 0.69 | 0.32 | |||
| Transect C2 | 0.57 | 0.51 | 0.58 | 0.62 | 0.22 | 0.88 | ||
| Transect C3 | 0.50 | 0.51 | 0.53 | 0.62 | 0.32 | 0.86 | 0.78 | |
| Transect C4 | 0.51 | 0.51 | 0.54 | 0.63 | 0.32 | 0.87 | 0.76 | 0.97 |
| Index | Polluted (n1) | Control (n2) | Confidence Interval (95%) | M-W U | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Taxa | 14 | 37 | 17 | 26 | 406 |
| Shannon’s | 2.64 | 3.61 | 0.7 | 1.47 | 406 |
| Simpson’s | 0.93 | 0.97 | 0.03 | 0.09 | 406 |
| Menhinick’s | 3.74 | 6.08 | 1.75 | 3.1 | 406 |
| Log10Chao-1 | 2.02 | 2.85 | 0.59 | 1.22 | 406 |
| Abundance | 1.25 | 1.45 | 0.01 | 0.48 | 329.5 |
| Maximum-Difference Bands | 9 | 10 | 28 | 8 | 30 |
| Wavelength | 436.99 | 447.17 | 630.32 | 426.8 | 650 |
| Difference | 195.96 | 170.87 | 123.1 | 122.48 | 102.25 |
| Minimum-Difference Bands | 49 | 46 | 40 | 47 | 38 |
| Wavelength | 844 | 813.48 | 752.43 | 823.65 | 732 |
| Difference | 1.79 | 3.49 | 3.67 | 4.11 | 6.22 |
| Stress-Insensitive Bands | 49 | 46 | 47 | 40 | 42 |
| Wavelength (nm) | 844 | 813.48 | 823.65 | 752.43 | 772.78 |
| Sensitivity | 0.00087 | 0.0018 | 0.002 | 0.002 | 0.0034 |
| Stress-Sensitive Bands | 10 | 9 | 8 | 28 | 29 |
| Wavelength (nm) | 447.17 | 436.99 | 426.8 | 630.32 | 640.5 |
| Sensitivity | 0.77 | 0.68 | 0.48 | 0.19 | 0.16 |
| Wavelength | Polluted (n1) | Control (n2) | Difference (n1–n2) | Confidence Interval (95%) | M-W U | |
|---|---|---|---|---|---|---|
| N = 17 | N = 16 | Lower Limit | Upper Limit | |||
| 426.8 nm | 372.43 | 238.83 | 118.56 | 75.47 | 164.8 | 406 |
| 436.99 nm | 453.35 | 270.36 | 175.62 | 134.52 | 237.25 | 421 |
| 447.17 nm | 392.74 | 221.87 | 164.2 | 110.1 | 219.4 | 407 |
| 630.32 nm | 763.24 | 640.14 | 124.82 | 91.36 | 154.47 | 420 |
| 640.5 nm | 725.66 | 624.38 | 115.71 | 83.26 | 140.94 | 402 |
| 650 nm | 740.05 | 637.8 | 114.28 | 59.6 | 154.48 | 396 |
| PLS | NPM | ||||||
|---|---|---|---|---|---|---|---|
| Response | Components Selected | R2 | PRESS | F | p | R2 | RSE |
| NDVVIs | |||||||
| Shannon’s | 2 | 0.67 | 12.3 | 17.56 | <0.05 | 0.71 | 0.61 |
| Simpson’s | 2 | 0.66 | 1.11 | 16.25 | <0.05 | 0.69 | 0.17 |
| Menhinick’s | 1 | 0.54 | 44 | 20.82 | <0.05 | 0.61 | 1.23 |
| Log(Chao-1) | 2 | 0.6 | 8.69 | 12.75 | <0.05 | 0.69 | 0.49 |
| Canopy Chlorophyll | 2 | 0.56 | 2181 | 10.92 | <0.05 | 0.58 | 9.08 |
| NBVIs | |||||||
| Shannon’s | 3 | 0.39 | 25.28 | 3.38 | <0.05 | 0.49 | 0.82 |
| Simpson’s | 1 | 0.3 | 1.71 | 8.23 | <0.05 | 0.46 | 0.23 |
| Menhinick’s | 4 | 0.48 | 67.78 | 3.54 | <0.05 | 0.58 | 1.31 |
| Log(Chao-1) | 3 | 0.50 | 11.74 | 5.35 | <0.05 | 0.55 | 0.58 |
| Canopy Chlorophyll | 1 | 0.11 | 4355 | 2.12 | ns | 0.59 | 8.89 |
| Response Variable | Model | F | p | R2 | RSE | RMSE | Bias |
|---|---|---|---|---|---|---|---|
| Shannon’s Diversity Index | 1A | 12.82 | <0.05 | 0.54 | 0.51 | 0.69 | −11.4 |
| 1B | 1.77 | ns | 0.14 | 0.69 | 0.9 | −16.2 | |
| 2A | 13.08 | <0.05 | 0.54 | 0.5 | 0.5 | −6.2 | |
| 2B | 2.67 | ns | 0.2 | 0.67 | 0.94 | −17.2 | |
| Simpson’s Diversity Index | 1A | 6.66 | <0.05 | 0.38 | 0.05 | 0.24 | −15.9 |
| 1B | 0.11 | ns | 0.01 | 0.07 | 0.22 | −18.1 | |
| 2A | 1.163 | 0.3 | 0.1 | 0.07 | 0.14 | −9.2 | |
| 2B | 0.09 | ns | 0.01 | 0.07 | 0.21 | −14.9 | |
| Menhinick’s Richness Index | 1A | 14.32 | <0.05 | 0.57 | 1.15 | 1.13 | −7.5 |
| 1B | 6.37 | <0.05 | 0.37 | 1.38 | 1.58 | −21.6 | |
| 2A | 5.35 | <0.05 | 0.33 | 1.42 | 1.32 | 1 | |
| 2B | 7.4 | <0.05 | 0.4 | 1.34 | 1.31 | −10.2 | |
| Log (Chao-1) | 1A | 8.55 | <0.05 | 0.44 | 0.24 | 0.57 | 3.3 |
| 1B | 2.12 | ns | 0.16 | 0.3 | 0.58 | −1.1 | |
| 2A | 10.16 | <0.05 | 0.48 | 0.23 | 0.56 | 2.1 | |
| 2B | 1.93 | ns | 0.15 | 0.3 | 0.51 | 3.2 | |
| Canopy Chlorophyll Content | 1A | 7.89 | <0.05 | 0.42 | 7.85 | 7.87 | 6.7 |
| 1B | 1.14 | ns | 0.09 | 9.79 | 9.29 | 4.7 | |
| 2A | 10.49 | <0.05 | 0.49 | 7.36 | 7.59 | 5.5 | |
| 2B | 1.64 | ns | 0.13 | 9.6 | 13.49 | 9.9 |
| Land Cover Type | Farmland | Forested | Mixed | Swamp | Waterbody |
|---|---|---|---|---|---|
| N | 7 | 5 | 10 | 6 | 2 |
| L8-NDVI | 0.17 | 0.2 | 0.18 | 0.13 | 0.11 |
| S2A-NDVI | 0.11 | 0.12 | 0.11 | 0.05 | 0.06 |
| NDVVI844,447 | 0.48 | 0.57 | 0.49 | 0.29 | 0.27 |
| NDVVI814,437 | 0.73 | 0.83 | 0.76 | 0.61 | 0.57 |
| NDVVI824,427 | 0.5 | 0.58 | 0.51 | 0.3 | 0.28 |
| NDVVI752,630 | 0.44 | 0.53 | 0.46 | 0.25 | 0.23 |
| NDVVI773,641 | 0.71 | 0.80 | 0.73 | 0.56 | 0.53 |
| NDVVI844,630 | 0.85 | 0.94 | 0.88 | 0.73 | 0.7 |
| Shannon’s | 2.59 | 3.42 | 2.77 | 0.88 | 0.94 |
| Simpson’s | 0.82 | 0.95 | 0.87 | 0.25 | 0.3 |
| Menhinick’s | 3.64 | 5.34 | 4.13 | 1.41 | 1.16 |
| LogChao-1 | 2.16 | 2.79 | 2.4 | 1 | 1.06 |
| Canopy Chlorophyll | 56.12 | 64.8 | 56.46 | 39.57 | 34.09 |
| Diversity Index | NDVILandsat-8 | NDVISentinel-2A |
|---|---|---|
| Shannon’s | 0.77 | 0.78 |
| Simpson’s | 0.73 | 0.75 |
| Menhinick’s | 0.78 | 0.79 |
| Chao-1 | 0.84 | 0.84 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyia, N.N.; Balzter, H.; Berrio, J.-C. Normalized Difference Vegetation Vigour Index: A New Remote Sensing Approach to Biodiversity Monitoring in Oil Polluted Regions. Remote Sens. 2018, 10, 897. https://doi.org/10.3390/rs10060897
Onyia NN, Balzter H, Berrio J-C. Normalized Difference Vegetation Vigour Index: A New Remote Sensing Approach to Biodiversity Monitoring in Oil Polluted Regions. Remote Sensing. 2018; 10(6):897. https://doi.org/10.3390/rs10060897
Chicago/Turabian StyleOnyia, Nkeiruka Nneti, Heiko Balzter, and Juan-Carlos Berrio. 2018. "Normalized Difference Vegetation Vigour Index: A New Remote Sensing Approach to Biodiversity Monitoring in Oil Polluted Regions" Remote Sensing 10, no. 6: 897. https://doi.org/10.3390/rs10060897
APA StyleOnyia, N. N., Balzter, H., & Berrio, J.-C. (2018). Normalized Difference Vegetation Vigour Index: A New Remote Sensing Approach to Biodiversity Monitoring in Oil Polluted Regions. Remote Sensing, 10(6), 897. https://doi.org/10.3390/rs10060897