Assessment of Portable Chlorophyll Meters for Measuring Crop Leaf Chlorophyll Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theoretical Basis of Portable Chlorophyll Meters
2.2. Leaf Chlorophyll Concentration Measurement
2.3. Statistical Analysis
2.4. Sensitivity Analysis
3. Results
3.1. Variability of LChl
3.2. Correlation among Leaf Pigment Concentrations
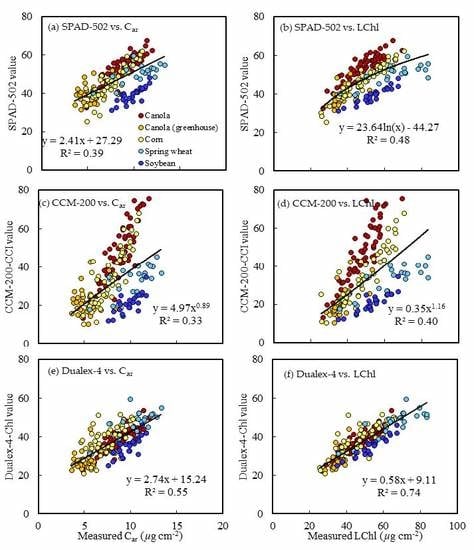
3.3. Estimation of Pigment Concentration from PCM Readings
3.4. Relationship of Meter Reading Averages and Deviations
3.5.1. Influence of Leaf Parameters
3.5.2. The Influence of Non-Uniform LChl Distribution
4. Discussion
5. Conclusions
- (1)
- SPAD-502 and CCM-200 readings of this study had larger dynamic ranges than Dualex-4 readings. The sources of error for both SPAD-502 and CCM-220 readings increased with increasing LChl, whereas they were relatively stable for Dualex-4;
- (2)
- Relationships between SPAD-502 and CCM-200 readings and the actual LChl were more sensitive to crop type than the relationships between Dualex-4 and LChl;
- (3)
- The sieve effect (caused by the heterogeneity of LChl distribution) would have more influence on PCM readings than the detour effect (caused by leaf parameters, such as leaf pigments and leaf internal structure) does. The ratio of light transmittance between the index and reference bands used in the Dualex-4-Chl was generally better at minimizing the interference factors;
- (4)
- Our results suggest that Dualex-4 is a better choice for collecting LChl measurements for different crops in the field, compared with the SPAD-502 and the CCM-200.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Crop | Types | Mean | CV (%) a | Range (Min–Max) b | |
|---|---|---|---|---|---|
| Canola (Field, n = 57) | Chlorophyll meter | SPAD-502 | 53.9 | 12.5 | 32.8–67.8 |
| CCM-200-CCI | 47.2 | 32.1 | 13.4–75.8 | ||
| Dualex-4-Chl | 38.7 | 15.8 | 22.3–58.8 | ||
| Dualex-4-Flav | 1.7 | 13.3 | 1.1–2.0 | ||
| Dualex-4-Anth | 0.1 | 50.4 | 0.0–0.1 | ||
| Dualex-4-NBI | 24.1 | 23.7 | 11.6–36.6 | ||
| Lab chemical measurement | Car (µg cm−2) | 8.7 | 18.7 | 4.8–12.0 | |
| Chla (µg cm−2) | 36.4 | 18.4 | 20.0–52.6 | ||
| Chlb (µg cm−2) | 12.0 | 19.2 | 6.4–16.6 | ||
| Chla/Chlb ratio | 3.1 | 6.6 | 2.7–3.8 | ||
| LChl (µg cm−2) | 48.6 | 18.4 | 26.4–69.2 | ||
| Canola (Greenhouse, n = 41) | Chlorophyll meter | SPAD-502 | 41.1 | 13.4 | 31.8–53.6 |
| CCM-200-CCI | 23.0 | 34.3 | 8.7–46.9 | ||
| Dualex-4-Chl | 31.2 | 21.1 | 22.8–52.9 | ||
| Dualex-4-Flav | 0.3 | 19.3 | 0.2–0.5 | ||
| Dualex-4-Anth | 0.1 | 31.9 | 0.0–0.1 | ||
| Dualex-4-NBI | 90.7 | 12.2 | 65.6–122.5 | ||
| Lab chemical measurement | Car (µg cm−2) | 5.3 | 19.4 | 3.6–8.3 | |
| Chla (µg cm−2) | 27.6 | 14.9 | 19.9–40.2 | ||
| Chlb (µg cm−2) | 9.6 | 16.6 | 7.8–15.9 | ||
| Chla/Chlb ratio | 2.9 | 9.5 | 2.2–3.3 | ||
| LChl (µg cm−2) | 30.3 | 37.0 | 11.4–53.2 | ||
| Corn (n = 52) | Chlorophyll meter | SPAD-502 | 46.7 | 18.4 | 25.5–62.2 |
| CCM-200-CCI | 34.5 | 42.6 | 20.4–68.2 | ||
| Dualex-4-Chl | 39.2 | 20.9 | 21.1–52.4 | ||
| Dualex-4-Flav | 1.4 | 22.4 | 0.7–1.8 | ||
| Dualex-4-Anth | 0.1 | 33.4 | 0.0–0.2 | ||
| Dualex-4-NBI | 30.3 | 29.5 | 12.4–53.8 | ||
| Lab chemical measurement | Car (µg cm−2) | 7.7 | 22.1 | 4.8–10.9 | |
| Chla (µg cm−2) | 38.6 | 24.1 | 20.8–56.5 | ||
| Chlb (µg cm−2) | 9.7 | 24.0 | 4.6–14.0 | ||
| Chla/Chlb ratio | 4.0 | 5.8 | 3.2–4.6 | ||
| LChl (µg cm−2) | 49.0 | 25.1 | 25.6–70.5 | ||
| Soybean (n = 25) | Chlorophyll meter | SPAD-502 | 39.3 | 10.2 | 31.4–48.3 |
| CCM-200-CCI | 21.1 | 23.8 | 12.1–34.7 | ||
| Dualex-4-Chl | 35.3 | 14.2 | 25.0–46.2 | ||
| Dualex-4-Flav | 1.47 | 8.2 | 1.2–1.7 | ||
| Dualex-4-Anth | 0.1 | 40.7 | 0.0–0.1 | ||
| Dualex-4-NBI | 24.1 | 12.4 | 16.8–29.9 | ||
| Lab chemical measurement | Car (µg cm−2) | 9.8 | 11.5 | 7.6–11.6 | |
| Chla (µg cm−2) | 41.3 | 15.3 | 27.2–51.8 | ||
| Chlb (µg cm−2) | 12.2 | 15.9 | 7.7–15.9 | ||
| Chla/Chlb ratio | 3.4 | 4.4 | 3.0–3.8 | ||
| LChl (µg cm−2) | 53.2 | 15.4 | 34.9–67.3 | ||
| Spring wheat (n = 20) | Chlorophyll meter | SPAD-502 | 50.8 | 10.8 | 39.7–59.6 |
| CCM-200-CCI | 34.5 | 23.2 | 17.7–47.5 | ||
| Dualex-4-Chl | 47.8 | 16.9 | 31.2–61.0 | ||
| Dualex-4-Flav | 1.2 | 8.2 | 1.1–1.5 | ||
| Dualex-4-Anth | 0.1 | 48.4 | 0.0–0.1 | ||
| Dualex-4-NBI | 38.9 | 19.6 | 25.1–56.6 | ||
| Lab chemical measurement | Car (µg cm−2) | 10.5 | 18.9 | 5.5–13.3 | |
| Chla (µg cm−2) | 49.8 | 20.6 | 26.3–62.8 | ||
| Chlb (µg cm−2) | 15.5 | 22.9 | 9.2–21.1 | ||
| Chla/Chlb ratio | 3.2 | 6.3 | 2.9–3.6 | ||
| LChl (µg cm−2) | 64.4 | 27.0 | 32.3–97.8 |
References
- Corti, M.; Cavalli, D.; Cabassi, G.; Marino Gallina, P.; Bechini, L. Does remote and proximal optical sensing successfully estimate maize variables? A review. Eur. J. Agron. 2018, 99, 37–50. [Google Scholar] [CrossRef]
- Padilla, F.M.; Gallardo, M.; Peña-Fleitas, M.T.; De Souza, R.; Thompson, R.B. Proximal optical sensors for nitrogen management of vegetable crops: A review. Sensor 2018, 18, 2083. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Shang, J.; Liu, J.; Qian, B.; Jing, Q.; Ma, B.; Huffman, T.; Geng, X.; Sow, A.; Shi, Y.; et al. Using RapidEye imagery to identify within-field variability of crop growth and yield in Ontario, Canada. Precis. Agric. 2019, 20, 1231–1250. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M. Leaf Pigment Content. In Comprehensive Remote Sensing; Liang, S., Ed.; Elsevier: Oxford, UK, 2018; pp. 117–142. [Google Scholar]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Houlès, V.; Guérif, M.; Mary, B. Elaboration of a nitrogen nutrition indicator for winter wheat based on leaf area index and chlorophyll content for making nitrogen recommendations. Eur. J. Agron. 2007, 27, 1–11. [Google Scholar] [CrossRef]
- Houborg, R.; Cescatti, A.; Migliavacca, M.; Kustas, W. Satellite retrievals of leaf chlorophyll and photosynthetic capacity for improved modeling of GPP. Agric. For. Meteorol. 2013, 177, 10–23. [Google Scholar] [CrossRef]
- Houborg, R.; McCabe, M.F.; Cescatti, A.; Gitelson, A.A. Leaf chlorophyll constraint on model simulated gross primary productivity in agricultural systems. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 160–176. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Peng, Y.; Viña, A.; Arkebauer, T.; Schepers, J.S. Efficiency of chlorophyll in gross primary productivity: A proof of concept and application in crops. J. Plant Physiol. 2016, 201, 101–110. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Y.-B.; Lyapustin, A.I.; Wang, Y.; Gao, F.; Suyker, A.; Verma, S.; Middleton, E.M. Estimation of crop gross primary production (GPP): fAPARchl versus MOD15A2 FPAR. Remote Sens. Environ. 2014, 153, 1–6. [Google Scholar] [CrossRef]
- Escobar-Gutiérrez, A.J.; Combe, L. Senescence in field-grown maize: From flowering to harvest. Field Crop. Res. 2012, 134, 47–58. [Google Scholar] [CrossRef]
- Shah, S.H.; Houborg, R.; McCabe, M.F. Response of Chlorophyll, Carotenoid and SPAD-502 Measurement to Salinity and Nutrient Stress in Wheat (Triticum aestivum L.). Agronomy 2017, 7, 61. [Google Scholar] [CrossRef]
- Daughtry, C.; Walthall, C.; Kim, M.; De Colstoun, E.B.; McMurtrey, J. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Féret, J.-B.; Gitelson, A.; Noble, S.; Jacquemoud, S. PROSPECT-D: Towards modeling leaf optical properties through a complete lifecycle. Remote Sens. Environ. 2017, 193, 204–215. [Google Scholar] [CrossRef]
- Houborg, R.; McCabe, M.F. Adapting a regularized canopy reflectance model (REGFLEC) for the retrieval challenges of dryland agricultural systems. Remote Sens. Environ. 2016, 186, 105–120. [Google Scholar] [CrossRef]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P. Sentinel-2: ESA’s optical high-resolution mission for GMES operational services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Shang, J.; Liu, J.; Ma, B.; Zhao, T.; Jiao, X.; Geng, X.; Huffman, T.; Kovacs, J.M.; Walters, D. Mapping spatial variability of crop growth conditions using RapidEye data in Northern Ontario, Canada. Remote Sens. Environ. 2015, 168, 113–125. [Google Scholar] [CrossRef]
- Transon, J.; d’Andrimont, R.; Maugnard, A.; Defourny, P. Survey of hyperspectral earth observation applications from space in the sentinel-2 context. Remote Sens. 2018, 10, 157. [Google Scholar] [CrossRef]
- Aasen, H.; Honkavaara, E.; Lucieer, A.; Zarco-Tejada, P.J. Quantitative Remote Sensing at Ultra-High Resolution with UAV Spectroscopy: A Review of Sensor Technology, Measurement Procedures, and Data Correction Workflows. Remote Sens. 2018, 10, 1091. [Google Scholar] [CrossRef]
- Castelli, F.; Contillo, R.; Miceli, F. Non-destructive Determination of Leaf Chlorophyll Content in Four Crop Species. J. Agron. Crop Sci. 1996, 177, 275–283. [Google Scholar] [CrossRef]
- Markwell, J.; Osterman, J.C.; Mitchell, J.L. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth. Res. 1995, 46, 467–472. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 603, 142–196. [Google Scholar] [CrossRef]
- Minocha, R.; Martinez, G.; Lyons, B.; Long, S. Development of a standardized methodology for quantifying total chlorophyll and carotenoids from foliage of hardwood and conifer tree species. Can. J. For. Res. 2009, 39, 849–861. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Gitelson, A.; Lang, M. Non-destructive determination of chlorophyll content of leaves of a green and an aurea mutant of tobacco by reflectance measurements. J. Plant Physiol. 1996, 148, 483–493. [Google Scholar] [CrossRef]
- Datt, B. Remote Sensing of Chlorophyll a, Chlorophyll b, Chlorophyll a+b, and Total Carotenoid Content in Eucalyptus Leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Ciganda, V.; Gitelson, A.; Schepers, J. Non-destructive determination of maize leaf and canopy chlorophyll content. J. Plant Physiol. 2009, 166, 157–167. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C. A comparison of two techniques for nondestructive measurement of chlorophyll content in grapevine leaves. Agron. J. 2008, 100, 779–782. [Google Scholar] [CrossRef]
- Casa, R.; Castaldi, F.; Pascucci, S.; Pignatti, S. Chlorophyll estimation in field crops: An assessment of handheld leaf meters and spectral reflectance measurements. J. Agric. Sci. 2015, 153, 876–890. [Google Scholar] [CrossRef]
- Marenco, R.A.; Antezana-Vera, S.A.; Nascimento, H.C.S. Relationship between specific leaf area, leaf thickness, leaf water content and SPAD-502 readings in six Amazonian tree species. Photosynthetica 2009, 47, 184–190. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Féret, J.-B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.; Ustin, S.L.; Le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Grasso, R.; Gallardo, M.; Thompson, R.B. Influence of time of day on measurement with chlorophyll meters and canopy reflectance sensors of different crop N status. Precis. Agric. 2019, 20, 1087–1106. [Google Scholar] [CrossRef] [Green Version]
- Higa, T.; Wada, M. Chloroplast avoidance movement is not functional in plants grown under strong sunlight. PlantCell Environ. 2016, 39, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef]
- Parry, C.; Blonquist, J.; Bugbee, B. In situ measurement of leaf chlorophyll concentration: Analysis of the optical/absolute relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef]
- Nauš, J.; Prokopová, J.; Řebíček, J.; Špundová, M. SPAD chlorophyll meter reading can be pronouncedly affected by chloroplast movement. Photosynth. Res. 2010, 105, 265–271. [Google Scholar] [CrossRef]
- McClendon, J.H.; Fukshansky, L. On the interpretation of absorption spectra of leaves-II. The non-absorbed ray of the sieve effect and the mean optical pathlength in the remainder of the leaf. Photochem. Photobiol. 1990, 51, 211–216. [Google Scholar] [CrossRef]
- McClendon, J.H.; Fukshansky, L. On the interpretation of absorption spectra of leaves–I. Introduction and the correction of leaf spectra for surface reflection. Photochem. Photobiol. 1990, 51, 203–210. [Google Scholar] [CrossRef]
- Baránková, B.; Lazár, D.; Nauš, J. Analysis of the effect of chloroplast arrangement on optical properties of green tobacco leaves. Remote Sens. Environ. 2016, 174, 181–196. [Google Scholar] [CrossRef]
- Barton, C.V. A theoretical analysis of the influence of heterogeneity in chlorophyll distribution on leaf reflectance. Tree Physiol. 2001, 21, 789–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef] [Green Version]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef]
- Overbeck, V.; Schmitz, M.; Tartachnyk, I.; Blanke, M. Identification of light availability in different sweet cherry orchards under cover by using non-destructive measurements with a Dualex™. Eur. J. Agron. 2018, 93, 50–56. [Google Scholar] [CrossRef]
- Meyer, S.; Cerovic, Z.G.; Goulas, Y.; Montpied, P.; Demotes-Mainard, S.; Bidel, L.P.R.; Moya, I.; Dreyer, E. Relationships between optically assessed polyphenols and chlorophyll contents, and leaf mass per area ratio in woody plants: A signature of the carbon–nitrogen balance within leaves? Plant Cell Environ. 2006, 29, 1338–1348. [Google Scholar] [CrossRef]
- Schepers, J.S.; Blackmer, T.M.; Wilhelm, W.W.; Resende, M. Transmittance and Reflectance Measurements of CornLeaves from Plants with Different Nitrogen and Water Supply. J. Plant Physiol. 1996, 148, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Apogee Instruments, I. CCM-200 plus Chlorophyll Meter, Product Manual; Apogee Instruments, Inc.: Logan, UT, USA, 2011. [Google Scholar]
- Konica, M. Spad 502 Plus Chlorophyll Meter, Product Manual; Konica Minolta: Chiyoda, Japan, 2011. [Google Scholar]
- Raymond Hunt, E.; Daughtry, C.S. Chlorophyll meter calibrations for chlorophyll content using measured and simulated leaf transmittances. Agron. J. 2014, 106, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.; Liu, J.; Shang, J.; Qian, B.; Ma, B.; Kovacs, J.M.; Walters, D.; Jiao, X.; Geng, X.; Shi, Y. Assessment of red-edge vegetation indices for crop leaf area index estimation. Remote Sens. Environ. 2019, 222, 133–143. [Google Scholar] [CrossRef]
- Liu, J.; Pattey, E.; Jégo, G. Assessment of vegetation indices for regional crop green LAI estimation from Landsat images over multiple growing seasons. Remote Sens. Environ. 2012, 123, 347–358. [Google Scholar] [CrossRef]
- Houborg, R.; Anderson, M.; Daughtry, C. Utility of an image-based canopy reflectance modeling tool for remote estimation of LAI and leaf chlorophyll content at the field scale. Remote Sens. Environ. 2009, 113, 259–274. [Google Scholar] [CrossRef]
- Fritschi, F.B.; Ray, J.D. Soybean leaf nitrogen, chlorophyll content, and chlorophyll a/b ratio. Photosynthetica 2007, 45, 92–98. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT+ SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Serrano, L. Effects of leaf structure on reflectance estimates of chlorophyll content. Int. J. Remote Sens. 2008, 29, 5265–5274. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer: Berlin, Germany, 2003. [Google Scholar]
- Kouril, R.; Ilík, P.; Naus, J.; Schoefs, B. On the limits of applicability of spectrophotometric and spectrofluorimetric methods for the determination of chlorophyll a/b ratio. Photosynth. Res. 1999, 62, 107–116. [Google Scholar] [CrossRef]
- Gitelson, A.; Solovchenko, A. Non-invasive quantification of foliar pigments: Possibilities and limitations of reflectance- and absorbance-based approaches. J. Photochem. Photobiol. B Biol. 2018, 178, 537–544. [Google Scholar] [CrossRef]
- Dong, T.; Liu, J.; Qian, B.; Jing, Q.; Croft, H.; Chen, J.; Wang, J.; Huffman, T.; Shang, J.; Chen, P. Deriving maximum light use efficiency from crop growth model and satellite data to improve crop biomass estimation. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2016, 10, 104–117. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Fei, P.; Song, J.; Li, D.; Ge, C.; Chen, W. Responses of rice leaf thickness, SPAD readings and chlorophyll a/b ratios to different nitrogen supply rates in paddy field. Field Crop. Res. 2009, 114, 426–432. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N. Signature analysis of leaf reflectance spectra: Algorithm development for remote sensing of chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Gallardo, M.; Giménez, C.; Thompson, R.B. Different responses of various chlorophyll meters to increasing nitrogen supply in sweet pepper. Front. Plant Sci. 2018, 9, 1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, P.A.; Caylor, S.; Whippo, C.W.; Hangarter, R.P. Changes in leaf optical properties associated with light-dependent chloroplast movements. PlantCell Environ. 2011, 34, 2047–2059. [Google Scholar] [CrossRef] [PubMed]
- Netto, A.T.; Campostrini, E.; Oliveira, J.G.d.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Stuckens, J.; Verstraeten, W.W.; Delalieux, S.; Swennen, R.; Coppin, P. A dorsiventral leaf radiative transfer model: Development, validation and improved model inversion techniques. Remote Sens. Environ. 2009, 113, 2560–2573. [Google Scholar] [CrossRef]







| Crop Type | Seeding Date | May 30 | June 16 | June 21 | July 07 | August 04 | Total | |
|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Corn | May 18 | - | - | 19 | 18 | 18 | 55 |
| Soybean | May 12 | 6 | 9 | 10 | 25 | |||
| Spring wheat | April 27 | - | - | 7 | 9 | 6 | 23 | |
| Canola | May 6 | 30 | 27 | Harvested | 57 | |||
| Experiment 2 (greenhouse) | Canola | May 2 | 30 | 30 | - | - | - | 60 |
| Total | - | 30 | 30 | 62 | 64 | 34 | 220 |
| Variable | Constant | Range | Step | Reference |
|---|---|---|---|---|
| Leaf structure parameter, Ns | 1.55 | 1.0–2.8 | 0.2 | [35] |
| Leaf chlorophyll concentration, LChl (µg cm−2) | 48.39 | 10–80 | 5 | Field collection |
| Leaf carotenoid concentration, Car (µg cm−2) | 8.04 | 3.6–12.6 | 1.0 | Field collection |
| Leaf water concentration, Cw (g cm−2) | 0.0113 | 0.004–0.04 | 0.004 | [35] |
| Leaf dry matter concentration, Cm (g cm−2) | 0.0053 | 0.0017–0.0137 | 0.00133 | [35] |
| Leaf anthocyanin concentration, Canth (µg cm−2) | 1.0 | 0–14.0 | 1.4 | [14] |
| Leaf brown pigment, Cbp | 0.0 | - | - | [55] |
| LChl | Types | Mean | CV (%) a | Min b | Max b |
|---|---|---|---|---|---|
| Portable chlorophyll meter | SPAD-502 | 46.6 | 18.3 | 25.5 | 67.8 |
| CCM-200-CCI | 33.1 | 46.3 | 8.7 | 75.8 | |
| Dualex-4-Chl | 37.4 | 22.6 | 22.1 | 61.0 | |
| Dualex-4-Flav | 1.2 | 42.5 | 0.2 | 2.0 | |
| Dualex-4-Anth | 0.1 | 46.3 | 0.0 | 0.2 | |
| Dualex-4-NBI | 41.1 | 65.3 | 11.6 | 122.5 | |
| Lab chemical measurement | Car (µg cm−2) | 8.0 | 28.0 | 3.6 | 13.3 |
| Chla (µg cm−2) | 37.1 | 26.0 | 19.9 | 62.8 | |
| Chlb (µg cm−2) | 11.3 | 25.9 | 4.6 | 21.1 | |
| Chla/Chlb ratio | 3.3 | 14.4 | 2.2 | 4.6 | |
| LChl (µg cm−2) | 48.4 | 25.3 | 25.6 | 83.6 |
| Handheld Chlorophyll Meter | Leaf Chlorophyll Concentration | Leaf Carotenoid Concentration | |||||
|---|---|---|---|---|---|---|---|
| Regression | R2 | RMSE | Regression | R2 | RMSE | ||
| Canola | SPAD-502 | y = 0.88x + 1.55 | 0.77 | 4.51 | y = 0.21x − 2.78 | 0.80 | 0.99 |
| CCM-200-CCI | y = 0.45x + 27.25 | 0.75 | 5.93 | y = 0.11x + 3.30 | 0.78 | 1.30 | |
| Dualex-4-Chl | y = 1.14x + 4.28 | 0.83 | 3.86 | y = 0.26x − 1.52 | 0.75 | 1.11 | |
| Corn | SPAD-502 | y = 1.31x − 13.02 | 0.90 | 3.68 | y = 0.17x − 0.47 | 0.74 | 0.93 |
| CCM-200-CCI | y = 0.72x + 23.18 | 0.81 | 5.04 | y = 0.10x + 4.30 | 0.68 | 0.99 | |
| Dualex-4-Chl | y = 1.21x + 0.37 | 0.69 | 6.33 | y = 0.14x + 1.94 | 0.46 | 1.27 | |
| Soybean | SPAD-502 | y = 1.91x − 21.71 | 0.88 | 2.79 | y = 0.23x + 0.71 | 0.68 | 0.63 |
| CCM-200-CCI | y = 8.53x0.60 | 0.84 | 3.44 | y = 3.22x0.37 | 0.59 | 0.72 | |
| Dualex-4-Chl | y = 1.55x − 1.20 | 0.90 | 2.56 | y = 0.18x + 3.45 | 0.64 | 0.66 | |
| Spring wheat | SPAD-502 | y = 10.71e0.04x | 0.66 | 8.88 | y = 2.54e0.03x | 0.48 | 1.48 |
| CCM-200-CCI | y = 4.58x0.76 | 0.74 | 7.57 | y = 1.20x0.62 | 0.58 | 1.28 | |
| Dualex-4-Chl | y =1.52x − 5.57 | 0.72 | 5.12 | y = 0.19x + 1.76 | 0.52 | 1.01 | |
| All crops | SPAD-502 | y = 18.29e0.02x | 0.48 | 9.31 | y = 0.16x + 0.47 | 0.39 | 1.75 |
| CCM-200-CCI | y = 14.49x0.34 | 0.40 | 10.12 | y = 2.18x0.37 | 0.33 | 1.88 | |
| Dualex-4-Chl | y = 1.27x + 1.11 | 0.74 | 6.25 | y = 0.20x + 0.51 | 0.55 | 1.48 | |
| Ns | LChl | Car | Cw | Cm | Canth | Interactions | ||
|---|---|---|---|---|---|---|---|---|
| Index band | T650 (SPAD-502) | 11.36 | 79.73 | 0.00 | 0.00 | 0.01 | 0.00 | 8.90 |
| T653 (CCM-200) | 12.00 | 79.31 | 0.00 | 0.00 | 0.02 | 0.00 | 8.67 | |
| T710 (Dualex-4) | 42.57 | 55.96 | 0.00 | 0.00 | 0.33 | 0.00 | 1.14 | |
| Reference band | T940 (SPAD-502) | 95.74 | 0.00 | 0.00 | 0.35 | 3.53 | 0.00 | 0.38 |
| T931 (CCM-200) | 95.72 | 0.00 | 0.00 | 0.38 | 3.52 | 0.00 | 0.38 | |
| T850 (Dualex-4) | 95.85 | 0.00 | 0.00 | 0.01 | 3.75 | 0.00 | 0.39 | |
| Ratio | T940/T650 | 11.37 | 74.13 | 0.00 | 0.01 | 0.05 | 0.00 | 14.44 |
| Log(T940/T650) (SPAD-502) | 7.50 | 91.68 | 0.00 | 0.01 | 0.03 | 0.00 | 0.78 | |
| (T931/T653) (CCM-200) | 10.81 | 79.96 | 0.00 | 0.02 | 0.05 | 0.00 | 9.16 | |
| (T850/T710) (Dualex-4) | 10.91 | 84.39 | 0.00 | 0.00 | 0.17 | 0.00 | 4.53 |
© 2019 by Crown Copyright. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, T.; Shang, J.; Chen, J.M.; Liu, J.; Qian, B.; Ma, B.; Morrison, M.J.; Zhang, C.; Liu, Y.; Shi, Y.; et al. Assessment of Portable Chlorophyll Meters for Measuring Crop Leaf Chlorophyll Concentration. Remote Sens. 2019, 11, 2706. https://doi.org/10.3390/rs11222706
Dong T, Shang J, Chen JM, Liu J, Qian B, Ma B, Morrison MJ, Zhang C, Liu Y, Shi Y, et al. Assessment of Portable Chlorophyll Meters for Measuring Crop Leaf Chlorophyll Concentration. Remote Sensing. 2019; 11(22):2706. https://doi.org/10.3390/rs11222706
Chicago/Turabian StyleDong, Taifeng, Jiali Shang, Jing M. Chen, Jiangui Liu, Budong Qian, Baoluo Ma, Malcolm J. Morrison, Chao Zhang, Yupeng Liu, Yichao Shi, and et al. 2019. "Assessment of Portable Chlorophyll Meters for Measuring Crop Leaf Chlorophyll Concentration" Remote Sensing 11, no. 22: 2706. https://doi.org/10.3390/rs11222706
APA StyleDong, T., Shang, J., Chen, J. M., Liu, J., Qian, B., Ma, B., Morrison, M. J., Zhang, C., Liu, Y., Shi, Y., Pan, H., & Zhou, G. (2019). Assessment of Portable Chlorophyll Meters for Measuring Crop Leaf Chlorophyll Concentration. Remote Sensing, 11(22), 2706. https://doi.org/10.3390/rs11222706