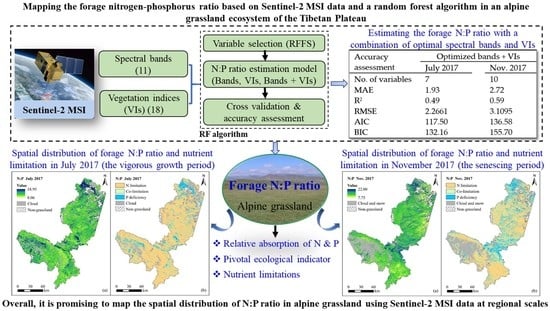
Mapping the Forage Nitrogen-Phosphorus Ratio Based on Sentinel-2 MSI Data and a Random Forest Algorithm in an Alpine Grassland Ecosystem of the Tibetan Plateau
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Grassland Observational Data
2.3. Chemical Analysis
2.4. Sentinel-2 MSI Data and Processing
2.5. Spectral Bands and Vegetation Indices
2.6. Random Forest
2.7. Variable Selection and Cross-Validation
2.8. Accuracy Assessment
3. Results
3.1. Variation in the Forage N:P Ratio
3.2. Predicting the Forage N:P Ratio with Spectral Bands
3.3. Predicting the Forage N:P Ratio with Sentinel-2 Vegetation Indices
3.4. Predicting the Forage N:P Ratio with a Combination of Spectral Bands and Vegetation Indices
3.5. Mapping of Potential Forage N and P Limitation
4. Discussion
4.1. Potential of Sentinel-2 Spectral Bands and Vegetation Indices in Estimating the Forage N:P Ratio
4.2. Effects of Different Seasons on Forage N:P Inversion in Natural Alpine Grasslands
4.3. Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blackburn, G.A. Spectral indices for estimating photosynthetic pigment concentrations: A test using senescent tree leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Ullah, S.; Si, Y.L.; Schlerf, M.; Skidmore, A.K.; Shafique, M.; Iqbal, I.K. Estimation of grassland biomass and nitrogen using MERIS data. Int. J. Appl. Earth Obs. Geoinf. 2012, 19, 196–204. [Google Scholar] [CrossRef]
- Richardson, A.J.; Everitt, J.H.; Gausman, H.W. Radiometric estimation of biomass and nitrogen content of alicia grass. Remote Sens. Environ. 1983, 13, 179–184. [Google Scholar] [CrossRef]
- Ramoelo, A.; Cho, M.A.; Mathieu, R.; Madonsela, S.; Van De Kerchove, R.; Kaszta, Z.; Wolff, E. Monitoring grass nutrients and biomass as indicators of rangeland quality and quantity using random forest modelling and WorldView-2 data. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 43–54. [Google Scholar] [CrossRef]
- Walker, A.P.; Beckerman, A.P.; Gu, L.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The relationship of leaf photosynthetic traits–vcmax and Jmax–to leaf nitrogen, leaf phosphorus, and specific leaf area: A meta-analysis and modeling study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.L.; Meng, B.P.; Liang, T.G.; Feng, Q.S.; Ge, J.; Yin, J.P.; Wu, C.X.; Cui, X.; Hou, M.J.; Liu, J.; et al. Modeling alpine grassland forage phosphorus based on hyperspectral remote sensing and a multi-factor machine learning algorithm in the east of Tibetan Plateau. China. ISPRS J. Photogramm. Remote Sens. 2019, 147, 104–117. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Olde Venterink, H.; Wassen, M.J.; Verkroost, A.W.M.; De Ruiter, P.C. Species richness-productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 2003, 84, 2191–2199. [Google Scholar] [CrossRef]
- He, J.S.; Wang, L.; Flynn, D.F.B.; Wang, X.P.; Ma, W.H.; Fang, J.Y. Leaf nitrogen:phosphorus stoichiometry across Chinese grassland biomes. Oecologia 2008, 155, 301–310. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar] [CrossRef]
- Ramoelo, A.; Skidmore, A.K.; Schlerf, M.; Heitkonig, I.M.; Mathieu, R.; Cho, M.A. Savanna grass nitrogen to phosphorous ratio estimation using field spectroscopy and the potential for estimation with imaging spectroscopy. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 334–343. [Google Scholar] [CrossRef]
- Wassen, M.J.; Venterink, H.O.; Lapshina, E.D.; Tanneberger, F. Endangered plants persist under phosphorus limitation. Nature 2005, 437, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Venterink, H.O.; van Bodegom, P.M.; Douma, J.C.; Heil, G.W.; Holzel, N.; Jablonska, E.; Kotowski, W.; Okruszko, T.; Pawlikowski, P.; et al. Low investment in sexual reproduction threatens plants adapted to phosphorus limitation. Nature 2014, 505, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Mutanga, O.; Dube, T.; Ahmed, F. Progress in remote sensing: Vegetation monitoring in South Africa. S. Afr. Geogr. J. 2016, 98, 461–471. [Google Scholar] [CrossRef]
- Ma, W.Y.; Wang, X.M. Progress on grassland chlorophyll content estimation by hyperspectral analysis. Prog. Geogr. 2016, 35, 25–34. [Google Scholar]
- Skidmore, A.K. Taxonomy of environmental models in the spatial sciences. In Environmental Modelling with GIS and Remote Sensing; Skidmore, A.K., Ed.; Taylor and Francis: London, UK, 2002; pp. 2–7. [Google Scholar]
- Wang, Z.H.; Skidmore, A.K.; Wang, T.J.; Darvishzadeh, R.; Hearne, J. Applicability of the PROSPECT model for estimating protein and cellulose + lignin in fresh leaves. Remote Sens. Environ. 2015, 168, 205–218. [Google Scholar] [CrossRef]
- Penuelas, J.; Gamon, J.A.; Freeden, A.L.; Merino, J.; Field, C. Reflectance indices associated with physiological changes in nitrogen and water-limited sunflower leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Mutanga, O. Hyperspectral Remote Sensing of Tropical Grass Quality and Quantity. Ph.D. Thesis, International Institute for Geoinformation Science and Earth Observation and Wageningen University, Wageningen, The Netherlands, 7 April 2004. [Google Scholar]
- Filella, I.; Penuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]
- Danson, F.M.; Plummer, S.E. Red edge response to forest leaf area index. Int. J. Remote Sens. 1995, 16, 183–188. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Macler, B.A.; Plummer, S.E. The effect of a Red leaf pigment on the relationship between red edge and chlorophyll concentration. Remote Sens. Environ. 1991, 35, 69–76. [Google Scholar] [CrossRef]
- Ramoelo, A. Savanna Grass Quality-Remote Sensing Estimation from Local to Regional Scale. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2012. [Google Scholar]
- Gao, J.L.; Liang, T.G.; Liu, J.; Yin, J.P.; Ge, J.; Hou, M.J.; Feng, Q.S.; Wu, C.X.; Xie, H.J. Potential of hyperspectral data and machine learning algorithms to estimate the forage carbon-nitrogen ratio in an alpine grassland ecosystem of the Tibetan Plateau. ISPRS J. Photogramm. Remote Sens. 2020, 163, 362–374. [Google Scholar] [CrossRef]
- Gökkaya, K.; Thomas, V.; Noland, T.; McCaughey, H.; Morrison, I.; Treitz, P. Mapping continuous forest type variation by means of correlating remotely sensed metrics to canopy N:P ratio in a boreal mixed wood forest. Appl. Veg. Sci. 2015, 18, 143–157. [Google Scholar] [CrossRef]
- Loozen, Y.; Karssenberg, D.; de Jong, S.M.; Wang, S.Q.; van Dijk, J.; Wassen, M.J.; Rebel, K.T. Exploring the use of vegetation indices to sense canopy nitrogen to phosphorous ratio in grasses. Int. J. Appl. Earth Obs. Geoinf. 2019, 75, 1–14. [Google Scholar] [CrossRef]
- Tong, Q.X.; Xue, Y.Q.; Zhang, L.F. Progress in hyperspectral remote sensing science and technology in China over the past three decades. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 7, 70–91. [Google Scholar] [CrossRef]
- Lehnert, L.W.; Meyer, H.; Wang, Y.; Miehe, G.; Thies, B.; Reudenbach, C.; Bendic, J. Retrieval of grassland plant coverage on the Tibetan Plateau based on a multiscale, multi-sensor and multi-method approach. Remote Sens. Environ. 2015, 164, 197–207. [Google Scholar] [CrossRef]
- Yang, S.X.; Feng, Q.S.; Liang, T.G.; Liu, B.K.; Zhang, W.J.; Xie, H.J. Modeling grassland above-ground biomass based on artificial neural network and remote sensing in the Three-River Headwaters Region. Remote Sens. Environ. 2017, 204, 448–455. [Google Scholar] [CrossRef]
- Meng, B.P.; Gao, J.L.; Liang, T.G.; Cui, X.; Ge, J.; Yin, J.P.; Feng, Q.S.; Xie, H.J. Modeling of alpine grassland cover based on unmanned aerial vehicle technology and multi-factor methods: A case study in the east of Tibetan Plateau, China. Remote Sens. 2018, 10, 320. [Google Scholar] [CrossRef] [Green Version]
- Ramoelo, A.; Skidmore, A.K.; Cho, M.A.; Schlerf, M.; Mathieu, R.; Heitkonig, I.M. Regional estimation of savanna grass nitrogen using the red-edge band of the spaceborne RapidEye sensor. Int. J. Appl. Earth Obs. Geoinf. 2012, 19, 151–162. [Google Scholar] [CrossRef]
- Shoko, C.; Mutanga, O. Examining the strength of the newly-launched Sentinel 2 MSI sensor in detecting and discriminating subtle differences between C3 and C4 grass species. ISPRS J. Photogramm. Remote Sens. 2017, 129, 32–40. [Google Scholar] [CrossRef]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahan, J.; Rundquist, D. Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Chemura, A.; Mutanga, O.; Odindi, J.; Kutywayo, D. Mapping spatial variability of foliar nitrogen in coffee (Coffea arabica L.) plantations with multispectral Sentinel-2 MSI data. ISPRS J. Photogramm. Remote Sens. 2018, 138, 1–11. [Google Scholar] [CrossRef]
- Singh, L.; Mutanga, O.; Mafongoya, P.; Peerbhay, K.Y. Multispectral mapping of key grassland nutrients in KwaZulu-Natal. S. Afr. J. Spat. Sci. 2018, 63, 155–172. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Gitelson, A.A. Remote estimation of crop and grass chlorophyll and nitrogen content using red-edge bands on sentinel-2 and-3. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Sakowska, K.; Juszczak, R.; Gianelle, D. Remote sensing of grassland biophysical parameters in the context of the Sentinel-2 satellite mission. J. Sens. 2016, 2016, 4612809. [Google Scholar] [CrossRef] [Green Version]
- Rouse, J.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium, Greenbelt, MD, USA, 10–14 December 1973. [Google Scholar]
- Hardisky, M.A.; Klemas, V.; Smart, R.M. The influences of soil salinity, growth form, and leaf moisture on the spectral reflectance of spartina alterniflora canopies. Photogramm. Eng. Remote Sens. 1983, 49, 77–83. [Google Scholar]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident detection of crop water stress, nitrogen status and canopy density using ground-based multispectral data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N. Quantitative estimation of chlorophyll using reflectance spectra. J. Photochem. Photobiol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOSMODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Frampton, W.J.; Dash, J.; Watmough, G.; Milton, E.J. Evaluating the capabilities of Sentinel-2 for quantitative estimation of biophysical variables in vegetation. ISPRS J. Photogramm. Remote Sens. 2013, 82, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Guyot, G.; Baret, F. Utilisation de la haute resolution spectrale pour suivre l’etat des couverts vegetaux. In Proceedings of the 4th International Colloquium Spectral Signatures of Objects in Remote Sensing, Aussois, France, 18–22 January 1988. [Google Scholar]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for nondestructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Lebedev, A.; Westman, E.; Van Westen, G.; Kramberger, M.; Lundervold, A.; Aarsland, D.; Soininen, H.; Kłoszewska, I.; Mecocci, P.; Tsolaki, M. Random Forest ensembles for detection and prediction of Alzheimer’s disease with a good between-cohort robustness. NeuroImage Clin. 2014, 6, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proceedings of the 14th International Joint Conference on Artificial Intelligence, Montreal, QC, Canada, 20–25 August 1995; Volume 2, pp. 1137–1143. [Google Scholar]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In International Symposium on Information Theory; Akademiai, K., Ed.; Springer: New York, NY, USA, 1973; pp. 610–624. [Google Scholar]
- Schwarz, G. Estimating the dimension of a model. Ann. Statist. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Ollinger, S.V.; Richardson, A.D.; Martin, M.E.; Hollinger, D.Y.; Frolking, S.E.; Reich, P.B.; Plourde, L.C.; Katul, G.G.; Munger, J.W.; Oren, R. Canopy nitrogen, carbon assimilation, and albedo in temperate and boreal forests: Functional relations and potential climate feedbacks. Proc. Natl. Acad. Sci. USA 2008, 105, 19336–19341. [Google Scholar] [CrossRef] [Green Version]
- Lepine, L.C.; Ollinger, S.V.; Ouimette, A.P.; Martin, M.E. Examining spectral reflectance features related to foliar nitrogen in forests: Implications for broadscale nitrogen mapping. Remote Sens. Environ. 2016, 173, 174–176. [Google Scholar] [CrossRef]
- Mutanga, O.; Kumar, L. Estimating and mapping grass phosphorus concentration in an African savanna using hyperspectral image data. Int. J. Remote Sens. 2007, 28, 4897–4911. [Google Scholar] [CrossRef]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Knox, N. Observing Temporal and Spatial Variability of Forage Quality. Ph.D. Thesis, Faculty Geo-Information Science and Earth Observation and Twente University, Enschede, The Netherlands, 28 October 2010. [Google Scholar]
- Ramoelo, A.; Skidmore, A.K.; Schlerf, M.; Mathieu, R.; Heitkönig, I.M.A. Water removed spectra increase the retrieval accuracy when estimating savanna grass nitrogen and phosphorus concentrations. ISPRS J. Photogramm. Remote Sens. 2011, 66, 408–417. [Google Scholar] [CrossRef]
- Daufresne, T.; Loreau, M. Plant–herbivore interactions and ecological stoichiometry: When do herbivores determine plant nutrient limitation. Ecol. Lett. 2001, 4, 196–206. [Google Scholar] [CrossRef]
- Elser, J.J.; Dobberfuhl, D.R.; MacKay, N.A.; Schampel, J.H. Organism size, life history, and N:P stoichiometry. BioScience 1996, 46, 674–684. [Google Scholar] [CrossRef] [Green Version]
- Aerts, R. Nutrients resorption from senescing leaves of perennials: Are these general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Wu, K.S.; Fu, H.; Zhang, X.Y.; Niu, D.C.; Ta, L.T. The seasonal dynamics of nutrient contents and the nutrition balanced values in the eight forage species in the Alxa desert grassland. Arid Zone Res. 2010, 2, 257–262. [Google Scholar] [CrossRef]
- Chen, J.X. Alpine Meadow Soil Nitrogen Seasonal Dynamics in Eastern Qinghai-Tibetan Plateau. Ph.D. Thesis, Sichuan Normal University, Sichuan, China, 1 May 2007. [Google Scholar]
- Gao, J.L.; Liang, T.G.; Yin, J.P.; Ge, J.; Feng, Q.S.; Wu, C.X.; Hou, M.J.; Liu, J.; Xie, H.J. Estimation of alpine grassland forage nitrogen coupled with hyperspectral characteristics during different growth periods on the Tibetan Plateau. Remote Sens. 2019, 11, 2085. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Ma, Y.L.; Ma, W.H.; Liang, C.Z.; Zhao, X.Q.; Fang, J.Y.; He, J.S. Large scale patterns of forage yield and quality across Chinese grasslands. Chin. Sci. Bull. 2013, 58, 1187–1199. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.X.; Cao, G.M. The nitrogen cycle in an alpine meadow ecosystem. Acta Ecol. Sin. 1999, 19, 509–513. [Google Scholar]
- Yang, X.H.; Guo, X.L. Quantifying responses of spectral vegetation indices to dead materials in mixed grasslands. Remote Sens. 2014, 6, 4289–4304. [Google Scholar] [CrossRef] [Green Version]
- Croft, H.; Chen, J.M.; Zhang, Y. The applicability of empirical vegetation indices for determining leaf chlorophyll content over different leaf and canopy structures. Ecol. Complex. 2014, 17, 119–130. [Google Scholar] [CrossRef]
- Schmidt, K.S.; Skidmore, A.K. Exploring spectral discrimination of grass species in African rangelands. Int. J. Remote Sens. 2001, 22, 3421–3434. [Google Scholar] [CrossRef]
- Cho, M.A.; Skidmore, A.K. A new technique for extracting the red edge position from hyperspectral data: The linear extrapolation method. Remote Sens. Environ. 2006, 101, 181–193. [Google Scholar] [CrossRef]
- Adjorlolo, C.; Mutanga, O.; Cho, M.A. Predicting C3 and C4 grass nutrient variability using in situ canopy reflectance and partial least squares regression. Int. J. Remote Sens. 2015, 36, 1743–1761. [Google Scholar] [CrossRef]
- Cho, M.A.; Debba, P.; Mutanga, O.; Dudeni-Tlhone, N.; Magadla, T.; Khuluse, S.A. Potential utility of the spectral red-edge region of SumbandilaSat imagery for assessing indigenous forest structure and health. Int. J. Appl. Earth Obs. Geoinf. 2012, 16, 85–93. [Google Scholar] [CrossRef]
- Mutanga, O.; Adam, E.; Adjorlolo, C.; Abdel-Rahman, E.M. Evaluating the robustness of models developed from field spectral data in predicting African grass foliar nitrogen concentration using WorldView-2 image as an independent test dataset. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 178–187. [Google Scholar] [CrossRef]
- Hardin, P.J.; Jackson, M.W. An unmanned aerial vehicle for rangeland photography. Rangel. Ecol. Manag. 2005, 58, 439–442. [Google Scholar] [CrossRef]
- Rango, A.; Laliberte, A.; Steele, C.; Herrick, J.E.; Bestelmeyer, B.; Schmugge, T.; Roanhorse, A.; Jenkins, V. Using unmanned aerial vehicles for rangelands: Current applications and future potentials. Environ. Pract. 2006, 8, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Houborg, R.; Soegaard, H.; Boegh, E. Combining vegetation index and model inversion methods for the extraction of key vegetation biophysical parameters using Terra and Aqua MODIS reflectance data. Remote Sens. Environ. 2007, 106, 39–58. [Google Scholar] [CrossRef]
- Le Maire, G.; Francois, C.; Dufrene, E. Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sens. Environ. 2004, 89, 1–28. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C. Inversion of a radiative transfer model for estimating vegetation LAI and chlorophyll in a heterogeneous grassland. Remote Sens. Environ. 2008, 112, 2592–2604. [Google Scholar] [CrossRef]





| Spectral Bands | Band Center (nm) | Bandwidth (nm) | Spatial Resolution (m) | Spectral Region |
|---|---|---|---|---|
| B2 | 490 | 65 | 10 | Blue |
| B3 | 560 | 35 | 10 | Green |
| B4 | 665 | 30 | 10 | Red |
| B5 | 705 | 15 | 20 | Red edge |
| B6 | 740 | 15 | 20 | Red edge |
| B7 | 783 | 20 | 20 | Red edge |
| B8 | 842 | 115 | 10 | NIR |
| B8A | 865 | 20 | 20 | NIR |
| B9 | 945 | 20 | 60 | NIR |
| B11 | 1375 | 30 | 20 | SWIR |
| B12 | 2190 | 180 | 20 | SWIR |
| Index | Name | Formulation | Bands | Reference |
|---|---|---|---|---|
| NDVI | Normalized difference vegetation index | (R842 − R665)/(R842 + R665) | B8, B4 | [38] |
| NDII | Normalized difference infrared index | (R842 − R1610)/(R842 + R1610) | B8, B11 | [39] |
| NDWI | Normalized difference water index | (R865 − R1610)/(R865 + R1610) | B8A, B11 | [40] |
| NDRE1 | Normalized difference red-edge 1 | (R740 − R705)/(R740 + R705) | B6, B5 | [41] |
| NDRE2 | Normalized difference red-edge 2 | (R783 − R705)/(R783 + R705) | B7, B5 | [42] |
| RNDVI | Renormalized normalized difference vegetation index | B8, B4 | [43] | |
| GNDVI | Green normalized difference vegetation index | (R865 − R560)/(R865 + R560) | B8A, B3 | [44] |
| EVI | Enhanced vegetation index | 2.5 × (R865 − R665)/(1 + R865 + 6 × R665 − 7.5 × R490) | B8A, B4, B2 | [45] |
| REP1 | Red-edge position 1 | 705 + 35 × {[(R842 + R665) × 0.5 − R705]/(R740 − R705)} | B8, B4, B5, B6 | [46] |
| REP2 | Red-edge position 2 | 705 + 35 × {[(R783 + R665) × 0.5 − R705]/(R740 − R705)} | B7, B4, B5, B6 | [47] |
| MTCI | MERRIS terrestrial chlorophyll index | (R842 − R705)/(R705 − R665) | B8, B5, B4 | [48] |
| IRECI | Inverted red-edge chlorophyll index | (R842 − R665)/(R740/R705) | B8, B4, B6, B5 | [46] |
| GCI 1 | Green chlorophyll index 1 | (R842/R560) − 1 | B8, B3 | [49] |
| GCI 2 | Green chlorophyll index 2 | (R783/R560) − 1 | B7, B3 | [50] |
| GCI 3 | Green chlorophyll index 3 | (R865/R560) − 1 | B8A, B3 | [50] |
| RECI 1 | Red-edge chlorophyll index 1 | (R842/R705) − 1 | B8, B5 | [49] |
| RECI 2 | Red-edge chlorophyll index 2 | (R865/R705) − 1 | B8A, B5 | [50] |
| WDRVI | Wide dynamic range vegetation index | (0.1 × R865 − R665)/(0.1 × R865 + R665) | B8A, B4 | [51] |
| Nutrient | Data Sets | Min | Max | Mean | Median | STDEV | CV (%) | SE | No. of Samples |
|---|---|---|---|---|---|---|---|---|---|
| N | July 2017 | 1.15 | 2.78 | 1.87 | 1.85 | 0.30 | 16 | 0.04 | 66 |
| Nov. 2017 | 0.32 | 1.41 | 0.80 | 0.80 | 0.19 | 23 | 0.02 | 57 | |
| P | July 2017 | 0.09 | 0.29 | 0.17 | 0.17 | 0.04 | 26 | 0.01 | 66 |
| Nov. 2017 | 0.03 | 0.13 | 0.06 | 0.06 | 0.02 | 32 | 0.00 | 57 | |
| N:P | July 2017 | 6.3 | 21.8 | 12.0 | 12.0 | 3.3 | 27 | 0.4 | 66 |
| Nov. 2017 | 5.2 | 24.8 | 14.1 | 13.6 | 4.4 | 31 | 0.6 | 57 |
| Periods | Nutrient | N | P | N:P |
|---|---|---|---|---|
| July 2017 | N | / | p = 0.1647 | p = 0.0005 |
| P | r = 0.17 | / | p = 0.0000 | |
| N:P | r = 0.42 | r = −0.74 | / | |
| Nov. 2017 | N | / | p = 0.0154 | p = 0.0000 |
| P | r = 0.32 | / | p = 0.0000 | |
| N:P | r = 0.51 | r = −0.59 | / |
| Accuracy Assessment | July 2017 | Nov. 2017 | ||
|---|---|---|---|---|
| All Bands | Optimized Bands | All Bands | Optimized Bands | |
| No. of variables | 11 | 3 | 11 | 5 |
| MAE | 2.19 | 2.1 | 3.08 | 2.67 |
| R2 | 0.41 | 0.43 | 0.54 | 0.55 |
| RMSE | 2.5433 | 2.4783 | 3.5299 | 3.1352 |
| AIC | 138.36 | 121.29 | 151.19 | 127.09 |
| BIC | 161.39 | 127.58 | 172.23 | 136.65 |
| Accuracy Assessment | July 2017 | Nov. 2017 | ||
|---|---|---|---|---|
| All VIs | Optimized VIs | All VIs | Optimized VIs | |
| No. of variables | 18 | 4 | 18 | 5 |
| MAE | 2.06 | 2.02 | 3.46 | 2.94 |
| R2 | 0.42 | 0.43 | 0.41 | 0.42 |
| RMSE | 2.4592 | 2.4756 | 4.0742 | 3.4881 |
| AIC | 146.93 | 118.68 | 180.48 | 137.60 |
| BIC | 184.63 | 127.06 | 214.9 | 147.16 |
| Accuracy Assessment | Optimized Bands + VIs | |
|---|---|---|
| July 2017 | Nov. 2017 | |
| No. of variables | 7 | 10 |
| MAE | 1.93 | 2.72 |
| R2 | 0.49 | 0.59 |
| RMSE | 2.2661 | 3.1095 |
| AIC | 117.50 | 136.58 |
| BIC | 132.16 | 155.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Liu, J.; Liang, T.; Hou, M.; Ge, J.; Feng, Q.; Wu, C.; Li, W. Mapping the Forage Nitrogen-Phosphorus Ratio Based on Sentinel-2 MSI Data and a Random Forest Algorithm in an Alpine Grassland Ecosystem of the Tibetan Plateau. Remote Sens. 2020, 12, 2929. https://doi.org/10.3390/rs12182929
Gao J, Liu J, Liang T, Hou M, Ge J, Feng Q, Wu C, Li W. Mapping the Forage Nitrogen-Phosphorus Ratio Based on Sentinel-2 MSI Data and a Random Forest Algorithm in an Alpine Grassland Ecosystem of the Tibetan Plateau. Remote Sensing. 2020; 12(18):2929. https://doi.org/10.3390/rs12182929
Chicago/Turabian StyleGao, Jinlong, Jie Liu, Tiangang Liang, Mengjing Hou, Jing Ge, Qisheng Feng, Caixia Wu, and Wenlong Li. 2020. "Mapping the Forage Nitrogen-Phosphorus Ratio Based on Sentinel-2 MSI Data and a Random Forest Algorithm in an Alpine Grassland Ecosystem of the Tibetan Plateau" Remote Sensing 12, no. 18: 2929. https://doi.org/10.3390/rs12182929
APA StyleGao, J., Liu, J., Liang, T., Hou, M., Ge, J., Feng, Q., Wu, C., & Li, W. (2020). Mapping the Forage Nitrogen-Phosphorus Ratio Based on Sentinel-2 MSI Data and a Random Forest Algorithm in an Alpine Grassland Ecosystem of the Tibetan Plateau. Remote Sensing, 12(18), 2929. https://doi.org/10.3390/rs12182929