Monitoring Wheat Leaf Rust and Stripe Rust in Winter Wheat Using High-Resolution UAV-Based Red-Green-Blue Imagery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Disease Monitoring
2.2. High-Resolution UAV Imagery
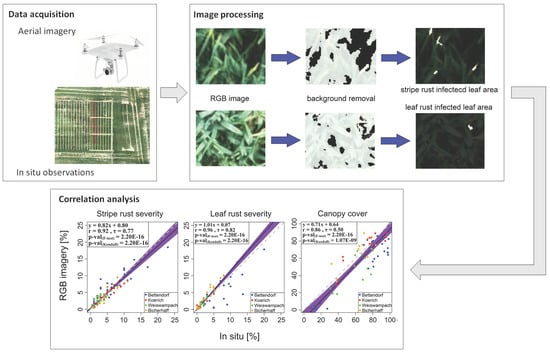
2.3. UAV-Based Estimation of Canopy Cover, Wheat Stripe Rust and Leaf Rust Severities
2.4. Evaluating the RGB-Derived Indices Using Independent Datasets
2.5. Data Analyses
3. Results and Discussion
3.1. Disease Levels During the 2018–2019 Cropping Season
3.2. Variability of RGB Imagery-Derived Wheat Leaf Rust and Stripe Severities and Canopy Cover
3.3. Relationships between Observations and RGB Imagery-Derived Values
3.4. Evaluating the RGB Imagery-Derived Indices Using Independent Multispectral Data
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. World Food Situation—FAO Cereal Supply and Demand Brief (Release Date: 03/09/2020); Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2020. [Google Scholar]
- USDA. World Agricultural Production. Circular Series WAP 9–20. Spetember 2020; United States Department of Agriculture (USDA) Foreign Agricultural Service, Gobal Market Analysis: Washington, DC, USA, 2020.
- Kolmer, J.A. Tracking wheat rust on a continental scale. Curr. Opin. Plant Biol. 2005, 8, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Hovmøller, M.S.; Walter, S.; Justesen, A.F. Escalating threat of wheat rusts. Science 2010, 329, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Jarroudi, M.; Kouadio, L.; Giraud, F.; Delfosse, P.; Tychon, B. Brown rust disease control in winter wheat: II. Exploring the optimization of fungicide sprays through a decision support system. Environ. Sci. Pollut. Res. 2014, 21, 4809–4818. [Google Scholar] [CrossRef] [Green Version]
- Beddow, J.M.; Pardey, P.G.; Chai, Y.; Hurley, T.M.; Kriticos, D.J.; Braun, H.-J.; Park, R.F.; Cuddy, W.S.; Yonow, T. Research investment implications of shifts in the global geography of wheat stripe rust. Nat. Plants 2015, 1, 15132. [Google Scholar] [CrossRef] [PubMed]
- El Jarroudi, M.; Kouadio, L.; Beyer, M.; Junk, J.; Hoffmann, L.; Tychon, B.; Maraite, H.; Bock, C.H.; Delfosse, P. Economics of a decision–support system for managing the main fungal diseases of winter wheat in the Grand-Duchy of Luxembourg. Field Crops Res. 2015, 172, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Rodriguez-Algaba, J.; Thach, T.; Sørensen, C.K.; Hansen, J.G.; Lassen, P.; Nazari, K.; Hodson, D.P.; Justesen, A.F.; Hovmøller, M.S. Yellow rust epidemics worldwide were caused by pathogen races from divergent genetic lineages. Front. Plant Sci. 2017, 8, 1057. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Espino, J.; Singh, R.P.; German, S.; McCallum, B.D.; Park, R.F.; Chen, W.; Bhardwaj, S.C.; Goyeau, H. Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar]
- Freier, B.; Boller, E.F. Integrated Pest Management in Europe—History, Policy, Achievements and Implementation. In Integrated Pest Management: Dissemination and Impact; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 435–454. [Google Scholar]
- Mohanty, S.P.; Hughes, D.P.; Salathé, M. Using Deep Learning for Image-based plant disease detection. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Bock, C.H.; Poole, G.H.; Parker, P.E.; Gottwald, T.R. Plant disease severity estimated visually, by digital photography and image analysis, and by hyperspectral imaging. Crit. Rev. Plant Sci. 2010, 29, 59–107. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Kouadio, A.L.; Mackels, C.; Tychon, B.; Delfosse, P.; Bock, C.H. A comparison between visual estimates and image analysis measurements to determine Septoria leaf blotch severity in winter wheat. Plant Pathol. 2014, 355–364. [Google Scholar] [CrossRef]
- Moshou, D.; Bravo, C.; West, J.; Wahlen, S.; McCartney, A.; Ramon, H. Automatic detection of ‘yellow rust’ in wheat using reflectance measurements and neural networks. Comp. Electron. Agric. 2004, 44, 173–188. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J.M. The application of small unmanned aerial systems for precision agriculture: A review. Precision Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Bohnenkamp, D.; Behmann, J.; Mahlein, A.-K. In-field detection of yellow rust in wheat on the ground canopy and UAV scale. Remote Sens. 2019, 11, 2495. [Google Scholar] [CrossRef] [Green Version]
- Boulent, J.; Foucher, S.; Théau, J.; St-Charles, P.-L. Convolutional Neural Networks for the automatic identification of plant diseases. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Dang, L.M.; Wang, H.; Li, Y.; Min, K.; Kwak, J.T.; Lee, O.N.; Park, H.; Moon, H. Fusarium wilt of radish detection using RGB and near infrared images from Unmanned Aerial Vehicles. Remote Sens. 2020, 12, 2863. [Google Scholar] [CrossRef]
- Singh, A.; Jones, S.; Ganapathysubramanian, B.; Sarkar, S.; Mueller, D.; Sandhu, K.; Nagasubramanian, K. Challenges and opportunities in machine-augmented plant stress phenotyping. Trends Plant Sci. 2020. [Google Scholar] [CrossRef]
- Franke, J.; Menz, G.; Oerke, E.-C.; Rascher, U. Comparison of multi- and hyperspectral imaging data of leaf rust infected wheat plants. In Remote Sensing for Agriculture, Ecosystems, and Hydrology VII.; SPIE: Bellingham, WA, USA, 2005; Volume 5976. [Google Scholar] [CrossRef]
- Su, J.; Liu, C.; Coombes, M.; Hu, X.; Wang, C.; Xu, X.; Li, Q.; Guo, L.; Chen, W.-H. Wheat yellow rust monitoring by learning from multispectral UAV aerial imagery. Comp. Electron. Agric. 2018, 155, 157–166. [Google Scholar] [CrossRef]
- Moshou, D.; Bravo, C.; Wahlen, S.; West, J.; McCartney, A.; De Baerdemaeker, J.; Ramon, H. Simultaneous identification of plant stresses and diseases in arable crops using proximal optical sensing and self-organising maps. Precision Agric. 2006, 7, 149–164. [Google Scholar] [CrossRef]
- Huang, W.; Lamb, D.W.; Niu, Z.; Zhang, Y.; Liu, L.; Wang, J. Identification of yellow rust in wheat using in-situ spectral reflectance measurements and airborne hyperspectral imaging. Precision Agric. 2007, 8, 187–197. [Google Scholar] [CrossRef]
- Moshou, D.; Bravo, C.; Oberti, R.; West, J.; Bodria, L.; McCartney, A.; Ramon, H. Plant disease detection based on data fusion of hyper-spectral and multi-spectral fluorescence imaging using Kohonen maps. Real-Time Imaging 2005, 11, 75–83. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Maja, J.M.; Ehsani, R. Visible-near infrared spectroscopy for detection of Huanglongbing in citrus orchards. Comp. Electron. Agric. 2011, 77, 127–134. [Google Scholar] [CrossRef]
- Bravo, C.; Moshou, D.; West, J.; McCartney, A.; Ramon, H. Early disease detection in wheat fields using spectral reflectance. Biosyst. Eng. 2003, 84, 137–145. [Google Scholar] [CrossRef]
- Whetton, R.L.; Waine, T.W.; Mouazen, A.M. Hyperspectral measurements of yellow rust and fusarium head blight in cereal crops: Part 2: On-line field measurement. Biosyst. Eng. 2018, 167, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Mahlein, A.K.; Rumpf, T.; Welke, P.; Dehne, H.W.; Plümer, L.; Steiner, U.; Oerke, E.C. Development of spectral indices for detecting and identifying plant diseases. Remote Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Das, P.K.; Laxman, B.; Rao, S.V.C.K.; Seshasai, M.V.R.; Dadhwal, V.K. Monitoring of bacterial leaf blight in rice using ground-based hyperspectral and LISS IV satellite data in Kurnool, Andhra Pradesh, India. Int. J. Pest Manag. 2015, 61, 359–368. [Google Scholar] [CrossRef]
- Heidarian Dehkordi, R.; Burgeon, V.; Fouche, J.; Placencia Gomez, E.; Cornelis, J.-T.; Nguyen, F.; Denis, A.; Meersmans, J. Using UAV collected RGB and multispectral images to evaluate winter wheat performance across a site characterized by century-old biochar patches in Belgium. Remote Sens. 2020, 12, 2504. [Google Scholar] [CrossRef]
- Heidarian Dehkordi, R.; Denis, A.; Fouche, J.; Burgeon, V.; Cornelis, J.T.; Tychon, B.; Placencia Gomez, E.; Meersmans, J. Remotely-sensed assessment of the impact of century-old biochar on chicory crop growth using high-resolution UAV-based imagery. Int. J. Appl. Earth. Obs. Geoinf. 2020, 91, 102147. [Google Scholar] [CrossRef]
- Dam, D.; Pallez-Barthel, M.; El Jarroudi, M.; Eickermann, M.; Beyer, M. The debate on a loss of biodiversity: Can we derive evidence from the monitoring of major plant pests and diseases in major crops? J. Plant Dis. Prot. 2020. [Google Scholar] [CrossRef]
- James, C.A. An illustrated series of assessment keys for plant diseases, their preparation and usage. Can. Plant Dis. Surv. 1971, 51, 39–65. [Google Scholar]
- Tomerlin, J.R.; Howell, A. DISTRAIN: A computer program for training people to estimate disease severity on cereal leaves. Plant Dis. 1988, 72, 455–459. [Google Scholar]
- BSA. Beschreibende Sortenliste 2018. Getreide, Mais, Ölfrüchte, Leguminosen (Großkörnig) Hackfrüchte (Außer Kartoffeln); Deutscher Landwirtschaftsverlag GmbH.: Hannover, Germany, 2016. [Google Scholar]
- Hou, Y.-C. Visual cryptography for color images. Pattern Recognit. 2003, 36, 1619–1629. [Google Scholar] [CrossRef]
- Liu, M.; Yu, T.; Gu, X.; Sun, Z.; Yang, J.; Zhang, Z.; Mi, X.; Cao, W.; Li, J. The impact of spatial resolution on the classification of vegetation types in highly fragmented planting areas based on unmanned aerial vehicle hyperspectral images. Remote Sens. 2020, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Lovell, D.J.; Parker, S.R.; Hunter, T.; Royle, D.J.; Coker, R.R. Influence of crop growth and structure on the risk of epidemics by Mycosphaerella graminicola (Septoria tritici) in winter wheat. Plant Pathol. 1997, 46, 126–138. [Google Scholar] [CrossRef]
- Lovell, D.J.; Parker, S.R.; Hunter, T.; Welham, S.J.; Nichols, A.R. Position of inoculum in the canopy affects the risk of septoria tritici blotch epidemics in winter wheat. Plant Pathol. 2004, 53, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Kendall, M.G. Partial rank correlation. Biometrika 1942, 32, 277–283. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- El Jarroudi, M.; Kouadio, L.; Bertrand, M.; Curnel, Y.; Giraud, F.; Delfosse, P.; Hoffmann, L.; Oger, R.; Tychon, B. Integrating the impact of wheat fungal diseases in the Belgian crop yield forecasting system (B-CYFS). Eur. J. Agron. 2012, 40, 8–17. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Kouadio, L.; Bock, C.H.; El Jarroudi, M.; Junk, J.; Pasquali, M.; Maraite, H.; Delfosse, P. A threshold-based weather model for predicting stripe rust infection in winter wheat. Plant Dis. 2017, 101, 693–703. [Google Scholar] [CrossRef] [Green Version]
- Junk, J.; Kouadio, L.; Delfosse, P.; El Jarroudi, M. Effects of regional climate change on brown rust disease in winter wheat. Clim. Chang. 2016, 135, 439–451. [Google Scholar] [CrossRef] [Green Version]
- El Jarroudi, M.; Kouadio, L.; Delfosse, P.; Tychon, B. Brown rust disease control in winter wheat: I. Exploring an approach for disease progression based on night weather conditions. Environ. Sci. Pollut. Res. 2014, 21, 4797–4808. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Moreno, V.M.; Jiménez-Lagunes, A.; Estrada-Avalos, J.; Mauricio-Ruvalcaba, J.E.; Padilla-Ramírez, J.S. Weather-data-based model: An approach for forecasting leaf and stripe rust on winter wheat. Meteorol. Appl. 2020, 27, e1896. [Google Scholar] [CrossRef] [Green Version]
- Gooding, M.J.; Dimmock, J.P.R.E.; France, J.; Jones, S.A. Green leaf area decline of wheat flag leaves: The influence of fungicides and relationships with mean grain weight and grain yield. Ann. Appl. Biol. 2000, 136, 77–84. [Google Scholar] [CrossRef]
- Adams, M.L.; Philpot, W.D.; Norvell, W.A. Yellowness index: An application of spectral second derivatives to estimate chlorosis of leaves in stressed vegetation. Int. J. Remote Sens. 1999, 20, 3663–3675. [Google Scholar] [CrossRef]









| Location | Cultivars | Sowing Date | Disease Susceptibility 1 | Treatment 2 | Growth Stage (GS) at Fungicide Application | Fungicide Mixture 3 | |
|---|---|---|---|---|---|---|---|
| WSR | WLR | ||||||
| Bicherhaff | Kerubino | 18 October 2018 | 7 | 5 | Control | no fungicide treatment | |
| T1 | GS43 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | |||||
| T2 | GS37 | 0.75 L ha−1 Opus team® + 1.00 L ha−1 Bravo® | |||||
| GS53 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | ||||||
| T3 | GS37 | 0.75 L ha−1 Opus team® + 1.00 L ha−1 Bravo® | |||||
| GS40 | 1.50 L ha−1 Fandango® + 1.00 L ha−1 Bravo® | ||||||
| GS53 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | ||||||
| Bettendorf | Desamo Kerubino | 24 October 2018 | 2 7 | 2 5 | Control | no fungicide treatment | |
| T1 | GS49 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | |||||
| T2 | GS32 | 0.75 L ha−1 Opus team® + 1.00 L ha−1 Bravo® | |||||
| GS59 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | ||||||
| T3 | GS32 | 0.75 L ha−1 Opus team® + 1.00 L ha−1 Bravo® | |||||
| GS37 | 1.50 L ha−1 Fandango® + 1.00 L ha−1 Bravo® | ||||||
| GS65 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | ||||||
| Koerich | Kerubino | 16 October 2018 | 7 | 5 | Control | no fungicide treatment | |
| T1 | GS50 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | |||||
| T2 | GS32 | 0.75 L ha−1 Opus team® + 1.00 L ha−1 Bravo® | |||||
| GS53 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | ||||||
| T3 | GS32 | 0.75 L ha−1 Opus team® + 1.00 L ha−1 Bravo® | |||||
| GS37 | 1.50 L ha−1 Fandango® + 1.00 L ha−1 Bravo® | ||||||
| GS50 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | ||||||
| Weiswampach | Kerubino | 12 October 2018 | 7 | 5 | Control | no fungicide treatment | |
| T1 | GS49 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | |||||
| T2 | GS32 | 0.75 L ha−1 Opus team® + 1.00 L ha−1 Bravo® | |||||
| GS59 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | ||||||
| T3 | GS32 | 0.75 l ha-1 Opus team® + 1.00 L ha−1 Bravo® | |||||
| GS37 | 1.50 L ha−1 Fandango® + 1.00 L ha−1 Bravo® | ||||||
| GS59 | 1.50 L ha−1 Swing Gold® + 1.00 L ha−1 Bravo® | ||||||
| Date | Site | Local Times (start-end) at UAV Flight | Plant Growth Stage 2 | Illumination Condition |
|---|---|---|---|---|
| 15 May | Bettendorf | 12:05–12:37 | GS37 | Sunny |
| Bicherhaff | 13:15–13:30 1 | GS37 | ||
| Koerich | 14:17–14:35 | GS32 | ||
| Weiswampach | 15:11–15:28 | GS32 | ||
| 24 May | Bettendorf | 11:14–11:40 | GS42 | Sunny |
| Bicherhaff | 12:30–12:44 | GS39 | ||
| Koerich | 13:22–13:36 | GS37 | ||
| Weiswampach | 14:02–14:15 | GS39 | ||
| 5 June | Bettendorf | 12:15–12:48 | GS57 | Cloudy |
| Bicherhaff | 13:35–13:53 | GS55 | ||
| Koerich | 14:33–14:53 | GS52 | ||
| Weiswampach | 15:40–15:57 | GS45 | ||
| 3 July | Bettendorf | 12:07–12:41 | GS82 | Sunny |
| Bicherhaff | 13:22–13:40 | GS80 | ||
| Koerich | 14:21–14:44 | GS77 | ||
| Weiswampach | 15:15–15:32 | GS77 |
| Statistics | GS32–GS37 | GS32–GS37 | GS32–GS37 | GS32–GS37 | |
|---|---|---|---|---|---|
| Wheat Stripe Rust | r | 0.76 | 0.83 | 0.89 | 0.91 |
| p-value (F-test) | 0.001 | <0.0001 | <0.0001 | <0.0001 | |
| τ | 0.71 | 0.72 | 0.66 | 0.75 | |
| p-value (Kendall) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Wheat Leaf Rust | r | 0.96 | 0.88 | 0.99 | 0.96 |
| p-value (F-test) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| τ | 0.86 | 0.86 | 0.83 | 0.79 | |
| p-value (Kendall) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Canopy Cover | r | 0.01 | 0.15 | 0.61 | 0.84 |
| p-value (F-test) | 0.637 | 0.551 | 0.009 | <0.0001 | |
| τ | 0.10 | 0.07 | 0.38 | 0.64 | |
| p-value (Kendall) | 0.597 | 0.715 | 0.034 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidarian Dehkordi, R.; El Jarroudi, M.; Kouadio, L.; Meersmans, J.; Beyer, M. Monitoring Wheat Leaf Rust and Stripe Rust in Winter Wheat Using High-Resolution UAV-Based Red-Green-Blue Imagery. Remote Sens. 2020, 12, 3696. https://doi.org/10.3390/rs12223696
Heidarian Dehkordi R, El Jarroudi M, Kouadio L, Meersmans J, Beyer M. Monitoring Wheat Leaf Rust and Stripe Rust in Winter Wheat Using High-Resolution UAV-Based Red-Green-Blue Imagery. Remote Sensing. 2020; 12(22):3696. https://doi.org/10.3390/rs12223696
Chicago/Turabian StyleHeidarian Dehkordi, Ramin, Moussa El Jarroudi, Louis Kouadio, Jeroen Meersmans, and Marco Beyer. 2020. "Monitoring Wheat Leaf Rust and Stripe Rust in Winter Wheat Using High-Resolution UAV-Based Red-Green-Blue Imagery" Remote Sensing 12, no. 22: 3696. https://doi.org/10.3390/rs12223696
APA StyleHeidarian Dehkordi, R., El Jarroudi, M., Kouadio, L., Meersmans, J., & Beyer, M. (2020). Monitoring Wheat Leaf Rust and Stripe Rust in Winter Wheat Using High-Resolution UAV-Based Red-Green-Blue Imagery. Remote Sensing, 12(22), 3696. https://doi.org/10.3390/rs12223696