Habitat-Suitability Model for the Yellow Rail (Coturnicops noveboracensis) in the Northern Gulf Coast of Alabama and Mississippi, USA
Abstract
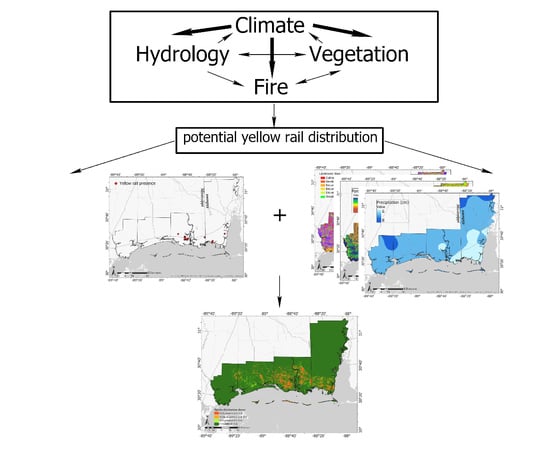
:1. Introduction
2. Materials and Methods
| 1. Distance to estuarine emergent wetland | Euclidean distance to Coastal Change Analysis Program (C-CAP) land cover type. Derived from the Coastal Change Analysis Program 2010 Regional Land Cover dataset [42]. |
| 2. Distance to estuarine forest wetland | |
| 3. Distance to estuarine shrub/scrub wetland | |
| 4. Distance to forest | |
| 5. Distance to grassland | |
| 6. Distance to palustrine emergent wetland | |
| 7. Distance to palustrine forest wetland | |
| 8. Distance to palustrine shrub/scrub wetland | |
| 9. Distance to shrub/scrub | |
| 10. Distance to water | |
| 11. Frequency of estuarine emergent wetland | Frequency of C-CAP land cover type 100 m radius. Cover derived from the Coastal Change Analysis Program 2010 Regional Land Cover dataset [42]. |
| 12. Frequency of estuarine forest wetland | |
| 13. Frequency of estuarine shrub/scrub wetland | |
| 14. Frequency of forest | |
| 15. Frequency of grassland | |
| 16. Frequency of palustrine emergent wetland | |
| 17. Frequency of palustrine forest wetland | |
| 18. Frequency of palustrine shrub/scrub wetland | |
| 19. Frequency of shrub/scrub | |
| 20. Frequency of water | |
| 21. Wetland soil landscapes (PWSL) | Gridded Soil Survey Geographic (gSSURGO) by State [44]. |
| 22. Precipitation in January | Monthly precipitation averaged from 1981–2010. Derived from PRISM Climate Data: 1981–2010 Monthly Average Precipitation by State [45]. |
| 23. Precipitation in February | |
| 24. Precipitation in March | |
| 25. Precipitation in April | |
| 26. Precipitation in May | |
| 27. Precipitation in June | |
| 28. Precipitation in July | |
| 29. Precipitation in August | |
| 30. Precipitation in September | |
| 31. Precipitation in October | |
| 32. Precipitation in November | |
| 33. Precipitation in December |
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leston, L.; Bookhout, T.A. Yellow rail (Coturnicops noveboracensis), version 2.0. In The Birds of North America; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2015. [Google Scholar]
- Austin, J.E.; Buhl, D.A. Relating yellow rail (Coturnicops noveboracensis) occupancy to habitat and landscape features in the context of fire. Waterbirds 2013, 36, 199–213. [Google Scholar] [CrossRef] [Green Version]
- Morris, K.M.; Woodrey, M.S.; Hereford, S.G.; Soehren, E.C.; Conkling, T.J.; Rush, S.A. Yellow rail (Coturnicops noveboracensis) occupancy in the context of fire in Mississippi and Alabama. Waterbirds 2017, 40, 5–104. [Google Scholar] [CrossRef] [Green Version]
- Soehren, E.C.; Hereford, S.G.; Morris, K.M.; Trent, J.A.; Walker, J.; Woodrey, M.S.; Rush, S.A. Winter use of wet pine savannas by yellow rail (Coturnicops noveboracensis) along coastal Alabama and Mississippi. Wilson J. Ornithol. 2018, 130, 615–625. [Google Scholar] [CrossRef]
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC). COSEWIC Assessment and Status Report on the Yellow Rail in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2009. [Google Scholar]
- U.S. Fish and Wildlife Service. Birds of Conservation Concern 2008; Division of Migratory Bird Management: Arlington, VA, USA, 2008. [Google Scholar]
- Grace, J.B.; Allain, L.K.; Baldwin, H.Q.; Billock, A.G.; Eddleman, W.R.; Given, A.M.; Jeske, C.W.; Moss, R. Effects of Prescribed Fire in the Coastal Prairies of Texas; U.S. Department of the Interior, Fish and Wildlife Service, Region 2, U.S. Geological Survey: Reston, VA, USA, 2005.
- Mizell, K.L. Effects of Fire and Grazing of Yellow Rail Habitat in a Texas Coastal Marsh. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1998. [Google Scholar]
- Post, W. Winter ecology of yellow rails based on South Carolina specimens. Wilson J. Ornithol. 2008, 120, 606–610. [Google Scholar] [CrossRef]
- Butler, C.J.; Pham, L.H.; Stinedurf, J.N.; Roy, C.L.; Judd, E.L.; Burgess, N.J.; Caddell, G.M. Yellow rails wintering in Oklahoma. Wilson J. Ornithol. 2010, 122, 385–387. [Google Scholar] [CrossRef]
- Butler, C.J.; Wilson, J.K.; Frazee, S.R.; Kelly, J.F. A comparison of the origins of yellow rails (Coturnicops noveboracensis) wintering in Oklahoma and Texas, USA. Waterbirds 2016, 39, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Wayne, A.T. Notes on certain birds taken or seen near Charleston, South Carolina. Auk 1905, 22, 395–400. [Google Scholar]
- Heck, B.A.; Arbour, W.D. The yellow rail in Oklahoma. Bull. Oklahoma Ornith. Soc. 2008, 41, 13–15. [Google Scholar]
- Peet, R.K.; Allard, D.J. Longleaf pine vegetation of the southern Atlantic and eastern Gulf Coast regions: A preliminary classification. Proc. Tall Timbers Fire Ecol. Conf. 1993, 18, 45–81. [Google Scholar]
- Ratnam, J.; Bond, W.J.; Fensham, R.J.; Hoffmann, W.A.; Archibald, S.; Lehmann, C.E.R.; Anderson, M.T.; Higgins, S.I.; Sankaran, M. When is a ‘forest’ a savanna, and why does it matter? Global Ecol. Biogeogr. 2011, 20, 653–660. [Google Scholar] [CrossRef]
- Platt, W.J.; Entrup, A.K.; Babl, E.K.; Coryell-Turpin, C.; Dao, V.; Hebert, J.A.; LaBarbera, C.D.; Noto, J.F.L.; Ogundare, S.O.; Timilsina, N. Short-term effects of herbicides and a prescribed fire on restoration of a shrub-encroached pine savanna. Restor. Ecol. 2015, 23, 909–917. [Google Scholar] [CrossRef]
- Van Langevelde, F.; Van De Vijver, C.A.D.M.; Kumar, L.; Van De Kopper, J.; De Ridder, N.; Van Andel, J.; Skidmore, A.K.; Hearne, J.W.; Stroosnijder, L.; Bond, W.J.; et al. Effects of fire and herbivory on the stability of savanna ecosystems. Ecology 2003, 84, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Gilliam, F.S.; Platt, W.J. Conservation and restoration of the Pinus palustris ecosystem. Appl. Veg. Sci. 2006, 9, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Noss, R.F. Longleaf pine and wiregrass: Keystone components of an endangered ecosystem. Nat. Areas J. 1989, 9, 211–213. [Google Scholar]
- Simberloff, D. Species-area and fragmentation effects on old-growth forests: Prospects for longleaf pine communities. Proc. Tall Timbers Fire Ecol. Conf. 1993, 18, 1–13. [Google Scholar]
- Brockway, D.G.; Lewis, C.E. Long-term effects of dormant-season prescribed fire on plant community diversity, structure and productivity in a longleaf pine wiregrass ecosystem. For. Ecol. Manag. 1997, 96, 167–183. [Google Scholar] [CrossRef]
- Frost, C.C. Four centuries of changing landscape patterns in the longleaf pine ecosystem. Proc. Tall Timbers Fire Ecol. Conf. 1993, 18, 17–43. [Google Scholar]
- Means, D.B.; Palis, J.G.; Baggett, M. Effects of slash pine silviculture on a Florida population of flatwoods salamander. Conserv. Biol. 1996, 10, 426–437. [Google Scholar] [CrossRef]
- Glitzenstein, J.S.; Platt, W.J.; Streng, D.R. Effects of fire regime and habitat on tree dynamics in north Florida longleaf pine savannas. Ecol. Monogr. 1995, 65, 441–476. [Google Scholar] [CrossRef]
- Olson, M.S.; Platt, W.J. Effects of habitat and growing season fires on resprouting of shrubs in longleaf pine savannas. Vegetatio 1995, 119, 101–118. [Google Scholar] [CrossRef]
- Palmquist, K.A.; Peet, R.K.; Weakley, A.S. Changes in plant species richness following reduced fire frequency and drought in one of the most species-rich savannas in North America. J. Veg. Sci. 2014, 25, 1426–1437. [Google Scholar] [CrossRef]
- Engler, R.; Guisan, A.; Rechsteiner, L. An improved approach for predicting the distribution of rare and endangered species from occurrence and pseudo-absence data. J. Appl. Ecol. 2004, 41, 263–274. [Google Scholar] [CrossRef]
- Engstrom, R.T.; Vickery, P.D.; Perkins, D.W.; Shriver, W.G. Effects of fire regime on birds in southeastern pine savannas and native prairies. Stud. Avian Biol. Ser. 2005, 30, 147–160. [Google Scholar]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: How to compute habitat-suitability maps without absence data? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Warren, D.L.; Wright, A.N.; Seifert, S.N.; Shaffer, H.B. Incorporating model complexity and spatial sampling bias into ecological niche models of climate change risks faced by 90 California vertebrate species of concern. Divers. Distrib. 2014, 20, 334–343. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Norquist, H.C. A Comparative Study of the Soils and Vegetation of Savannas in Mississippi. Ph.D. Thesis, Mississippi State University, Mississippi State, MS, USA, 1984. [Google Scholar]
- Greller, A.M. Correlation of warmth and temperateness with the distributional limits of zonal forests in eastern North America. Bull. Torrey Bot. Club 1989, 116, 145–163. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. Comprehensive Conservation Plan for Mississippi Sandhill Crane National Wildlife Refuge, Jackson County, Mississippi; U.S. Department of Interior, U.S. Fish and Wildlife Service, Southeast Region: Atlanta, GA, USA, 2007. [Google Scholar]
- Folkerts, G.W. A preliminary classification of pitcher plant habitats in the southeastern United States. J. Ala. Acad. Sci. 1991, 62, 199–225. [Google Scholar]
- Freeman, J.E.; Kobziar, L.N.; Leone, E.H.; Williges, K. Drivers of plant functional group richness and beta diversity in fire-dependent pine savannas. Divers. Distrib. 2019, 25, 1024–1044. [Google Scholar] [CrossRef]
- Morris, K.M. Ecology of Yellow Rail (Coturnicops noveboracensis) Overwintering in Coastal Pine Savannas of the Northern Gulf of Mexico. Master’s Thesis, Mississippi State University, Mississippi State, MS, USA, 2015. [Google Scholar]
- Veloz, S.D. Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. J. Biogeogr. 2009, 36, 2290–2299. [Google Scholar] [CrossRef]
- Brown, J.L. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- National Oceanic and Atmospheric Administration. C-CAP Regional Land Cover. Coastal Change Analysis Program (C-CAP) Regional Land Cover. 2010. Available online: http://www.coast.noaa.gov/ccapftp (accessed on 1 August 2019).
- Noss, R. Forgotten Grasslands of the South: Natural History and Conservation; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- U.S. Department of Agriculture, Natural Resources Conservation Service. Gridded Soil Survey Geographic (gSSURGO) Database for Alabama and Mississippi. Available online: https://gdg.sc.egov.usda.gov/ (accessed on 1 August 2019).
- PRISM Climate Group. Average Monthly and Average Annual Precipitation 1961–1990, 1971–2000 and 1981–2010 by State. Oregon State University. Available online: http://prism.oregonstate.edu (accessed on 1 August 2019).
- Akaike, H. A new look at the statistical model identification. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1974; pp. 215–222. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multi-Model Inference; Springer: New York, NY, USA, 2002. [Google Scholar]
- Jueterbock, A.; Smolina, I.; Coyer, J.A.; Hoarau, G. The fate of the Arctic seaweed Fucus distichus under climate change: An ecological niche modeling approach. Ecol. Evol. 2016, 6, 1712–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, H.M.; Ghoddousi, A. Water availability limits brown bear distribution at the southern edge of its global range. Ursus 2018, 29, 13–25. [Google Scholar] [CrossRef]
- Yang, X.Q.; Kushwaha, S.P.S.; Saran, S.; Xu, J.; Roy, P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, F.; Li, G.; Qin, W.; Li, S.; Gao, H.; Cai, Z.; Lin, G.; Zhang, T. Maxent modeling for predicting the spatial distribution of three raptors in the Sanjiangyuan National Park, China. Ecol. Evol. 2019, 9, 6643–6654. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, B.C.; Ackerly, D.D.; Klos, P.Z.; Natali, J.; Dawson, T.E.; Thompson, S.E. Hydrologic refugia, plants, and climate change. Glob. Chang. Biol. 2017, 23, 2941–2961. [Google Scholar] [CrossRef] [Green Version]
- Greene, R.E.; Iglay, R.B.; Evans, K.O.; Miller, D.A.; Wigley, T.B.; Riffell, S.K. A meta-analysis of biodiversity responses to management of southeastern pine forests—Opportunities for open pine conservation. For. Ecol. Manag. 2016, 360, 30–39. [Google Scholar] [CrossRef]
- Honda, E.A.; Durigan, G. Woody encroachment and its consequences on hydrological processes in the savannah. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckage, B.; Gross, L.J.; Platt, W.J. Modeling responses of pine savannas to climate change and large-scale disturbance. Appl. Veg. Sci. 2006, 9, 75–82. [Google Scholar] [CrossRef]
- Wuebbles, D.; Meehl, G.; Hayhoe, K.; Karl, T.R.; Kunkel, K.; Santer, B.; Wehner, M.; Colle, B.; Fischer, E.M.; Fu, R.; et al. CMIP5 climate model analyses: Climate extremes in the United States. Bull. Am. Meteorol. Soc. 2014, 95, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.J.; Liu, Y.; O’Brien, J.J.; Elliott, K.J.; Starr, G.; Miniat, C.F.; Hiers, J.K. Future climate and fire interactions in the southeastern region of the United States. For. Ecol. Manag. 2014, 327, 316–326. [Google Scholar] [CrossRef]
- Owuor, S.O.; Butterbach-Bahl, K.; Guzha, A.C.; Rufino, M.C.; Pelster, D.E.; Díaz-Pinés, E.; Breuer, L. Groundwater recharge rates and surface runoff response to land use and land cover changes in semi-arid environments. Ecol. Process. 2016, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Slocum, M.G.; Beckage, B.; Platt, W.J.; Orzell, S.L.; Taylor, W. Effect of climate on wildfire size: A cross-scale analysis. Ecosystems 2010, 13, 828–840. [Google Scholar] [CrossRef]
- Varner, J.M., III; Gordon, D.R.; Putz, F.E.; Hiers, J.K. Restoring fire to long-unburned Pinus palustris ecosystems: Novel fire effects and consequences for long-unburned ecosystems. Restor. Ecol. 2005, 13, 536–544. [Google Scholar] [CrossRef]



| Features a | β b | Test AUC c | Training AUC d | AUC Difference e | Mean Omission Rate f | Mean Minimum Omission Rate g | Number of Parameters h |
|---|---|---|---|---|---|---|---|
| H | 1 | 0.99 ± 0.006 SE | 0.991 ± 0.003 SE | 0.005 | 0.093 | 0.008 | 17 |
| Environmental Variable | Percent Contribution | Variable Description and Source |
|---|---|---|
| Frequency of palustrine emergent wetland | 33.1 | Frequency of palustrine emergent wetland habitat within a specified radius. Derived from the Coastal Change Analysis Program 2010 Regional Land Cover dataset [42]. |
| Frequency of palustrine shrub/scrub wetland | 21.8 | Frequency of palustrine shrub/scrub wetland habitat within a specified radius. Derived from the Coastal Change Analysis Program 2010 Regional Land Cover dataset [42]. |
| June precipitation | 16.9 | Monthly precipitation averaged from 1981–2010. Derived from PRISM Climate Data: 1981–2010 Monthly Average Precipitation by State [45]. |
| August precipitation | 10.6 | Monthly precipitation averaged from 1981–2010. Derived from PRISM Climate Data: 1981–2010 Monthly Average Precipitation by State [45]. |
| September precipitation | 10.5 | Monthly precipitation averaged from 1981–2010. Derived from PRISM Climate Data: 1981–2010 Monthly Average Precipitation by State [45]. |
| Distance to palustrine emergent wetland | 7.1 | Euclidean distance to palustrine emergent wetland habitat. Derived from the Coastal Change Analysis Program 2010 Regional Land Cover dataset [42]. |
| Species Distribution Classes | Estimated Area (ha) | Proportion of the Study Area (%) |
|---|---|---|
| High potential (0.6 ≤ 1.0) | 2436 | 0.31 |
| Moderate potential (0.4 ≤ 0.6) | 1356 | 0.17 |
| Low potential (0.2 ≤ 0.4) | 4851 | 0.62 |
| Unsuitable (0 ≤ 0.2) | 776,014 | 98.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, K.M.; Soehren, E.C.; Woodrey, M.S.; Rush, S.A. Habitat-Suitability Model for the Yellow Rail (Coturnicops noveboracensis) in the Northern Gulf Coast of Alabama and Mississippi, USA. Remote Sens. 2020, 12, 848. https://doi.org/10.3390/rs12050848
Morris KM, Soehren EC, Woodrey MS, Rush SA. Habitat-Suitability Model for the Yellow Rail (Coturnicops noveboracensis) in the Northern Gulf Coast of Alabama and Mississippi, USA. Remote Sensing. 2020; 12(5):848. https://doi.org/10.3390/rs12050848
Chicago/Turabian StyleMorris, Kelly M., Eric C. Soehren, Mark S. Woodrey, and Scott A. Rush. 2020. "Habitat-Suitability Model for the Yellow Rail (Coturnicops noveboracensis) in the Northern Gulf Coast of Alabama and Mississippi, USA" Remote Sensing 12, no. 5: 848. https://doi.org/10.3390/rs12050848
APA StyleMorris, K. M., Soehren, E. C., Woodrey, M. S., & Rush, S. A. (2020). Habitat-Suitability Model for the Yellow Rail (Coturnicops noveboracensis) in the Northern Gulf Coast of Alabama and Mississippi, USA. Remote Sensing, 12(5), 848. https://doi.org/10.3390/rs12050848