Mapping Mangrove Zonation Changes in Senegal with Landsat Imagery Using an OBIA Approach Combined with Linear Spectral Unmixing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Satellite Data Acquisition and Preprocessing
2.3. Pansharpening
2.4. Isolating the Mangrove
2.5. Stacked Unsupervised Classification
2.6. Linear Spectral Unmixing and Plant Fraction
2.7. Endmember Selection
2.8. OBIA
- -
- High riverine mangrove dominated by Rhizophora racemose: High mangrove.
- -
- Low and dense mangrove dominated by Rhizophora mangle: Low and dense mangrove.
- -
- Low and open mangrove with mixed Rhizophora mangle and Avicennia germinans: Low and open mangrove.
2.9. Mapping of Spatiotemporal Trajectories
3. Results
3.1. Accuracy Assessment
3.2. Contribution of Each Formation to Overall Change
3.2.1. Reorganisation of Surfaces
3.2.2. Conversion of Surfaces
3.3. Spatial Analysis of Changes
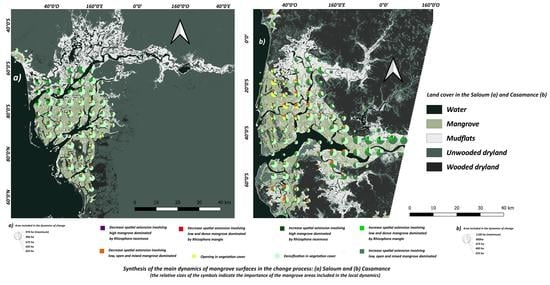
3.3.1. Summary Maps of Regeneration
3.3.2. Path Dependence
4. Discussion
4.1. Accuracy and Uncertainty Margin
4.2. Summary of Trajectories in the Mangrove Regeneration Process: Densification in the Saloum and Colonisation in Casamance
4.3. Factors in the Dynamics of Plant Formations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friess, D.A.; Rogers, K.; Lovelock, C.E.; Krauss, K.W.; Hamilton, S.E.; Lee, S.Y.; Lucas, R.; Primavera, J.; Rajkaran, A.; Shi, S. The State of the World’s Mangrove Forests: Past, Present, and Future. Annu. Rev. Environ. Resour. 2019, 44, 89–115. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Hamilton, S.; Barbier, E.; Primavera, J.; Lewis, R. Better restoration policies are needed to conserve mangrove ecosystems. Nat. Ecol. Evol. 2019, 3, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Otero, V.; Van De Kerchove, R.; Satyanarayana, B.; Mohd-Lokman, H.; Lucas, R.; Dahdouh-Guebas, F. An analysis of the early regeneration of mangrove forests using landsat time series in the Matang mangrove forest reserve, Peninsular Malaysia. Remote Sens. 2020, 11, 774. [Google Scholar] [CrossRef] [Green Version]
- Duke, N.C.; Meynecke, J.-O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A World Without Mangroves? Science 2007, 317, 41–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Brondizio, E.S., Settele, J., Díaz, S., Ngo, H.T., Eds.; IPBES secretariat: Bonn, Germany, 2019. [Google Scholar]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Alongi, D.M. Introduction in the Energetics of Mangrove Forests; Springer Science and Business Media BV: New York, NY, USA, 2012. [Google Scholar]
- Friess, D.A.; Yando, E.S.; Abuchahla, G.M.O.; Adams, J.B.; Cannicci, S.; Canty, S.W.J.; Wee, A.K.S. Mangroves give cause for conservation optimism, for now. Curr. Biol. 2020, 30, R153–R154. [Google Scholar] [CrossRef] [Green Version]
- Saenger, P. Mangrove Ecology, Silviculture and Conservation; Kluwer Academic Publishers: Dordrecht, Netherlands, 2002; pp. 11–18. [Google Scholar]
- Spalding, M.; Kainuma, M.; Collins, L. World Atlas of Mangroves; A Collaborative Project of ITTO, ISME, FAO, UNEP-WCMC, UNESCO-MAB, UNU-INWEH and TNC. 2010; Earthscan: London, UK, 2010; p. 319. [Google Scholar]
- Gallup, L.; Sonnenfeld, D.A.; Dahdouh-Guebas, F. Mangrove use and management within the Sine-Saloum Delta, Senegal. Ocean Coast. Manag. 2020, 185, 105001. [Google Scholar] [CrossRef] [Green Version]
- Walters, B.B.; Rönnbäck, P.; Kovacs, J.M.; Crona, B.; Hussain, S.A.; Badola, R.; Primavera, J.H.; Barbier, E.; Dahdouh-Guebas, F. Ethnobiology, socio-economics and management of mangrove forests: A review. Aquat. Bot. 2008, 89, 220–236. [Google Scholar] [CrossRef] [Green Version]
- Cormier-Salem, M. Dynamique et Usages de la Mangrove Dans les Pays des Rivières du Sud, du Sénégal à la Sierra Leone; IRD Éditions: Marseille, France, 1991. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Collin, S.; Seen, D.L.; Rönnbäck, P.; Depommier, D.; Ravishankar, T.; Koedam, N. Analysing ethnobotanical and fishery-related importance of mangroves of East-Godavari Delta (Andhra Pradesh, India) for conservation and management purposes. J. Ethnobiol. Ethnomed. 2006, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Edwards, P. Aquaculture environment interactions: Past, present and likely future trends. Aquaculture 2015, 447, 2–14. [Google Scholar] [CrossRef]
- FAO. The World’s Mangroves 1980–2005; Étude FAO: Rome, Italy, 2007. [Google Scholar]
- Bryan-Brown, D.N.; Connolly, R.M.; Richards, D.R. Global trends in mangrove forest fragmentation. Sci. Rep. 2020, 10, 7117. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Forest Resources Assessment 2020: Main Report; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Andrieu, J. Land cover changes on the West-African coastline from the Saloum Delta (Senegal) to Rio Geba (Guinea-Bissau) between 1979 and 2015. Eur. J. Remote Sens. 2018, 51, 314–325. [Google Scholar] [CrossRef] [Green Version]
- Andrieu, J.; Lombard, F.; Fall, A.; Thior, M.; Demba Ba, B.; Ephrem, A.; Dieme, B. Botanical field-study and remote sensing to describe mangrove resilience in the Saloum Delta (Senegal) after 30 years of degradation narrative. For. Ecol. Manag. 2020, 461, 117963. [Google Scholar] [CrossRef]
- Dieye, E.H.B.; Diaw, A.T.; Sané, T.; Ndour, N.; Ndour, N. Dynamique de la mangrove de l’estuaire du Saloum (Sénégal) entre 1972 et 2010. Cybergeo Eur. J. Geogr. 2013, doc 629. [Google Scholar] [CrossRef]
- Fent, A.; Bardou, R.; Carney, J.; Cavanaugh, K. Transborder political ecology of mangroves in Senegal and The Gambia. Glob. Environ. Chang. 2019, 54, 214–226. [Google Scholar] [CrossRef]
- Descroix, L.; Sané, Y.; Thior, M.; Manga, S.-P.; Ba, B.D.; Mingou, J.; Mendy, V.; Coly, S.; Dièye, A.; Badiane, A.; et al. Inverse Estuaries in West Africa: Evidence of the Rainfall Recovery? Water 2020, 12, 647. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, S.E. The West African Sahel: A review of recent studies on the rainfall regime and its interannual variability (Article ID 453521). ISRN Meteorol. 2013, 32. [Google Scholar] [CrossRef]
- Mahé, G.; Lienou, G.; Descroix, L.; Bamba, L.; Paturel, J.-E.; Laraque, A.; Meddi, M.; Habaieb, M.; Adeaga, O.; Dieulin, C.; et al. The rivers of Africa: Witness of climate change and human impact on the environment. Hydrol. Process. 2013, 27, 2105–2114. [Google Scholar] [CrossRef]
- Moreno-de las Heras, M.; Díaz-Sierra, R.; Turnbull, L.; Wainwright, J. Assessing vegetation structure and ANPP dynamics in a grassland–shrubland Chihuahuan ecotone using NDVI–rainfall relationships. Biogeosciences 2015, 12, 2907–2925. [Google Scholar] [CrossRef] [Green Version]
- Thoma, D.P.; Munson, S.M.; Irvine, K.M.; Witwicki, D.L.; Bunting, E.L. Semi-arid vegetation response to antecedent climate and water balance windows. Appl. Veg. Sci. 2016, 19, 413–429. [Google Scholar] [CrossRef]
- Zhou, J.; Jia, L.; Menenti, M.; Van Hoek, M.; Lu, J.; Zheng, C.; Wu, H.; Yuan, X. Characterizing vegetation response to rainfall at multiple temporal scales in the Sahel-Sudano-Guinean region using transfer function analysis. Remote Sens. Environ. 2021, 252, 112108. [Google Scholar] [CrossRef]
- Holling, C.S. Adaptive Environmental Assessment and Management; John Wiley and Sons: Chichester, UK, 1978. [Google Scholar]
- Conchedda, G.; Durieux, L.; Mayaux, P. An object-based method for mapping and change analysis in mangrove ecosystems. ISPRS J. Photogramm. Remote Sens. 2008, 63, 578–589. [Google Scholar] [CrossRef]
- Cormier-Salem, M.C. Mangrove Grabbing: An Exploration of Changes in Mangrove Tenure from a Political Ecology Perspective; The Sea within: Maritime Tenure and Cosmopolitical Debates; Artaud, H., Surallés, A., Eds.; IWGIA: Copenhagen, Denmark, 2017; pp. 143–162. [Google Scholar]
- Cormier-Salem, M.-C.; Panfili, J. Mangrove reforestation in question: Greening or grabbing coastal zones and deltas? Afr. J. Aquat. Sci. 2016, 41, 89–98. [Google Scholar] [CrossRef]
- Arumugam, M.; Niyomugabo, R.; Dahdouh-Guebas, F.; Hugé, J. The perceptions of stakeholders on current management of mangroves in the Sine-Saloum Delta, Senegal, Estuarine. Coast. Shelf Sci. 2021, 248, 107160. [Google Scholar] [CrossRef]
- Lombard, F.; Andrieu, J.; Descroix, L. La population d’Avicennia germinans du delta du Saloum est-elle relictuelle depuis la dernière période humide? Bois et Forêts des Trop. 2020, 346, 51–64. [Google Scholar] [CrossRef]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Kuenzer, C.; Bluemel, A.; Gebhardt, S.; Quoc, T.V.; Dech, S. Remote Sensing of Mangrove Ecosystems: A Review. Remote Sens. 2011, 3, 878–928. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wan, B.; Qiu, P.; Su, Y.; Guo, Q.; Wang, R.; Sun, F.; Wu, X. Evaluating the Performance of Sentinel-2, Landsat 8 and Pléiades-1 in Mapping Mangrove Extent and Species. Remote Sens. 2018, 10, 1468. [Google Scholar] [CrossRef] [Green Version]
- Taureau, F.; Robin, M.; Proisy, C.; Fromard, F.; Imbert, D.; Debaine, F. Mapping the Mangrove Forest Canopy Using Spectral Unmixing of Very High Spatial Resolution Satellite Images. Remote Sens. 2019, 11, 367. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Sousa, W.P.; Gong, P. Integration of object-based and pixel-based classification for mapping mangroves with IKONOS imagery. Int. J. Remote Sens. 2004, 25, 5655–5668. [Google Scholar] [CrossRef]
- Lombard, F.; Andrieu, J.; Josselin, D. Object-oriented classification-based mapping of mangrove zonation in Casamance and Sine-Saloum (Senegal) using Sentinel-2 images. Geo-Spat. Inf. Sci. 2021. submitted for publication. [Google Scholar]
- Johnson, B. Effects of Pansharpening on Vegetation Indices. ISPRS Int. J. Geo-Inf. 2014, 3, 507–522. [Google Scholar] [CrossRef]
- Kasawani, I.; Kim, A.; Shah, A.; Sulong, I.; Ismail, M.H.; Jusoff, K. Coastal Change Detection using GIS in Setiu Lagoon, Terengganu, Malaysia. J. GIS Trends 2010, 1, 20–26. [Google Scholar]
- Yuvaraj, E.; Dharanirajan, K.; Saravanan, N.; Karpoorasundarapandian, N. Evaluation of Vegetation density of the Mangrove forest in South Andaman Island using Remote Sensing and GIS techniques. Int. Res. J. Environ. Sci. 2014, 3, 19–25. [Google Scholar]
- Sari, S.P.; Rosalina, D. Mapping and Monitoring of Mangrove Density Changes on tin Mining Area. Procedia Environ. Sci. 2016, 33, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Myint, S.; Chandra, G.; Wang, L.; Gillette, S. Identifying Mangrove Species and Their Surrounding Land Use and Land Cover Classes Using an Object-Oriented Approach with a Lacunarity Spatial Measure. GISci. Remote Sens. 2008, 45, 188–208. [Google Scholar] [CrossRef]
- Taureau, F. Cartographie Harmonisée des Mangroves de L’outre-Mer Français. Thèse de Doctorat. Sciences de L’environnement; Université de Nantes (UNAM), Nantes, France, 2017. Doctorat Thèse, Sciences de L’environnement. Université de Nantes (UNAM), Nantes, France, 2017. [Google Scholar]
- Bullock, E.L.; Fagherazzi, S.; Nardin, W.; Vo-Luong, P.; Nguyen, P.; Woodcock, C.E. Temporal patterns in species zonation in a mangrove forest in the Mekong Delta, Vietnam, using a time series of Landsat imagery. Cont. Shelf Res. 2017, 147, 144–154. [Google Scholar] [CrossRef]
- Gudex-Cross, D.; Pontius, J.; Adams, A. Enhanced forest cover mapping using spectral unmixing and object-based classification of multi-temporal Landsat imagery. Remote Sens. Environ. 2017, 196, 193–204. [Google Scholar] [CrossRef]
- Descroix, L. Processus et Enjeux D’eau en Afrique de L’ouest Soudano-Sahélienne; EAC: Paris, France, 2018; p. 320. ISBN 978-2-8130-0314-0. [Google Scholar]
- Andrieu, J.; Cormier-Salem, M.C.; Descroix, L.; Diéye, E.H.B.; Ndour, N.; Sané, T. Correctly assessing forest change in a priority West African mangrove ecosystem: 1986–2010 An answer to Carney et al., 2014 paper “Assessing forest change in a priority West African mangrove ecosystem: 1986–2010”. Remote Sens. App. Soc. Environ. 2019, 13, 337–347. [Google Scholar] [CrossRef]
- Datta, D.; Roy, A.K.; Kundu, A.; Dutta, D.; Neogy, S. An alternative approach to delineate wetland influence zone of a tropical intertidal mudflat using geo-information technology. Estuar. Coast. Shelf Sci. 2021, 253, 107308. [Google Scholar] [CrossRef]
- Taureau, F. Evaluation des Données Sentinel Pour la Mise en Place D’un Système de Surveillance des Mangroves à Mayotte; Université de Nantes: Nantes, France, 2018; p. 116. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, P.; Foody, G.M.; Herold, M.; Stehman, S.V.; Woodcock, C.E.; Wulder, M.A. Good practices for estimating area an assessing accuracy of land change. Remote Sens. Environ. 2014, 148, 42–57. [Google Scholar] [CrossRef]
- Sakho, I.; Mesnage, V.; Deloffre, J.; Lafite, R.; Niang, I.; Faye, G. The influence of natural and anthropogenic factors on mangrove dynamics over 60 years: The Somone Estuary Senegal. Estuar. Coast. Shelf Sci. 2011, 94, 93–101. [Google Scholar] [CrossRef]
- Solly, B.; Dièye, E.H.B.; Sané, T.; Diaw, A.T. Dynamique de la Mangrove de Thiobon dans l’estuaire de la Casamance (Sénégal) entre 1972 et 2017. Eur. Sci. J. 2018, 14, 118–133. [Google Scholar] [CrossRef]
- Soumaré, S.; Fall, F.; Andrieu, J.; Marega, O.; Dieme, B. Dynamique spatio-temporelle de la mangrove de Kafountine dans l’estuaire de la Basse-Casamance des années 1972 à nos jours: Approche par télédétection. IOSR J. Eng. (IOSRJEN) 2020, 10, 1–14. [Google Scholar]
- Hogarth, P.J. The Biology of Mangroves and Seagrasses; Biology of Habitats, 2 ed.; Oxford University Press: Oxford, UK, 2007; ISBN 978-0-19-856870-4. [Google Scholar]
- Bassene, O.; Cubizolle, H.; Cormier-Salem, M.C.; Sy, B.A. L’impact des changements démographiques et socio-économiques sur la perception et la gestion de la mangrove en Basse Casamance (Sénégal). Géocarrefour 2013, 88, 299–315. [Google Scholar] [CrossRef]
- Barusseau, J.-P.; Diop, E.S.; Saos, J.-L. Evidence of dynamics reversal in tropical estuaries, geomorphological and sedimentological consequences (Salum and Casamance Rivers, Senegal). Sedimentology 1985, 32, 543–552. [Google Scholar] [CrossRef]
- Diop, E.S. La Côte Ouest Africaine du Saloum (Sénégal) à la Mellacorée (République de Guinée). Ph.D. Thesis, Université Louis Pasteur, Strasbourg, France, 1990. [Google Scholar]
- Marius, C. Mangroves du Sénégal et de la Gambie. Ecologie, Pédologie, Géochimie, Mise en Valeur et Aménagement. Ph.D. Thesis, Louis Pasteur Strasbourg University, Strasbourg, France, 1985; p. 335. [Google Scholar]
- Diouf, A.C. Dynamiques Des Ressources Naturelles et Adaptations Des Sociétés Dans la Réserve de Biosphère du Delta du Saloum au Sénégal. Ph.D. Thesis, Université Gaston Bergé, Dakar, Senegal, 2019. [Google Scholar]
- Olivry, J.-C. Les conséquences durables de la sécheresse actuelle sur l’écoulement du fleuve Sénégal et l’hypersalinisation de la Basse-Casamance. In Influence of Climate Change and Climatic Variability on the Hydrologie Regime and Water Resources; IAHS Publisher: Wallingford, UK, 1987. [Google Scholar]











| Dates of Acquisition by Path/Row | |||||
|---|---|---|---|---|---|
| 205/50 and 205/51 | Capture | Collection and Level of Product | Spatial Resolution | Bands | Spectral Range(μm) |
| 08/12/2000 | ETM+ | C2/L2 | 30 m | 1 | 0.45–0.515 |
| C2/L2 | 30 m | 2 | 0.525–0.605 | ||
| C2/L2 | 30 m | 3 | 0.63–0.69 | ||
| C2/L2 | 30 m | 4 | 0.775–0.90 | ||
| C2/L2 | 30 m | 5 | 1.55–1.75 | ||
| C2/L2 | 30 m | 7 | 2.08–2.35 | ||
| C2/L1 | 15 m | 8 | 0.52–0.9 | ||
| 18/12/2018 | OLI | C2/L2 | 30 m | 2 | 0.45–0.515 |
| C2/L2 | 30 m | 3 | 0.525–0.600 | ||
| C2/L2 | 30 m | 4 | 0.630–0.680 | ||
| C2/L2 | 30 m | 5 | 0.845–0.885 | ||
| C2/L2 | 30 m | 6 | 1.560–1.660 | ||
| C2/L2 | 30 m | 7 | 2.100–2.300 | ||
| C2/L1 | 15 m | 8 | 0.500–0.680 |
| Saloum | Casamance | |||
|---|---|---|---|---|
| Training | Control | Training | Control | |
| high mangrove | 1109 | 1055 | 1812 | 1335 |
| low and dense mangrove | 1827 | 891 | 2213 | 2344 |
| Low and open mangrove | 1382 | 1002 | 2620 | 1863 |
| Total (pixel) | 7266 | 12,187 | ||
| Saloum | High Mangrove | Low and Dense Mangrove | Low and Open Mangrove | Total | Commission |
|---|---|---|---|---|---|
| High mangrove | 983 | 236 | 2 | 1221 | 0.19 |
| Low and dense mangrove | 58 | 646 | 47 | 751 | 0.13 |
| Low and open mangrove | 14 | 8 | 924 | 946 | 0.02 |
| Total | 1055 | 891 | 1002 | 2948 | |
| Omission | 0.06 | 0.27 | 0.07 | 0.13 |
| Casamance | High Mangrove | Low and Dense Mangrove | Low and Open Mangrove | Total | Commission |
|---|---|---|---|---|---|
| High mangrove | 1202 | 128 | 2 | 1332 | 0.09 |
| Low and dense mangrove | 90 | 2033 | 133 | 2256 | 0.09 |
| Low and open mangrove | 43 | 177 | 1696 | 946 | 0.11 |
| Total | 1335 | 2344 | 1863 | 5542 | |
| Omission | 0.09 | 0.13 | 0.08 | 0.11 |
| Overall Accuracy | Kappa |
|---|---|
| Saloum 2018 | 0.80 |
| Casamance 2018 | 0.83 |
| Saloum 2018 | Mapped (ha) | Estimated (ha) | Relative Difference | MoE at 95% Confidence |
|---|---|---|---|---|
| High and dense mangrove | 13,549 | 7603 | 78% | 91% |
| Low and dense mangrove | 30,962 | 16,829 | 84% | 41% |
| Low and open mangrove | 18,770 | 21,719 | −14% | 32% |
| Casamance 2018 | Mapped (ha) | Estimated (ha) | Relative Difference | MoE at 95% Confidence |
| High and dense mangrove | 10,943 | 10,941 | 0% | 61% |
| Low and dense mangrove | 33,689 | 24,450 | 38% | 27% |
| Low and open mangrove | 36,221 | 23,575 | 54% | 28% |
| Saloum | No Mangrove | High Mangrove | Low and Dense Mangrove | Low and Open Mangrove | Total |
|---|---|---|---|---|---|
| No mangrove | 323 ha | 426 ha | 2248 ha | 2996 ha | |
| High mangrove | 1410 ha | 6246 ha | 3925 ha | 4050 ha | 15,631 ha |
| Low and dense mangrove | 2534 ha | 3000 ha | 13,148 ha | 13,764 ha | 32,446 ha |
| Low and open mangrove | 4382 ha | 73 ha | 1287 ha | 9923 ha | 15,665 ha |
| Total | 8326 ha | 9641 ha | 18,786 ha | 29,986 ha | |
| Legend | |||||
| Decrease spatial extension involving high mangrove dominated by Rhizophora racemosa | |||||
| Decrease spatial extension involving low and dense mangrove dominated by Rhizophora mangle | |||||
| Decrease spatial extension involving low, open and mixed mangrove dominated by Rhizophora mangle et Avicennia germinans | |||||
| Opening in vegetation cover | |||||
| Increase in spatial extension involving high mangrove dominated by Rhizophora racemosa | |||||
| Increase spatial extension involving low and dense mangrove dominated by Rhizophora mangle | |||||
| Increase spatial extension involving low, open and mixed mangrove dominated by Rhizophora mangle et Avicennia germinans | |||||
| Densification in vegetation cover | |||||
| Stability in vegetation cover | |||||
| Casamance | No Mangrove | High Mangrove | Low and Dense Mangrove | Low and Open Mangrove | Total |
|---|---|---|---|---|---|
| No mangrove | 70 ha | 436 ha | 4611 ha | 5118 ha | |
| High mangrove | 984 ha | 4659 ha | 4336 ha | 2262 ha | 12,242 ha |
| Low and dense mangrove | 4284 ha | 3263 ha | 15,574 ha | 12,370 ha | 35,491 ha |
| Low and open mangrove | 11,464 ha | 124 ha | 3472 ha | 17,364 ha | 32,424 ha |
| Total | 16,732 ha | 8116 ha | 23,819 ha | 36,607 ha | |
| Legend | |||||
| Decrease spatial extension involving high mangrove dominated by Rhizophora racemosa | |||||
| Decrease spatial extension involving low and dense mangrove dominated by Rhizophora mangle | |||||
| Decrease spatial extension involving low, open and mixed mangrove dominated by Rhizophora mangle et Avicennia germinans | |||||
| Opening in vegetation cover | |||||
| Increase in spatial extension involving high mangrove dominated by Rhizophora racemosa | |||||
| Increase spatial extension involving low and dense mangrove dominated by Rhizophora mangle | |||||
| Increase spatial extension involving low, open and mixed mangrove dominated by Rhizophora mangle et Avicennia germinans | |||||
| Densification in vegetation cover | |||||
| Stability in vegetation cover | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombard, F.; Andrieu, J. Mapping Mangrove Zonation Changes in Senegal with Landsat Imagery Using an OBIA Approach Combined with Linear Spectral Unmixing. Remote Sens. 2021, 13, 1961. https://doi.org/10.3390/rs13101961
Lombard F, Andrieu J. Mapping Mangrove Zonation Changes in Senegal with Landsat Imagery Using an OBIA Approach Combined with Linear Spectral Unmixing. Remote Sensing. 2021; 13(10):1961. https://doi.org/10.3390/rs13101961
Chicago/Turabian StyleLombard, Florent, and Julien Andrieu. 2021. "Mapping Mangrove Zonation Changes in Senegal with Landsat Imagery Using an OBIA Approach Combined with Linear Spectral Unmixing" Remote Sensing 13, no. 10: 1961. https://doi.org/10.3390/rs13101961
APA StyleLombard, F., & Andrieu, J. (2021). Mapping Mangrove Zonation Changes in Senegal with Landsat Imagery Using an OBIA Approach Combined with Linear Spectral Unmixing. Remote Sensing, 13(10), 1961. https://doi.org/10.3390/rs13101961