Specific Drivers and Responses to Land Surface Phenology of Different Vegetation Types in the Qinling Mountains, Central China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Datasets
2.3. Retrieval of Phenology Metrics from NDVI Time Series Data
2.4. Method and Statistical Analysis
2.4.1. Trend Analysis
2.4.2. Change Pattern and Relative Attribution Analysis
2.4.3. Analysis of the Relative Importance of Different Drivers
3. Results
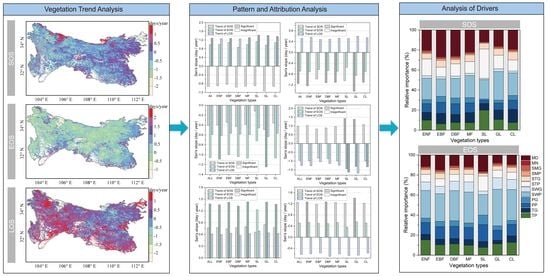
3.1. Spatiotemporal Variations of Phenology Metrics in the QMs
3.2. Change Pattern of LSP and Relative Attribution Analysis
3.3. Drivers of Interannual Variations in LSP
4. Discussion
4.1. Dynamics Changes in LSP in the QMs
4.2. Asymmetry in Contributions of SOS and EOS Trends to LOS
4.3. Analysis of the Drivers of Interannual Variations in LSP
4.4. Evaluation of RF Model
5. Conclusions
- (1)
- The average advance of SOS across QMs was 1.5 days/decade, with a significant advance in SOS observed for 27.5% of pixels. EOS was delayed by 2.4 days/decade, with a significant delay in EOS observed for 42.1% of pixels. LOS was lengthened by 3.9 days/decade, with a significant LOS lengthening observed for 40.3% of pixels.
- (2)
- The dominant pattern of change in the growing season for different vegetation types was advanced SOS, delayed EOS, and lengthened LOS, and this pattern had the highest percentage in evergreen broadleaved forests. The percentage of area shows that the patterns of delayed SOS and EOS and lengthened LOS were the highest percentage in shrubs.
- (3)
- For the whole QMs, LOS changes were mainly controlled by EOS, and the percentage was 51.4%. For deciduous broadleaved forests and grasses, LOS changes were attributed to SOS, while for other vegetation types, they were attributed to EOS.
- (4)
- SWP was found to be the most important factor influencing SOS and EOS, and grass and crop most influenced by SWP. Interannual variations in SOS were more influenced by biological factors (MD) than in EOS. The interannual variability of EOS is more influenced by preseason precipitation (PP) than SOS.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LSP | Land surface phenology |
| QMs | Qinling Mountains |
| NDVI | Normalized difference vegetation index |
| SOS | The start of the growing season |
| EOS | The end of the growing season |
| LOS | The length of the growing season |
| ENF | Evergreen needleleaved forest |
| EBF | Evergreen broadleaved forest |
| DBF | Deciduous broadleaved forest |
| MF | Mixed forest |
| SL | Shrubland |
| GL | Grassland |
| CL | Cropland |
| TP | Preseason average temperature |
| TG | Growing season average temperature |
| PP | Preseason total precipitation |
| PG | Growing season total precipitation |
| SWP | Preseason mean shortwave radiation |
| SWG | Growing season mean shortwave radiation |
| STP | Preseason soil temperature |
| STG | Growing season soil temperature |
| SMP | Preseason soil moisture |
| SMG | Growing season soil moisture |
| MD | The middle date of the growing season |
| MN | Maximum NDVI during growing season |
| RF | Random forest |
| OOB | Out of bag |
References
- Güsewell, S.; Furrer, R.; Gehrig, R.; Pietragalla, B. Changes in temperature sensitivity of spring phenology with recent climate warming in Switzerland are related to shifts of the preseason. Glob. Chang. Biol. 2017, 23, 5189–5202. [Google Scholar] [CrossRef]
- Fu, Y.H.; Piao, S.; Vitasse, Y.; Zhao, H.; De Boeck, H.J.; Liu, Q.; Yang, H.; Weber, U.; Hänninen, H.; Janssens, I.A. Increased heat requirement for leaf flushing in temperate woody species over 1980–2012: Effects of chilling, precipitation and insolation. Glob. Chang. Biol. 2015, 21, 2687–2697. [Google Scholar] [CrossRef]
- Lang, W.; Chen, X.; Qian, S.; Liu, G.; Piao, S. A new process-based model for predicting autumn phenology: How is leaf senescence controlled by photoperiod and temperature coupling? Agric. For. Meteorol. 2019, 268, 124–135. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Henrys, P.A.; Hemming, D.; Bell, J.R.; Botham, M.S.; Burthe, S.; Helaouet, P.; Johns, D.G.; Jones, I.D.; Leech, D.I.; et al. Phenological sensitivity to climate across taxa and trophic levels. Nature 2016, 535, 241–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Z.; Piao, S.; Li, L.; Zhou, L.; Ciais, P.; Wang, T.; Li, Y.; Lian, X.; Wood, E.; Mao, J. Climate mitigation from vegetation biophysical feedbacks during the past three decades. Nat. Clim. Chang. 2017, 7, 432–436. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.F.; Sanchez-Castillo, M.; Dash, J.; Atkinson, P.M.; Ojeda-Zujar, J. Modelling interannual variation in the spring and autumn land surface phenology of the European forest. Biogeosciences 2016, 13, 3305–3317. [Google Scholar] [CrossRef] [Green Version]
- Zu, J.; Zhang, Y.; Huang, K.; Liu, Y.; Chen, N.; Cong, N. Biological and climate factors co-regulated spatial-temporal dynamics of vegetation autumn phenology on the Tibetan Plateau. Int. J. Appl. Earth Obs. Geoinf. 2018, 69, 198–205. [Google Scholar] [CrossRef]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; Keefe, J.O.; Schmid, H.P.; Wing, I.S. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Clim. Chang. 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Wu, C.; Hou, X.; Peng, D.; Gonsamo, A.; Xu, S. Land surface phenology of China’s temperate ecosystems over 1999–2013: Spatial–temporal patterns, interaction effects, covariation with climate and implications for productivity. Agric. For. Meteorol. 2016, 216, 177–187. [Google Scholar] [CrossRef]
- Beurs, K.M.D.; Henebry, G.M. Land surface phenology and temperature variation in the International Geosphere-Biosphere Program high-latitude transects. Glob. Chang. Biol. 2010, 11, 779–790. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Stoeckli, R.; Vidale, P.L. European plant phenology and climate as seen in a 20-year AVHRR land-surface parameter dataset. Int. J. Remote Sens. 2004, 25, 3303–3330. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Zhou, L.; Ciais, P.; Zhu, B. Variations in satellite-derived phenology in China’s temperate vegetation. Glob. Chang. Biol. 2010, 12, 672–685. [Google Scholar] [CrossRef]
- Jeong, S.J.; Chang-Hoi, H.O.; Gim, H.J.; Brown, M.E. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob. Chang. Biol. 2011, 17, 2385–2399. [Google Scholar] [CrossRef]
- Shen, M.; Tang, Y.; Chen, J.; Zhu, X.; Zheng, Y. Influences of temperature and precipitation before the growing season on spring phenology in grasslands of the central and eastern Qinghai-Tibetan Plateau. Agric. For. Meteorol. 2011, 151, 1711–1722. [Google Scholar] [CrossRef]
- Yuan, M.; Zhao, L.; Lin, A.; Li, Q.; Qu, S. How do climatic and non-climatic factors contribute to the dynamics of vegetation autumn phenology in the Yellow River Basin, China? Ecol. Indic. 2020, 112, 106112. [Google Scholar] [CrossRef]
- Wang, C.; Guo, H.; Zhang, L.; Liu, S.; Qiu, Y.; Sun, Z. Assessing phenological change and climatic control of alpine grasslands in the Tibetan Plateau with MODIS time series. Int. J. Biometeorol. 2015, 59, 11–23. [Google Scholar] [CrossRef]
- Flynn, D.; Wolkovich, E.M. Temperature and photoperiod drive spring phenology across all species in a temperate forest community. New Phytol. 2018, 219, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Piao, S.; Fu, Y.H.; Gao, M.; Peñuelas, J.; Janssens, I.A. Climatic Warming Increases Spatial Synchrony in Spring Vegetation Phenology Across the Northern Hemisphere. Geophys. Res. Lett. 2019, 46, 1641–1650. [Google Scholar] [CrossRef] [Green Version]
- Prevéy, J.S.; Seastedt, T.R. Seasonality of precipitation interacts with exotic species to alter composition and phenology of a semi-arid grassland. J. Ecol. 2014, 102, 1549–1561. [Google Scholar] [CrossRef]
- Way, D.A.; Montgomery, R.A. Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant Cell Environ. 2015, 38, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Ztürk, M.; Bolat, L.; Ergün, A. Influence of air-soil temperature on leaf expansion and LAI of Carpinus betulus trees in a temperate urban forest patch. Agric. For. Meteorol. 2015, 200, 185–191. [Google Scholar] [CrossRef]
- Jin, Z.; Zhuang, Q.; He, J.S.; Luo, T.; Shi, Y. Phenology shift from 1989 to 2008 on the Tibetan Plateau: An analysis with a process-based soil physical model and remote sensing data. Clim. Chang. 2013, 119, 435–449. [Google Scholar] [CrossRef]
- Zhou, Y. Asymmetric Behavior of Vegetation Seasonal Growth and the Climatic Cause: Evidence from Long-Term NDVI Dataset in Northeast China. Remote Sens. 2019, 11, 2107. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Nelson, F.E.; Shiklomanov, N.I.; Guo, D.; Wan, G. Permafrost degradation and its environmental effects on the Tibetan Plateau: A review of recent research. Earth-Sci. Rev. 2010, 103, 31–44. [Google Scholar] [CrossRef]
- Park, H.; Jeong, S.J.; Ho, C.H.; Kim, J.; Brown, M.E.; Schaepman, M.E. Nonlinear response of vegetation green-up to local temperature variations in temperate and boreal forests in the Northern Hemisphere. Remote Sens. Environ. 2015, 165, 100–108. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liang, M.; Li, Z.; Pierre, M.; Tong, X.; Zhang, J.; Dong, L.; Zheng, Y.; Ma, W.; Zhao, L. Plant functional groups mediate effects of climate and soil factors on species richness and community biomass in grasslands of Mongolian Plateau. J. Plant Ecol. 2021, 14, 679–691. [Google Scholar] [CrossRef]
- Zhao, M.; Peng, C.; Xiang, W.; Deng, X.; Tian, D.; Zhou, X.; Yu, G.; He, H.; Zhao, Z. Plant phenological modeling and its application in global climate change research: Overview and future challenges. Environ. Rev. 2013, 21, 1–14. [Google Scholar] [CrossRef]
- Deng, C.; Bai, H.; Ma, X.; Zhao, T.; Huang, X. Spatiotemporal differences in the climatic growing season in the Qinling Mountains of China under the influence of global warming from 1964 to 2015. Theor. Appl. Climatol. 2019, 138, 1899–1911. [Google Scholar] [CrossRef]
- Bai, W.G.; Zhang, L. Phytocoenological characteristics and community classification of Abies fargesii forests in Qinling Mountains. J. Beijing For. Univ. 2007, S2, 222–226. [Google Scholar]
- Myneni, R.B.; Keeling, C.D.; Tucker, C.J.; Asrar, G.; Nemani, R.R. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 1997, 386, 698–702. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, J.; Li, X.; Cheng, G.; Ma, M.; Zhu, G.; Altaf Arain, M.; Andrew Black, T.; Jassal, R.S. No trends in spring and autumn phenology during the global warming hiatus. Nat. Commun. 2019, 10, 2389. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Yang, C.; Shen, M.; Chen, J.; Ji, Z.; Cong, W.; Wei, Y. A simple method to improve the quality of NDVI time-series data by integrating spatiotemporal information with the Savitzky-Golay filter. Remote Sens. Environ. 2018, 217, 244–257. [Google Scholar] [CrossRef]
- Kaur, R.; Kiran, G.S.; Shah, M.N.; Mistry, N.V.; Mohan, S. Applicability of Smoothing Techniques in Generation of Phenological Metrics of Tectona grandis L. Using NDVI Time Series Data. Remote Sens. 2021, 13, 3343. [Google Scholar]
- Gocic, M.; Trajkovic, S. Analysis of changes in meteorological variables using Mann-Kendall and Sen’s slope estimator statistical tests in Serbia. Glob. Planet. Chang. 2013, 100, 172–182. [Google Scholar] [CrossRef]
- Mann, H.B. Nonparametric test against trend. Econometrica 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Garonna, I.; Jong, R.D.; Wit, A.D.; Mücher, C.A.; Schmid, B.; Schaepman, M.E. Strong contribution of autumn phenology to changes in satellite-derived growing season length estimates across Europe (1982–2011). Glob. Chang. Biol. 2015, 20, 3457–3470. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.M.; Ahmed, F.B.; Ismail, R. Random forest regression and spectral band selection for estimating sugarcane leaf nitrogen concentration using EO-1 Hyperion hyperspectral data. Int. J. Remote Sens. 2013, 34, 712–728. [Google Scholar] [CrossRef]
- Tian, F.; Cai, Z.; Jin, H.; Hufkens, K.; Scheifinger, H.; Tagesson, T.; Smets, B.; Van Hoolst, R.; Bonte, K.; Ivits, E.; et al. Calibrating vegetation phenology from Sentinel-2 using eddy covariance, PhenoCam, and PEP725 networks across Europe. Remote Sens. Environ. 2021, 260, 112456. [Google Scholar] [CrossRef]
- Sun, Q.; Li, B.; Zhou, G.; Jiang, Y.; Yuan, Y. Delayed autumn leaf senescence date prolongs the growing season length of herbaceous plants on the Qinghai-Tibetan Plateau. Agric. For. Meteorol. 2020, 284, 107896. [Google Scholar] [CrossRef]
- Wang, X.; Piao, S.; Xu, X.; Ciais, P.; Macbean, N.; Myneni, R.B.; Li, L. Has the advancing onset of spring vegetation green-up slowed down or changed abruptly over the last three decades? Glob. Ecol. Biogeogr. 2015, 24, 621–631. [Google Scholar] [CrossRef]
- Zeng, H.; Jia, G.; Epstein, H. Recent changes in phenology over the northern high latitudes detected from multi-satellite data. Environ. Res. Lett. 2011, 6, 45508–45518. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Qin, Y.; Feng, G.; Meng, Q.; Liu, G. Forest Phenology Dynamics to Climate Change and Topography in a Geographic and Climate Transition Zone: The Qinling Mountains in Central China. Forests 2019, 10, 1007. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ge, Q.; Dai, J.; Tao, Z. Geographical pattern in first bloom variability and its relation to temperature sensitivity in the USA and China. Int. J. Biometeorol. 2015, 59, 961–969. [Google Scholar] [CrossRef]
- Panchen, Z.A.; Primack, R.B.; Nordt, B.; Ellwood, E.R.; Stevens, A.D.; Renner, S.S.; Willis, C.G.; Fahey, R.; Whittemore, A.; Du, Y. Leaf out times of temperate woody plants are related to phylogeny, deciduousness, growth habit and wood anatomy. New Phytol. 2014, 203, 1208–1219. [Google Scholar] [CrossRef]
- Kurukulasuriya, P.; Mendelsohn, R. Impact And Adaptation Of South-East Asian Farmers To Climate Change: Conclusions And Policy Recommendations. Clim. Chang. Econ. 2017, 8, 1740007. [Google Scholar] [CrossRef]
- Qiu, B.W.; Zhong, M. Spatiotemporal variability of vegetation phenology with reference to altitude and climate in the subtropical mountain and hill region, China. Chin. Sci. Bull. 2013, 58, 2883–2892. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Zhu, W.; Chen, G.; Jiang, N.; Fan, D.; Zhang, D. Continuous but diverse advancement of spring-summer phenology in response to climate warming across the Qinghai-Tibetan Plateau. Agric. For. Meteorol. 2016, 223, 194–202. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, H.; Rutishauser, T.; Dai, J. Phenological response to climate change in China: A meta-analysis. Glob. Chang. Biol. 2015, 21, 265–274. [Google Scholar] [CrossRef]
- Park, T.; Ganguly, S.; T Mmervik, H.; Euskirchen, E.S.; H Gda, K.A.; Karlsen, S.R.; Brovkin, V.; Nemani, R.R.; Myneni, R.B. Changes in growing season duration and productivity of northern vegetation inferred from long-term remote sensing data. Environ. Res. Lett. 2016, 11, 84001. [Google Scholar] [CrossRef]
- Shen, M.; Piao, S.; Dorji, T.; Liu, Q.; Cong, N.; Chen, X.; An, S.; Wang, S.; Wang, T.; Zhang, G. Plant phenological responses to climate change on the Tibetan Plateau: Research status and challenges. Natl. Sci. Rev. 2015, 2, 454–467. [Google Scholar] [CrossRef] [Green Version]
- Cong, N.; Huang, K.; Zhang, Y. Unsynchronized Driving Mechanisms of Spring and Autumn Phenology Over Northern Hemisphere Grasslands. Front. For. Glob. Chang. 2021, 3, 610162. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Xiong, J.; He, D.; Cheng, W.; Ye, C.; Yong, Z.; Huang, X. Dynamics and Drivers of Vegetation Phenology in Three-River Headwaters Region Based on the Google Earth Engine. Remote Sens. 2021, 13, 2528. [Google Scholar] [CrossRef]
- Mcwatters, H.G.; Devlin, P.F. Timing in plants—A rhythmic arrangement. Febs. Lett. 2011, 585, 1474–1484. [Google Scholar] [CrossRef] [Green Version]
- Stone, P.J.; Sorensen, I.B.; Jamieson, P.D. Effect of soil temperature on phenology, canopy development, biomass and yield of maize in a cool-temperate climate. Field Crop. Res. 1999, 63, 169–178. [Google Scholar] [CrossRef]
- Chen, X.; Ciais, P.; Maignan, F.; Zhang, Y.; Zhang, H. Vapor Pressure Deficit and Sunlight Explain Seasonality of Leaf Phenology and Photosynthesis Across Amazonian Evergreen Broadleaved Forest. Glob. Biogeochem. Cycles 2021, 35, e2020GB006893. [Google Scholar] [CrossRef]
- Diez, J.M.; Ibáñez, I.; Miller-Rushing, A.J.; Mazer, S.J.; Crimmins, T.M.; Crimmins, M.A.; Bertelsen, C.D.; Inouye, D.W. Forecasting phenology: From species variability to community patterns. Ecol. Lett. 2012, 15, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Wang, C.; Sun, Q.; Rong, G.; Tong, Z.; Liu, X.; Zhang, J. Spring Phenological Sensitivity to Climate Change in the Northern Hemisphere: Comprehensive Evaluation and Driving Force Analysis. Remote Sens. 2021, 10, 1972. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, A.; Cao, S.; Liu, X.; Liu, J.; Cheng, D. Responses of vegetation productivity to multi-scale drought in Loess Plateau, China. Catena 2017, 163, 165–171. [Google Scholar] [CrossRef]
- Chuine, I. A unified model for budburst of trees. J. Theor. Biol. 2000, 207, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Z.; Zhang, H.F.; Yan, J.W.; Wang, G.Y.; Zhou, T.M.; Xu, G.; Innes, J.L.; Che, M.L.; Dou, X.M. Spatial and temporal variations in the end date of the vegetation growing season throughout the Qinghai-Tibetan Plateau from 1982 to 2011. Agric. For. Meteorol. 2014, 189–190, 81–90. [Google Scholar]
- Gerten, D.; Schaphoff, S.; Lucht, W. Potential future changes in water limitations of the terrestrial biosphere. Clim. Chang. 2007, 80, 277–299. [Google Scholar] [CrossRef]









| Dataset | Spatial Resolution | Temporal Resolution | Time Span | Source |
|---|---|---|---|---|
| MODIS13A2 NDVI | 1 km | 16 days | 2001–2019 | The Level-1 and Atmosphere Archive and Distribution System Distributed Active Archive Center (LAADS DAAC) (https://search.earthdata.nasa.gov/search/, accessed on 15 October 2020). |
| Land cover (CCI-LC) | 300 m | Yearly | 2001–2019 | http://maps.elie.ucl.ac.be/CCI/viewer/index.php, accessed on 20 October 2020 |
| Temperature | 0.1° | hourly | 2001–2019 | The Reanalysis (ERA5) climatic datasets (https://cds.climate.copernicus.eu, accessed on 12 November 2020) |
| Precipitation | 0.1° | hourly | 2001–2019 | |
| Shortwave radiation | 0.1° | hourly | 2001–2019 | |
| Soil temperature | 0.1° | hourly | 2001–2019 | |
| Soil moisture | 0.1° | hourly | 2001–2019 |
| Change Pattern | Trend of SOS | Trend of EOS | Trend of LOS |
|---|---|---|---|
| I | Advanced (−) | Delayed (+) | Lengthened (+) |
| II | Advanced (−) | Advanced (−) | Lengthened (+) |
| III | Advanced (−) | Advanced (−) | Shortened (−) |
| IV | Delayed (+) | Advanced (−) | Shortened (−) |
| V | Delayed (+) | Delayed (+) | Lengthened (+) |
| VI | Delayed (+) | Delayed (+) | Shortened (−) |
| Variables | SOS Drivers | EOS Drivers |
|---|---|---|
| Meteorological factors | Preseason average temperature * (TP) | Preseason average temperature ** (TP) |
| Growing season average temperature (TG) | Growing season average temperature (TG) | |
| Preseason total precipitation * (PP) | Preseason total precipitation ** (PP) | |
| Growing season total precipitation (PG) | Growing season total precipitation (PG) | |
| Preseason mean shortwave radiation * (SWP) | Preseason mean shortwave radiation ** (SWP) | |
| Growing season mean shortwave radiation (SWG) | Growing season mean shortwave radiation (SWG) | |
| Soil factors | Preseason soil temperature * (STP) | Preseason soil temperature ** (STP) |
| Growing season soil temperature (STG) | Growing season soil temperature (STG) | |
| Preseason soil moisture * (SMP) | Preseason soil moisture ** (SMP) | |
| Growing season soil moisture (SMG) | Growing season soil moisture (SMG) | |
| Biological factors | Maximum NDVI during growing season (MN) | Maximum NDVI during growing season (MN) |
| Middle season date (MD) | Middle season date (MD) |
| Change Patterns | All the Vegetation Types | ENF | EBF | DBF | MF | SL | GL | CL |
|---|---|---|---|---|---|---|---|---|
| I | 48.4% | 46.6% | 50.1% | 49.3% | 47.2% | 43.2% | 44.8% | 48.7% |
| II | 12.0% | 10.9% | 9.5% | 13.7% | 11.8% | 6.9% | 15.2% | 10.5% |
| III | 7.3% | 6.8% | 6.2% | 8.6% | 7.3% | 3.1% | 7.6% | 5.9% |
| IV | 8.7% | 9.0% | 7.7% | 7.9% | 8.8% | 15.5% | 12.2% | 9.3% |
| V | 15.2% | 16.8% | 18.0% | 12.4% | 16.5% | 23.5% | 12.7% | 17.2% |
| VI | 8.5% | 9.9% | 8.5% | 8.1% | 8.4% | 7.8% | 7.5% | 8.6% |
| Total | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% |
| All the Vegetation Types | SOS Controlled | EOS Controlled | Total |
|---|---|---|---|
| ENF | 46.9% | 53.1% | 100% |
| EBF | 43.4% | 56.6% | 100% |
| DBF | 53.4% | 46.6% | 100% |
| MF | 47.2% | 52.8% | 100% |
| SL | 41.7% | 58.3% | 100% |
| GL | 52.0% | 48.0% | 100% |
| CL | 45.9% | 54.1% | 100% |
| The whole area | 48.6% | 51.4% | 100% |
| LSP | All the Vegetation Types | First Dominant Driver | Second Dominant Driver | Third Dominant Driver |
|---|---|---|---|---|
| SOS | ENF | SWP | MD | STP |
| EBF | MD | SWP | PP | |
| DBF | MD | SWP | STP | |
| MF | SWP | MD | STP | |
| SL | STP | TP | SWP | |
| GL | SWP | MD | STP | |
| CL | SWP | MD | TG | |
| the whole area | SWP | MD | STP | |
| EOS | ENF | SWP | TP | PP |
| EBF | SWP | MD | PP | |
| DBF | SWP | PP | TP | |
| MF | SWP | PP | MD | |
| SL | SWP | PP | MD | |
| GL | SWP | SWG | TP | |
| CL | SWP | TP | PP | |
| the whole area | SWP | PP | MD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Liu, X.; Ge, W.; Ni, X.; Ma, W.; Lu, Q.; Xing, X. Specific Drivers and Responses to Land Surface Phenology of Different Vegetation Types in the Qinling Mountains, Central China. Remote Sens. 2021, 13, 4538. https://doi.org/10.3390/rs13224538
Guo J, Liu X, Ge W, Ni X, Ma W, Lu Q, Xing X. Specific Drivers and Responses to Land Surface Phenology of Different Vegetation Types in the Qinling Mountains, Central China. Remote Sensing. 2021; 13(22):4538. https://doi.org/10.3390/rs13224538
Chicago/Turabian StyleGuo, Jiaqi, Xiaohong Liu, Wensen Ge, Xiaofeng Ni, Wenyuan Ma, Qiangqiang Lu, and Xiaoyu Xing. 2021. "Specific Drivers and Responses to Land Surface Phenology of Different Vegetation Types in the Qinling Mountains, Central China" Remote Sensing 13, no. 22: 4538. https://doi.org/10.3390/rs13224538
APA StyleGuo, J., Liu, X., Ge, W., Ni, X., Ma, W., Lu, Q., & Xing, X. (2021). Specific Drivers and Responses to Land Surface Phenology of Different Vegetation Types in the Qinling Mountains, Central China. Remote Sensing, 13(22), 4538. https://doi.org/10.3390/rs13224538