Yield Prediction in Soybean Crop Grown under Different Levels of Water Availability Using Reflectance Spectroscopy and Partial Least Squares Regression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Spectral Data Acquisition and Processing
2.3. Statistical Analysis
Partial Least Squares Regression—PLSR
3. Results and Discussion
3.1. Effect of Experimental Treatments on Climatic Water Balance, Soil Moisture, Grain Yield and Leaf Reflectance
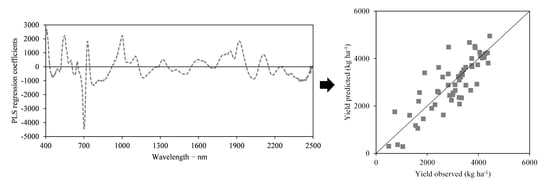
3.2. Predicting Soybean Grain Yield through Partial Least Squares Regression—PLSR
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CONAB (National Company of Food Supply). Brazilian Crop Assessment–Grain, 2019/2020 Crops, Sixth Inventory Survey, March/2020. 2020. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 25 March 2020).
- USDA (United States Department of Agriculture). World Agricultural Production. Circular Series WAP 3–20 March 2020. 2020. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 25 March 2020).
- Battisti, R.; Sentelhas, P.C.; Pascoalino, J.A.L.; Sako, H.; Dantas, J.P.D.S.; Moraes, M.F. Soybean Yield Gap in the Areas of Yield Contest in Brazil. Int. J. Plant Prod. 2018, 12, 159–168. [Google Scholar] [CrossRef]
- Sentelhas, P.C.; Battisti, R.; Câmara, G.M.S.; Farias, J.R.B.; Hampf, A.C.; Nendel, C. The soybean yield gap in Brazil—magnitude, causes and possible solutions for sustainable production. J. Agric. Sci. 2015, 153, 1394–1411. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.C. Quantificação das Perdas por Seca na Cultura da Soja o Brasil. Ph.D. Thesis, Universidade Estadual de Londrina, Londrina, Brazil, 2016. [Google Scholar]
- Rio, A.D.; Sentelhas, P.C.; Farias, J.R.B.; Sibaldelli, R.N.R.; Ferreira, R.C. Alternative sowing dates as a mitigation measure to reduce climate change impacts on soybean yields in southern Brazil. Int. J. Clim. 2015, 36, 3664–3672. [Google Scholar] [CrossRef]
- Gusso, A.; Ducati, J.R. Algorithm for Soybean Classification Using Medium Resolution Satellite Images. Remote Sens. 2012, 4, 3127–3142. [Google Scholar] [CrossRef] [Green Version]
- De Souza, C.H.W.; Mercante, E.; Johann, J.A.; Lamparelli, R.A.C.; Uribe-Opazo, M.A. Mapping and discrimination of soya bean and corn crops using spectro-temporal profiles of vegetation indices. Int. J. Remote Sens. 2015, 36, 1809–1824. [Google Scholar] [CrossRef]
- Song, X.-P.; Potapov, P.V.; Krylov, A.; King, L.; Di Bella, C.M.; Hudson, A.; Khan, A.; Adusei, B.; Stehman, S.V.; Hansen, M.C. National-scale soybean mapping and area estimation in the United States using medium resolution satellite imagery and field survey. Remote Sens. Environ. 2017, 190, 383–395. [Google Scholar] [CrossRef]
- Da Silva, C.A.; Nanni, M.R.; Teodoro, P.E.; Silva, G.F.C. Vegetation Indices for Discrimination of Soybean Areas: A New Approach. Agron. J. 2017, 109, 1331–1343. [Google Scholar] [CrossRef]
- Bolton, D.K.; Friedl, M.A. Forecasting crop yield using remotely sensed vegetation indices and crop phenology metrics. Agric. For. Meteorol. 2013, 173, 74–84. [Google Scholar] [CrossRef]
- Johnson, D.M. An assessment of pre- and within-season remotely sensed variables for forecasting corn and soybean yields in the United States. Remote Sens. Environ. 2014, 141, 116–128. [Google Scholar] [CrossRef]
- Sakamoto, T. Incorporating environmental variables into a MODIS-based crop yield estimation method for United States corn and soybeans through the use of a random forest regression algorithm. ISPRS J. Photogramm. Remote Sens. 2020, 160, 208–228. [Google Scholar] [CrossRef]
- Yu, N.; Li, L.; Schmitz, N.; Tian, L.F.; Greenberg, J.A.; Diers, B.W. Development of methods to improve soybean yield estimation and predict plant maturity with an unmanned aerial vehicle based platform. Remote Sens. Environ. 2016, 187, 91–101. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Yang, G.; Liu, J.; Cao, J.; Li, C.; Zhao, X.; Gai, J. Establishment of Plot-Yield Prediction Models in Soybean Breeding Programs Using UAV-Based Hyperspectral Remote Sensing. Remote Sens. 2019, 11, 2752. [Google Scholar] [CrossRef] [Green Version]
- Maimaitijiang, M.; Sagan, V.; Sidike, P.; Hartling, S.; Esposito, F.; Fritschi, F.B. Soybean yield prediction from UAV using multimodal data fusion and deep learning. Remote Sens. Environ. 2020, 237, 111599. [Google Scholar] [CrossRef]
- Braga, P.; Crusiol, L.G.T.; Nanni, M.R.; Caranhato, A.L.H.; Fuhrmann, M.B.; Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B.; Koltun, A.; Gonçalves, L.S.A.; et al. Vegetation indices and NIR-SWIR spectral bands as a phenotyping tool for water status determination in soybean. Precis. Agric. 2021, 22, 249–266. [Google Scholar] [CrossRef]
- Zhou, Z.; Morel, J.; Parsons, D.; Kucheryavskiy, S.V.; Gustavsson, A.-M. Estimation of yield and quality of legume and grass mixtures using partial least squares and support vector machine analysis of spectral data. Comput. Electron. Agric. 2019, 162, 246–253. [Google Scholar] [CrossRef]
- Christenson, B.S.; Schapaugh, W.T.; An, N.; Price, K.P.; Prasad, V.; Fritz, A.K. Predicting Soybean Relative Maturity and Seed Yield Using Canopy Reflectance. Crop Sci. 2016, 56, 625–643. [Google Scholar] [CrossRef] [Green Version]
- Weber, V.S.; Araus, J.L.; Cairns, J.E.; Sanchez, C.; Melchinger, A.E.; Orsini, E. Prediction of grain yield using reflectance spectra of canopy and leaves in maize plants grown under different water regimes. Field Crops Res. 2012, 128, 82–90. [Google Scholar] [CrossRef]
- Herrmann, I.; Bdolach, E.; Montekyo, Y.; Rachmilevitch, S.; Townsend, P.A.; Karnieli, A. Assessment of maize yield and phenology by drone-mounted superspectral camera. Precis. Agric. 2020, 21, 51–76. [Google Scholar] [CrossRef]
- Hansen, P.M.; Jørgensen, J.R.; Thomsen, A. Predicting grain yield and protein content in winter wheat and spring barley using repeated canopy reflectance measurements and partial least squares regression. J. Agric. Sci. 2002, 139, 307–318. [Google Scholar] [CrossRef]
- Sharabian, V.R.; Noguchi, N.; Ishi, K. Significant wavelengths for prediction of winter wheat growth status and grain yield using multivariate analysis. Eng. Agric. Environ. Food 2014, 7, 14–21. [Google Scholar] [CrossRef]
- Barmeier, G.; Hofer, K.; Schmidhalter, U. Mid-season prediction of grain yield and protein content of spring barley cultivars using high-throughput spectral sensing. Eur. J. Agron. 2017, 90, 108–116. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y. Rapid estimation of seed yield using hyperspectral images of oilseed rape leaves. Ind. Crops Prod. 2013, 42, 416–420. [Google Scholar] [CrossRef]
- Ancin-Murguzur, F.J.; Taff, G.; Davids, C.; Tømmervik, H.; Mølmann, J.; Jørgensen, M. Yield Estimates by a Two-Step Approach Using Hyperspectral Methods in Grasslands at High Latitudes. Remote Sens. 2019, 11, 400. [Google Scholar] [CrossRef] [Green Version]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakaiya, E.; Zhu, Y.; Takahashi, W. Diagnostic mapping of canopy nitrogen content in rice based on hyperspectral measurements. Remote Sens. Environ. 2012, 126, 210–221. [Google Scholar] [CrossRef]
- Li, F.; Mistele, B.; Hu, Y.; Chen, X.; Schmidhalter, U. Reflectance estimation of canopy nitrogen content in winter wheat using optimised hyperspectral spectral indices and partial least squares regression. Eur. J. Agron. 2014, 52, 198–209. [Google Scholar] [CrossRef]
- Wrege, M.S.; Steinmetz, S.; Reiser, C., Jr.; de Almeida, I.R. Atlas Climático da Região Sul do Brasil: Estados do Paraná, Santa Catarina e Rio Grande do Sul; Embrapa Clima Temperado: Pelotas, Brazil; Embrapa Florestas: Colombo, Brazil, 2011. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- USDA (United States Department of Agriculture)—Natural Resources Conservation Service. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; USDA: Washington, DC, USA, 1999.
- Embrapa Soja. Tecnologias de Produção de Soja–Região Central do Brasil 2014; Embrapa Soja: Londrina, Brazil, 2013. [Google Scholar]
- Fehr, W.R.; Caviness, C.E. Stages of Soybean Development; Special Report 80; Iowa State University of Science and Technology: Ames, IA, USA, 1977. [Google Scholar]
- Sibaldelli, R.N.R.; Farias, J.R.B. Boletim Agrometeorológico da Embrapa Soja, Londrina, PR–2016; Embrapa Soja: Londrina, Brazil, 2017; Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1067152 (accessed on 15 June 2020).
- Sibaldelli, R.N.R.; Farias, J.R.B. Boletim Agrometeorológico da Embrapa Soja, Londrina, PR–2017; Embrapa Soja: Londrina, Brazil, 2018; Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1087963 (accessed on 15 June 2020).
- Sibaldelli, R.N.R.; Farias, J.R.B. Boletim Agrometeorológico da Embrapa Soja, Londrina, PR–2018; Embrapa Soja: Londrina, Brazil, 2019; Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1109091/1/DOC4111.pdf (accessed on 15 June 2020).
- Thornthwaite, C.W.; Mather, J.R. The Water Balance; Laboratory of Climatology: Centerton, AR, USA, 1955. [Google Scholar]
- Rumpf, T.; Mahlein, A.-K.; Steiner, U.; Oerke, E.-C.; Dehne, H.-W.; Plümer, L. Early detection and classification of plant diseases with Support Vector Machines based on hyperspectral reflectance. Comput. Electron. Agric. 2010, 74, 91–99. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Steiner, U.; Dehne, H.-W.; Oerke, E.-C. Spectral signatures of sugar beet leaves for the detection and differentiation of diseases. Precis. Agric. 2010, 11, 413–431. [Google Scholar] [CrossRef]
- Da Silva, C.A., Jr.; Nanni, M.R.; Shakir, M.; Teodoro, P.E.; De Oliveira, J.F., Jr.; Cézar, E.; De Gois, G.; Lima, M.; Wojciechowski, J.C.; Shiratsuchi, L.S. Soybean varieties discrimination using non-imaging hyperspectral sensor. Infrared Phys. Technol. 2018, 89, 338–350. [Google Scholar] [CrossRef]
- Streher, A.S.; da Silva Torres, R.; Morellato, L.P.C.; Silva, T.S.F. Accuracy and limitations for spectroscopic prediction of leaf traits in seasonally dry tropical environments. Remote Sens. Environ. 2020, 244, 111828. [Google Scholar] [CrossRef]
- Crusiol, L.G.T.; Nanni, M.R.; Furlanetto, R.H.; Sibaldelli, R.N.R.; Cezar, E.; Sun, L.; Foloni, J.S.S.; Mertz-Henning, L.M.; Nepomuceno, A.L.; Neumaier, N.; et al. Classification of Soybean Genotypes Assessed Under Different Water Availability and at Different Phenological Stages Using Leaf-Based Hyperspectral Reflectance. Remote Sens. 2021, 13, 172. [Google Scholar] [CrossRef]
- Schmidt, K.; Skidmore, A. Spectral discrimination of vegetation types in a coastal wetland. Remote Sens. Environ. 2003, 85, 92–108. [Google Scholar] [CrossRef]
- Peng, Y.; Fan, M.; Song, J.; Cui, T.; Li, R. Assessment of plant species diversity based on hyperspectral indices at a fine scale. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência e Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef] [Green Version]
- Yendrek, C.R.; Tomaz, T.; Montes, C.M.; Cao, Y.; Morse, A.M.; Brown, P.J.; McIntyre, L.M.; Leakey, A.D.; Ainsworth, E.A. High-Throughput Phenotyping of Maize Leaf Physiological and Biochemical Traits Using Hyperspectral Reflectance. Plant Physiol. 2017, 173, 614–626. [Google Scholar] [CrossRef]
- De Souza, A.M.; Breitkreitz, M.C.; Filgueiras, P.R.; Rohwedder, J.J.R.; Poppi, R.J. Experimento didático de quimiometria para calibração multivariada na determinação de paracetamol em comprimidos comerciais utilizando espectroscopia no infravermelho próximo: Um tutorial, parte II. Química Nova 2013, 36, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, S.D.S.S.; Nagai, V. Deficiência hídrica simulada nos diferentes estádios de desenvolvimento de um cultivar precoce de soja. Bragantia 1988, 47, 9–14. [Google Scholar] [CrossRef]
- Rolla, A.A.D.P.; Carvalho, J.D.F.C.; Fuganti-Pagliarini, R.; Engels, C.; Rio, A.D.; Marin, S.R.R.; De Oliveira, M.C.N.; Beneventi, M.A.; Marcelino-Guimarães, F.C.; Farias, J.R.B.; et al. Phenotyping soybean plants transformed with rd29A:AtDREB1A for drought tolerance in the greenhouse and field. Transgenic Res. 2013, 23, 75–87. [Google Scholar] [CrossRef]
- Carvalho, J.D.F.C.; Crusiol, L.G.T.; Perini, L.J.; Sibaldelli, R.N.R.; Ferreira, L.C.; Marcelino-Guimarães, F.C.; Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B. Phenotyping Soybeans for Drought Responses Using Remote Sensing Techniques and Non-Destructive Physiological Analysis. Glob. Sci. Technol. 2015, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Crusiol, L.G.T.; Carvalho, J.D.F.C.; Sibaldelli, R.N.R.; Neiverth, W.; Rio, A.D.; Ferreira, L.C.; Procópio, S.D.O.; Mertz-Henning, L.M.; Nepomuceno, A.L.; Neumaier, N.; et al. NDVI variation according to the time of measurement, sampling size, positioning of sensor and water regime in different soybean cultivars. Precis. Agric. 2016, 18, 470–490. [Google Scholar] [CrossRef] [Green Version]
- Damm, A.; Paul-Limoges, E.; Haghighi, E.; Simmer, C.; Morsdorf, F.; Schneider, F.; Van Der Tol, C.; Migliavacca, M.; Rascher, U. Remote sensing of plant-water relations: An overview and future perspectives. J. Plant Physiol. 2018, 227, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Maimaitiyiming, M.; Miller, A.J.; Ghulam, A. Discriminating Spectral Signatures among and within Two Closely Related Grapevine Species. Photogramm. Eng. Remote Sens. 2016, 82, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Simlat, M.; Ślęzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Pattaro, M.; Furlanetto, R.H.; Nanni, M.R.; Antunes, W.C. High resolution leaf spectral signature as a tool for foliar pigment estimation displaying potential for species differentiation. J. Plant Physiol. 2020, 249, 153161. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Melø, T.B.; Naqvi, K.R. Effect of anthocyanins, carotenoids, and flavonols on chlorophyll fluorescence excitation spectra in apple fruit: Signature analysis, assessment, modelling, and relevance to photoprotection. J. Exp. Bot. 2008, 59, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.; Solovchenko, A. Non-invasive quantification of foliar pigments: Possibilities and limitations of reflectance- and absorbance-based approaches. J. Photochem. Photobiol. B Biol. 2018, 178, 537–544. [Google Scholar] [CrossRef]
- Gitelson, A.; Chivkunova, O.; Zhigalova, T.; Solovchenko, A. In situ optical properties of foliar flavonoids: Implication for non-destructive estimation of flavonoid content. J. Plant Physiol. 2017, 218, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S.; Jay-Allemand, C.; Logan, B.A.; Baissac, Y.; Bidel, L.P. When are foliar anthocyanins useful to plants? Re-evaluation of the photoprotection hypothesis using Arabidopsis thaliana mutants that differ in anthocyanin accumulation. Environ. Exp. Bot. 2018, 154, 11–22. [Google Scholar] [CrossRef]
- Marinho, J.P.; Kanamori, N.; Ferreira, L.C.; Fuganti-Pagliarini, R.; Carvalho, J.D.F.C.; Freitas, R.A.; Marin, S.R.R.; Rodrigues, F.A.; Mertz-Henning, L.M.; Farias, J.R.B.; et al. Characterization of Molecular and Physiological Responses under Water Deficit of Genetically Modified Soybean Plants Overexpressing the AtAREB1 Transcription Factor. Plant Mol. Biol. Rep. 2015, 34, 410–426. [Google Scholar] [CrossRef]
- Honna, P.T.; Fuganti-Pagliarini, R.; Ferreira, L.C.; Molinari, M.D.C.; Marin, S.R.R.; De Oliveira, M.C.N.; Farias, J.R.B.; Neumaier, N.; Mertz-Henning, L.M.; Kanamori, N.; et al. Molecular, physiological, and agronomical characterization, in greenhouse and in field conditions, of soybean plants genetically modified with AtGolS2 gene for drought tolerance. Mol. Breed. 2016, 36, 157. [Google Scholar] [CrossRef] [Green Version]
- Daughtry, C.S.T.; Gallo, K.P.; Goward, S.N.; Prince, S.D.; Kustas, W.P. Spectral estimates of absorbed radiation and phytomass production in corn and soybean canopies. Remote Sens. Environ. 1992, 39, 141–152. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Singer, J.W.; Meek, D.W.; Sauer, T.J.; Prueger, J.H.; Hatfield, J.L. Variability of light interception and radiation use efficiency in maize and soybean. Field Crop. Res. 2011, 121, 147–152. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Peng, Y.; Arkebauer, T.J.; Suyker, A.E. Productivity, absorbed photosynthetically active radiation, and light use efficiency in crops: Implications for remote sensing of crop primary production. J. Plant Physiol. 2015, 177, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A. Remote estimation of fraction of radiation absorbed by photosynthetically active vegetation: Generic algorithm for maize and soybean. Remote Sens. Lett. 2018, 10, 283–291. [Google Scholar] [CrossRef]
- Moriwaki, T.; Falcioni, R.; Tanaka, F.A.O.; Cardoso, K.A.K.; Souza, L.A.; Benedito, E.; Nanni, M.R.; Bonato, C.M.; Antunes, W.C. Nitrogen-improved photosynthesis quantum yield is driven by increased thylakoid density, enhancing green light absorption. Plant Sci. 2019, 278, 1–11. [Google Scholar] [CrossRef]
- Manjunath, K.R.; Ray, S.S.; Panigrahy, S. Discrimination of Spectrally-Close Crops Using Ground-Based Hyperspectral Data. J. Indian Soc. Remote Sens. 2011, 39, 599–602. [Google Scholar] [CrossRef]
- Sahoo, R.N.; Ray, S.S.; Manjunath, K.R. Hyperspectral Remote Sensing of Agriculture. Curr. Sci. 2015, 108, 848–859. [Google Scholar]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral Properties of Plants. Appl. Opt. 1965, 4, 11–20. [Google Scholar] [CrossRef]
- Latimer, P. Apparent Shifts of Absorption Bands of Cell Suspensions and Selective Light Scattering. Science 1958, 127, 29–30. [Google Scholar] [CrossRef] [PubMed]
- Ustin, S.L.; Jacquemoud, S.; Govaerts, Y. Simulation of photon transport in a three-dimensional leaf: Implications for photosynthesis. Plant Cell Environ. 2001, 24, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.J. NMDI: A normalized multi-band drought index for monitoring soil and vegetation moisture with satellite remote sensing. Geophys. Res. Lett. 2007, 34, 20405. [Google Scholar] [CrossRef]
- Wang, J.; Xu, R.; Yang, S. Estimation of plant water content by spectral absorption features centered at 1450 nm and 1940 nm regions. Environ. Monit. Assess. 2008, 157, 459–469. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, B.-H.; Li, Z.-L. Retrieval of leaf water content from remotely sensed data using a vegetation index model constructed with shortwave infrared reflectances. Int. J. Remote Sens. 2018, 40, 2313–2323. [Google Scholar] [CrossRef]
- Kross, A.; Znoj, E.; Callegari, D.; Kaur, G.; Sunohara, M.; Lapen, D.R.; McNairn, H. Using Artificial Neural Networks and Remotely Sensed Data to Evaluate the Relative Importance of Variables for Prediction of Within-Field Corn and Soybean Yields. Remote Sens. 2020, 12, 2230. [Google Scholar] [CrossRef]
- Carneiro, F.M.; Furlani, C.E.A.; Zerbato, C.; De Menezes, P.C.; Gírio, L.A.D.S.; De Oliveira, M.F. Comparison between vegetation indices for detecting spatial and temporal variabilities in soybean crop using canopy sensors. Precis. Agric. 2019, 21, 979–1007. [Google Scholar] [CrossRef]
- Ferrio, J.; Villegas, D.; Zarco, J.; Aparicio, N.; Araus, J.; Royo, C. Assessment of durum wheat yield using visible and near-infrared reflectance spectra of canopies. Field Crop. Res. 2005, 94, 126–148. [Google Scholar] [CrossRef]
- Øvergaard, S.I.; Isaksson, T.; Kvaal, K.; Korsaeth, A. Comparisons of Two Hand-Held, Multispectral Field Radiometers and a Hyperspectral Airborne Imager in Terms of Predicting Spring Wheat Grain Yield and Quality by Means of Powered Partial Least Squares Regression. J. Near Infrared Spectrosc. 2010, 18, 247–261. [Google Scholar] [CrossRef]
- Fuganti-Pagliarini, R.; Ferreira, L.C.; Rodrigues, F.A.; Molinari, H.B.C.; Marin, S.R.R.; Molinari, M.D.C.; Marcolino-Gomes, J.; Mertz-Henning, L.M.; Farias, J.R.B.; De Oliveira, M.C.N.; et al. Characterization of Soybean Genetically Modified for Drought Tolerance in Field Conditions. Front. Plant Sci. 2017, 8, 448. [Google Scholar] [CrossRef] [Green Version]
- Stolf-Moreira, R.; Lemos, E.G.M.; Carareto-Alves, L.; Marcondes, J.; Pereira, S.S.; Rolla, A.A.P.; Pereira, R.M.; Neumaier, N.; Binneck, E.; Abdelnoor, R.V.; et al. Transcriptional Profiles of Roots of Different Soybean Genotypes Subjected to Drought Stress. Plant Mol. Biol. Rep. 2010, 29, 19–34. [Google Scholar] [CrossRef]









| pH | H++Al3+ | Al3+ | Ca2+ | Mg2+ | K+ | CTC | P | C | SB | V% | Clay |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O | cmolc dm−3 | mg dm−3 | g dm−3 | g kg−1 | |||||||
| 4.9 | 3.5 | 0.03 | 3.9 | 1.8 | 0.7 | 10.0 | 24.2 | 15.6 | 6.5 | 64.8 | 710 |
| Cropping Season | Sowing | Water Deficit Induced at Vegetative Stages | Water Deficit Induced at Reproductive Stages | Harvesting Period |
|---|---|---|---|---|
| 2016/2017 | 19 October 2016 | From 37 DAS to 54 DAS | From 54 DAS to the harvesting period | From 116 DAS |
| 2017/2018 | 18 October 2017 | From 33 DAS to 62 DAS | From 62 DAS to the harvesting period | From 139 DAS |
| 2018/2019 | 16 October 2018 | From 41 DAS to 64 DAS | From 41 DAS to 90 DAS | From 129 DAS |
| Cropping Season | Days after Sowing | Amount (mm) | Length (min) |
|---|---|---|---|
| 2016/2017 | 24 | 14.4 | 60 |
| 29 | 4.8 | 20 | |
| 30 | 7.2 | 30 | |
| 31 | 9.6 | 40 | |
| 34 | 4.8 | 20 | |
| 35 | 4.8 | 20 | |
| 36 | 4.8 | 20 | |
| 37 | 4.8 | 20 | |
| 38 | 14.4 | 60 | |
| 2018/2019 | 52 | 14.4 | 60 |
| 53 | 14.4 | 60 | |
| 57 | 11.5 | 48 | |
| 58 | 5.7 | 24 | |
| 59 | 5.7 | 24 | |
| 61 | 8.4 | 35 | |
| 66 | 2.9 | 12 | |
| 106 | 7.2 | 30 | |
| 109 | 8.4 | 35 | |
| 114 | 11.5 | 48 | |
| 115 | 2.9 | 12 | |
| 116 | 8.4 | 35 | |
| 119 | 4.8 | 20 |
| 2016/2017 Cropping Season | 2017/2018 Cropping Season | 2018/2019 Cropping Season | |||
|---|---|---|---|---|---|
| DAS | Spectral Samples | DAS | Spectral Samples | DAS | Spectral Samples |
| 28 | 64 | 29 | 80 | 41 | 80 |
| 33 | 64 | 37 | 80 | 50 | 80 |
| 44 | 64 | 42 | 80 | 57 | 80 |
| 57 | 64 | 57 | 80 | 64 | 80 |
| 69 | 64 | 78 | 80 | 79 | 80 |
| 89 | 64 | 96 | 80 | 87 | 80 |
| 112 | 60 | 106 | 80 | 94 | 80 |
| - | - | 113 | 80 | 101 | 80 |
| - | - | - | - | 107 | 80 |
| Cropping Season | Days after Sowing | Spectral Samples | Rc2 | Rcv2 | RMSEc (kg ha−1) | RMSEcv (kg ha−1) | RMSEc % | RMSEcv % | BIAScv |
|---|---|---|---|---|---|---|---|---|---|
| 2016/2017 | 28 | 64 | 0.168 | 0.088 | 588.43 | 625.72 | 19.96 | 21.22 | 7.286 |
| 33 | 64 | 0.134 | 0.083 | 600.12 | 627.55 | 20.36 | 21.29 | 0.854 | |
| 44 | 64 | 0.407 | 0.105 | 496.77 | 619.91 | 16.85 | 21.03 | 6.646 | |
| 57 | 64 | 0.573 | 0.316 | 421.44 | 541.83 | 14.29 | 18.38 | 4.036 | |
| 69 | 64 | 0.641 | 0.529 | 386.42 | 449.36 | 13.11 | 15.24 | 7.225 | |
| 89 | 64 | 0.731 | 0.595 | 334.11 | 416.81 | 11.33 | 14.14 | −6.651 | |
| 112 | 60 | 0.611 | 0.590 | 403.51 | 421.29 | 13.69 | 14.29 | 4.686 | |
| 2017/2018 | 29 | 80 | 0.510 | 0.265 | 893.46 | 1108.96 | 19.76 | 24.52 | −12.929 |
| 37 | 80 | 0.440 | 0.189 | 955.69 | 1164.52 | 21.13 | 25.75 | −13.176 | |
| 42 | 80 | 0.601 | 0.314 | 806.25 | 1070.67 | 17.83 | 23.67 | 7.477 | |
| 57 | 80 | 0.159 | 0.167 | 1171.35 | 1180.37 | 25.90 | 26.10 | 32.699 | |
| 78 | 80 | 0.913 | 0.881 | 374.97 | 445.10 | 8.29 | 9.84 | 2.867 | |
| 96 | 80 | 0.924 | 0.885 | 351.49 | 437.22 | 7.77 | 9.67 | 5.618 | |
| 106 | 80 | 0.921 | 0.866 | 358.21 | 473.19 | 7.92 | 10.46 | 3.037 | |
| 113 | 80 | 0.847 | 0.842 | 498.32 | 513.24 | 11.02 | 11.35 | 3.300 | |
| 2018/2019 | 41 | 80 | 0.542 | 0.329 | 826.84 | 1014.04 | 18.23 | 22.36 | −0.107 |
| 50 | 80 | 0.251 | 0.094 | 1057.83 | 1178.01 | 23.32 | 25.97 | −0.942 | |
| 57 | 80 | 0.751 | 0.494 | 609.89 | 880.60 | 13.45 | 19.41 | −6.239 | |
| 64 | 80 | 0.858 | 0.443 | 460.64 | 923.32 | 10.16 | 20.36 | −2.539 | |
| 79 | 80 | 0.868 | 0.801 | 443.18 | 551.14 | 9.77 | 12.15 | 2.740 | |
| 87 | 80 | 0.871 | 0.775 | 437.76 | 586.23 | 9.65 | 12.92 | −8.600 | |
| 94 | 80 | 0.891 | 0.810 | 403.59 | 538.47 | 8.90 | 11.87 | −7.726 | |
| 101 | 80 | 0.739 | 0.681 | 624.04 | 698.49 | 13.76 | 15.40 | 3.671 | |
| 107 | 80 | 0.839 | 0.733 | 489.73 | 638.78 | 10.80 | 14.08 | 1.618 |
| PLSR | R2 | RMSE (kg ha−1) | RMSE% | BIAS |
|---|---|---|---|---|
| Calibration | 0.775 | 574.52 | 11.38 | - |
| Cross-validation | 0.730 | 634.44 | 12.57 | −4.941 |
| External validation | 0.688 | 622.46 | 13.34 | - |
| Treatment | Spectral Samples | Rc2 | Rcv2 | RMSEc (kg ha−1) | RMSEcv (kg ha−1) | RMSEc% | RMSEcv % | BIAScv |
|---|---|---|---|---|---|---|---|---|
| IRR | 36 | 0.622 | 0.315 | 453.72 | 628.61 | 16.68 | 23.11 | −24.25 |
| NIRR | 76 | 0.691 | 0.625 | 378.62 | 422.79 | 13.11 | 14.64 | 4.79 |
| WDV | 56 | 0.812 | 0.738 | 367.04 | 441.83 | 11.06 | 13.32 | 4.07 |
| WDR | 56 | 0.795 | 0.624 | 298.39 | 412.20 | 11.33 | 15.65 | −16.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crusiol, L.G.T.; Nanni, M.R.; Furlanetto, R.H.; Sibaldelli, R.N.R.; Cezar, E.; Sun, L.; Foloni, J.S.S.; Mertz-Henning, L.M.; Nepomuceno, A.L.; Neumaier, N.; et al. Yield Prediction in Soybean Crop Grown under Different Levels of Water Availability Using Reflectance Spectroscopy and Partial Least Squares Regression. Remote Sens. 2021, 13, 977. https://doi.org/10.3390/rs13050977
Crusiol LGT, Nanni MR, Furlanetto RH, Sibaldelli RNR, Cezar E, Sun L, Foloni JSS, Mertz-Henning LM, Nepomuceno AL, Neumaier N, et al. Yield Prediction in Soybean Crop Grown under Different Levels of Water Availability Using Reflectance Spectroscopy and Partial Least Squares Regression. Remote Sensing. 2021; 13(5):977. https://doi.org/10.3390/rs13050977
Chicago/Turabian StyleCrusiol, Luís Guilherme Teixeira, Marcos Rafael Nanni, Renato Herrig Furlanetto, Rubson Natal Ribeiro Sibaldelli, Everson Cezar, Liang Sun, José Salvador Simonetto Foloni, Liliane Marcia Mertz-Henning, Alexandre Lima Nepomuceno, Norman Neumaier, and et al. 2021. "Yield Prediction in Soybean Crop Grown under Different Levels of Water Availability Using Reflectance Spectroscopy and Partial Least Squares Regression" Remote Sensing 13, no. 5: 977. https://doi.org/10.3390/rs13050977
APA StyleCrusiol, L. G. T., Nanni, M. R., Furlanetto, R. H., Sibaldelli, R. N. R., Cezar, E., Sun, L., Foloni, J. S. S., Mertz-Henning, L. M., Nepomuceno, A. L., Neumaier, N., & Farias, J. R. B. (2021). Yield Prediction in Soybean Crop Grown under Different Levels of Water Availability Using Reflectance Spectroscopy and Partial Least Squares Regression. Remote Sensing, 13(5), 977. https://doi.org/10.3390/rs13050977