Hyperspectral Monitoring of Non-Native Tropical Grasses over Phenological Seasons
Abstract
:1. Introduction
2. Materials and Method
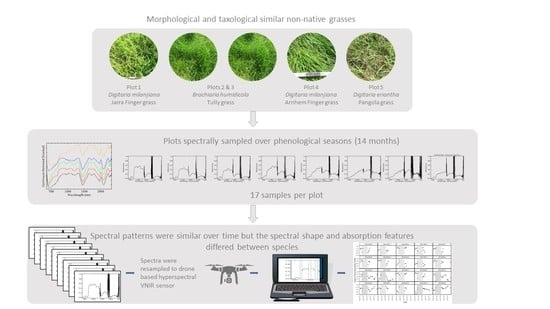
2.1. The Study Area and Plot Design
2.2. Field Data Collection
2.3. Data Analysis
3. Results
3.1. Meteorological Data
3.2. Summary Statistics
3.3. Temporal Analysis of the VNIR-SWIR Data–Reflectance and CR over Phenology
3.3.1. Spectra of the Late Dry Season. Sample Dates (1–5) –26/09 (1), 9/10 (2), 30/10 (3), 15/11 (4), 30/11 (5)
3.3.2. Spectra of the Late Wet Season/Early Dry Season. Sample Dates 11/4 (6), 23/4 (7), 10/5 (8), 22/5 (9), 12/6 (10)
3.3.3. Spectra of the Dry Season. Sample Dates 18/7 (11), 1/8 (12), 6/9 (13), 21/9 (14), 4/10 (15), 5/10 (16) 26/11 (17)
3.4. Spectral Feature Fitting Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oenema, O.; de Klein, C.; Alfaro, M. Intensification of grassland and forage use: Driving forces and constraints. Crop Pasture Sci. 2014, 65, 524–537. [Google Scholar] [CrossRef]
- O’Mara, F. The role of grasslands in food security and climate change. Annal. Bot. 2012, 110, 1263–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, 1–20. [Google Scholar] [CrossRef]
- Ferdinands, K.; Beggs, K.; Whitehead, P. Biodiversity and invasive grass species: Multiple-use or monoculture? Wildlife Res. 2005, 32, 447–457. [Google Scholar] [CrossRef]
- Mack, M.C.; D’Antonio, C.M. Impacts of biological invasions on disturbance regimes. Trend. Ecol. Evol. 1998, 13, 195–198. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. BioScience 2004, 54, 677–688. [Google Scholar] [CrossRef] [Green Version]
- Douglas, M.M.; Setterfield, S.A.; Rossiter, N.A.; Barratt, J.; Hutley, L. Effects of mission grass (Pennisetum polystachion (L) Schult.) invasion on fuel loads and nitrogen availability in a northern Australia tropical savanna. In Proceedings of the 14th Australian Weeds Conference; Wagga Wagga, New South Wales, Australia, 6–9 September 2004, Sindel, B., Johnson, S., Eds.; Charles Sturt University: New South Wales, Australia, 2004; pp. 179–181. [Google Scholar]
- Setterfield, S.A.; Rossiter-Rachor, N.A.; Hutley, L.B.; Douglas, M.M.; Williams, R.J. Biodiversity research: Turning up the heat: The impacts of Andropogon gayanus (gamba grass) invasion on fire behaviour in northern Australian savannas. Divers. Distrib. 2010, 16, 854–861. [Google Scholar] [CrossRef]
- Cook, G.; Dias, L. It was no accident: Deliberate plant introductions by Australian government agencies during the 20th century. Turner Review No. 12. Aust. J. Bot. 2006, 54, 601–625. [Google Scholar] [CrossRef]
- Grace, B.S.; Gardener, M.R.; Cameron, A.G. Pest or pasture? Introduced pasture grasses in the Northern Territory. In Proceedings of the 14th Australian Weeds Conference, Wagga Wagga, New South Wales, Australia, 6–9 September 2004; pp. 157–160. [Google Scholar]
- Lonsdale, W.M.; Lane, A.M. Tourist vehicles as vectors of weed seeds in Kakadu National Park, Northern Australia. Biolo. Conserv. 1994, 69, 277–283. [Google Scholar] [CrossRef]
- López-Granados, F. Weed detection for site-specific weed management: Mapping and real time approaches. Weed Res. 2010, 51, 1–11. [Google Scholar]
- Tucker, C.J. Asymptotic nature of grass canopy spectral reflectance. Appl. Optics. 1977, 16, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.J. Spectral estimation of grass canopy variables. Remote Sens. Environ. 1977, 6, 11–26. [Google Scholar] [CrossRef]
- Bao, S.; Cao, C.; Chen, W.; Tian, H. Spectral features and separability of alpine wetland grass species. Spectrosc. Lett. 2017, 50, 245–256. [Google Scholar] [CrossRef]
- Schmidt, K.; Skidmore, A. Exploring spectral discrimination of grass species in African rangelands. Int. J. Remote Sens. 2001, 22, 3421–3434. [Google Scholar] [CrossRef]
- Ali, I.; Cawkwell, F.; Dwyer, E.; Barrett, B.; Green, S. Satellite remote sensing of grasslands: From observation to management. J. Plant Ecol. 2016, 9, 649–671. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Casero, M.T.; Castillejo-González, I.L.; García-Ferrer, A.; Peña-Barragán, J.M.; Jurado-Expósito, M.; García-Torres, L.; López-Granados, F. Spectral discrimination of wild oat and canary grass in wheat fields for less herbicide application. Agron. Sustain. Dev. 2010, 30, 689–699. [Google Scholar] [CrossRef]
- Shoko, C.; Mutanga, O. Seasonal discrimination of C3 and C4 grasses functional types: An evaluation of the prospects of varying spectral configurations of new generation sensors. Int. J. Appl. Earth Obs. Geoinf. 2017, 62, 47–55. [Google Scholar] [CrossRef]
- Cho, M.A.; Skidmore, A.; Corsi, F.; Van Wieren, S.E.; Sobhan, I. Estimation of green grass/herb biomass from airborne hyperspectral imagery using spectral indices and partial least squares regression. Int. J. Appl. Earth Obs. Geoinf. 2007, 9, 414–424. [Google Scholar] [CrossRef]
- Pinar, A.; Curran, P. Technical note grass chlorophyll and the reflectance red edge. Int. J. Remote Sens. 1996, 17, 351–357. [Google Scholar] [CrossRef]
- Wang, Z.; Townsend, P.A.; Schweiger, A.K.; Couture, J.J.; Singh, A.; Hobbie, S.E.; Cavender-Bares, J. Mapping foliar functional traits and their uncertainties across three years in a grassland experiment. Remote Sens. Environ. 2019, 221, 405–416. [Google Scholar] [CrossRef]
- Yu, H.; Kong, B.; Wang, G.; Sun, H.; Wang, L. Hyperspectral database prediction of ecological characteristics for grass species of alpine grasslands. Rangel. J. 2018, 40, 19–29. [Google Scholar] [CrossRef]
- Fyfe, S.K. Spatial and Temporal Variation in Spectral Reflectance: Are Seagrass Species Spectrally Distinct? Limnol. Oceanogr. 2003, 48, 464–479. [Google Scholar] [CrossRef]
- Wessman, C.A.; Aber, J.D.; Peterson, D.L. An evaluation of imaging spectrometry for estimating forest canopy chemistry. Int. J. Remote Sens. 1989, 10, 1293–1316. [Google Scholar] [CrossRef]
- Wessman, C.A.; Aber, J.D.; Peterson, D.L.; Melillo, J.M. Remote sensing of canopy chemistry and nitrogen cycling in temperate forest ecosystems. Nature 1988, 335, 154. [Google Scholar] [CrossRef]
- Zwiggelaar, R. A review of spectral properties of plants and their potential use for crop/weed discrimination in row-crops. Crop Prot. 1998, 17, 189–206. [Google Scholar] [CrossRef]
- Asner, G.P. Biophysical and biochemical sources of variability in canopy reflectance. Remote Sens. Environ. 1998, 64, 234–253. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Red edge shift and biochemical content in grass canopies. ISPRS J. Photogramm. Remote Sens. 2007, 62, 34–42. [Google Scholar] [CrossRef]
- Li, F.; Mistele, B.; Hu, Y.C.; Chen, X.P.; Schmidhalter, U. Optimising Three-Band Spectral Indices to Assess Aerial N Concentration, N Uptake and Aboveground Biomass of Winter Wheat Remotely in China and Germany. ISPRS J. Photogramm. Remote Sens. 2014, 92, 112–123. [Google Scholar] [CrossRef]
- Sukhova, E.; Sukhov, V. Relation of Photochemical Reflectance Indices Based on Different Wavelengths to the Parameters of Light Reactions in Photosystems I and II in Pea Plants. Remote Sens. 2020, 12, 1312. [Google Scholar] [CrossRef] [Green Version]
- Lopatin, J.; Fassnacht, F.E.; Kattenborn, T.; Schmidtlein, S. Mapping plant species in mixed grassland communities using close range imaging spectroscopy. Remote Sens. Environ. 2017, 201, 12–23. [Google Scholar] [CrossRef]
- Matson, P.; Johnson, L.; Billow, C.; Miller, J.; Pu, R. Seasonal patterns and remote spectral estimation of canopy chemistry across the Oregon transect. Ecol. Appl. 1994, 4, 280–298. [Google Scholar] [CrossRef]
- Cundill, S.L.; van der Werff, H.M.A.; van der Meijde, M. Adjusting Spectral Indices for Spectral Response Function Differences of Very High Spatial Resolution Sensors Simulated from Field Spectra. Sensors 2015, 15, 6221–6240. [Google Scholar] [CrossRef] [PubMed]
- Kattenborn, T.; Fassnacht, F.E.; Schmidtlein, S. Differentiating plant functional types using reflectance: Which traits make the difference? Remote Sens. Ecol. Conserv. 2019, 5, 5–19. [Google Scholar] [CrossRef] [Green Version]
- Marcinkowska-Ochtyra, A.; Jarocińska, A.; Bzdęga, K.; Tokarska-Guzik, B. Classification of Expansive Grassland Species in Different Growth Stages Based on Hyperspectral and LiDAR Data. Remote Sens. 2018, 10, 2019. [Google Scholar] [CrossRef] [Green Version]
- Hakala, T.; Markelin, L.; Honkavaara, E.; Scott, B.; Theocharous, T.; Nevalainen, O.; Näsi, R.; Suomalainen, J.; Viljanen, N.; Greenwell, C.; et al. Direct reflectance measurements from drones: Sensor absolute radiometric calibration and system tests for forest reflectance characterization. Sensors 2018, 18, 1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saari, H.; Akujärvi, A.; Holmlund, C.; Ojanen, H.; Kaivosoja, J.; Nissinen, A.; Niemeläinen, O. Visible, very near IR and shortwave IR hyperspectral drone imaging system for agriculture and natural water applications. Int. Arch. Photogram. Remote Sens. Spat. Inf. Sci. 2017, XLII-3/W3, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Constantinescu, C.A.; Herbei, M.V.; Sala, F. Characterisation of some varieties of cereal grasses on the basis of spectral information from aerial images. Res. J. Agric. Sci. 2017, 49, 85–94. [Google Scholar]
- Michez, A.; Lejeune, P.; Bauwens, S.; Herinaina, A.A.L.; Blaise, Y.; Castro Muñoz, E.; Lebeau, F.; Bindelle, J. Mapping and Monitoring of Biomass and Grazing in Pasture with an Unmanned Aerial System. Remote Sens. 2019, 11, 473. [Google Scholar] [CrossRef] [Green Version]
- Aasen, H. The Acquisition of Hyperspectral Digital Surface Models of Crops from UAV Snapshot Cameras. PhD thesis, Universität zu Köln, Köln, Germany, 2016. [Google Scholar]
- BOM Bureau of Meteorology, Climate Data Online. Available online: http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=122&p_display_type=dailyDataFile&p_startYear=&p_c=&p_stn_num=014015 (accessed on 22 July 2020).
- Bureau of Meteorology (BOM) Mean Rainfall of Berrimah Farm. Available online: http://www.bom.gov.au/climate/data/ (accessed on 1 April 2020).
- Cameron, A.; Principal Pastures Agronomist, Darwin. Personal communication, 29 April 2019.
- Beggs, K.E. Effects of exotic pasture grasses on biodiversity in the Mary River Catchment, Northern Territory. PhD thesis, Charles Darwin University, Darwin, Australia, 2010. [Google Scholar]
- Tropical Forages. Available online: https://www.tropicalforages.info/text/entities/digitaria_eriantha.htm and https://www.tropicalforages.info/text/entities/digitaria_milanjiana.htm (accessed on 1 May 2019).
- Cook, B.G.; Pengelly, B.C.; Brown, S.D.; Donnelly, J.L.; Eagles, D.A.; Franco, M.A.; Hanson, J.; Mullen, B.F.; Partridge, I.J.; Peters, M.; et al. Tropical Forages: An Interactive Selection Tool CSIRO, DPI&F (Qld), CIAT and ILRI, Brisbane, Australia. Available online: https://www.tropicalforages.info/text/intro/index.html (accessed on 15 February 2021).
- Cameron, A.G. Pangola Grass (Digitaria eriantha) Agnote, No, E35, Department of Primary Industry, Fisheries and Mines Northern Territory Government. January 2013. Available online: https://industry.nt.gov.au/__data/assets/pdf_file/0015/233430/306.pdf (accessed on 15 February 2021).
- Cameron, A.G. Tully (Brachiaria humidicola). Agnote, No. E31, Department of Primary Industry, Fisheries and Mines. Northern Territory Government. January 2013. Available online: https://dpir.nt.gov.au/__data/assets/pdf_file/0005/233438/550.pdf (accessed on 15 February 2021).
- Cameron, A.G. Arnhem Finer Grass (Digitaria swynnertonii), Agnote, No. E63, Northern Territory Government. February 2019. Available online: https://industry.nt.gov.au/__data/assets/pdf_file/0005/233537/725.pdf (accessed on 15 February 2021).
- Cameron, A.G. Jarra Finer Grass (Digitaria milanjiana cv Jarra), Agnote, No. E55, Northern Territory Government. August 2010. Available online: https://industry.nt.gov.au/__data/assets/pdf_file/0019/233209/684.pdf (accessed on 15 February 2021).
- Pfitzner, K.; Bollhöfer, A.; Carr, G. A standard design for collecting vegetation reference spectra: Implementation and implications for data sharing. J. Spat. Sci. 2006, 52, 79–92. [Google Scholar] [CrossRef]
- Pfitzner, K.; Bartolo, R.; Carr, G.; Esparon, A.; Bollhöfer, A. Standards for Reflectance Spectral Measurement of Temporal Vegetation Plots; Supervising Scientist Report 195; Department of Sustainability, Environment, Water, Population and Communities: Darwin, NT, Australia, 2011. Available online: https://www.environment.gov.au/science/supervising-scientist/publications/ssr/standards-for-reflectance-spectral-measurement-of-temporal-vegetation-plots. (accessed on 15 February 2021).
- Rueda, C.A.; Wrona, A.F. SAMS Spectral Analysis and Management System Version 2.0 User’s Manual. Centre for Spatial Technologies and Remote Sensing, Department of Land, Air and Water Resources, University of California, Davis: Davis, CA, USA. 2003. Available online: https://storage.googleapis.com/google-code-archive-downloads/v2/code.google.com/cstars-sams/SAMS%202.0%20User%20Manual.pdf (accessed on 15 February 2021).
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res: Solid Earth 1984, 89, 6329–6340. [Google Scholar] [CrossRef]
- Clark, R.N.; Swayze, G.A. Mapping minerals, amorphous materials, environmental materials, vegetation, water, ice and snow, and other materials: The USGS tricorder algorithm. In Summaries of the Third Annual JPL Airborne Geoscience Workshop (Volume 1, pp. 39–40); JPL Publication 92–14; Jet Propulsion Laboratory: Pasadena, CA, USA, 1995. Available online: https://popo.jpl.nasa.gov/pub/docs/workshops/95_docs/12.PDF (accessed on 15 February 2021).
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Adão, T.; Peres, E.; Pádua, L.; Hruška, J.; Sousa, J.J.; Morais, R. UAS-based hyperspectral sensing methodology for continuous monitoring and early detection of vineyard anomalies. In Proceedings of the Small Unmanned Aerial Systems for Environmental Research, Vila Real, Portugal, 28–30 June 2017. [Google Scholar]
- Burkart, A.; Cogliati, S.; Schickling, A.; Rascher, U. A novel UAV-based ultra-light weight spectrometer for field spectroscopy. IEEE Sens. J. 2014, 14, 62–67. [Google Scholar] [CrossRef]
- Holasek, R.; Nakanishi, K.; Ziph-Schatzberg, L.; Santman, J.; Woodman, P.; Zacaroli, R.; Wiggins, R. The selectable hyperspectral airborne remote sensing kit (SHARK) as an enabler for precision agriculture. In Proceedings SPIE 10213, Hyperspectral Imaging Sensors: Innovative Applications and Sensor Standards Anaheim, Anaheim, CA, USA, 22 May 2017; p. 1021304. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, X.; Xu, Y.; Jia, T.; Cui, S.; Wei, L.; Ma, A.; Zhang, L. MINI-UAV borne hyperspectral remote sensing: A review. In Proceedings of 2017 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Fort Worth, TX, USA, 23–28 July 2017; pp. 5908–5911. [Google Scholar]
- Mitchell, J.J.; Glenn, N.F.; Sankey, T.T.; Derryberry, D.R.; Anderson, M.O.; Hruska, R.C. Spectroscopic detection of nitrogen concentrations in sagebrush. Remote Sens. Lett. 2012, 3, 285–294. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Peterson, D.L. Estimating the foliar biochemical concentration of leaves with reflectance spectrometry: Testing the Kokaly and Clark methodologies. Remote Sens. Environ. 2001, 76, 349–359. [Google Scholar] [CrossRef]
- Shi, T.; Wang, J.; Liu, H.; Wu, G. Estimating leaf nitrogen concentration in heterogeneous crop plants from hyperspectral reflectance. Int. J. Remote Sens. 2015, 36, 4652–4667. [Google Scholar] [CrossRef]
- Pan, Z.; Huang, J.; Wang, F. Multi range spectral feature fitting for hyperspectral imagery in extracting oilseed rape planting area. Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 21–29. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Despain, D.G.; Clark, R.N.; Livo, K.E. Mapping vegetation in Yellowstone National Park using spectral feature analysis of AVIRIS data. Remote Sens. Environ. 2003, 84, 437–456. [Google Scholar] [CrossRef] [Green Version]
- Mutanga, O.; Skidmore, A.K.; Prins, H.H.T. Predicting in situ pasture quality in the Kruger National Park, South Africa, using continuum-removed absorption features. Remote Sens. Environ. 2004, 89, 393–408. [Google Scholar] [CrossRef]
- Rossiter-Rachor, N.A.; Setterfield, S.A.; Douglas, M.M.; Hutley, L.B.; Cook, G.D. Andropogon gayanus (gamba grass) invasion increases fire-mediated nitrogen losses in the tropical savannas of northern Australia. Ecosystems 2008, 11, 77–88. [Google Scholar] [CrossRef]
- Head, L.; Atchison, J. Governing invasive plants: Policy and practice in managing the Gamba grass (Andropogon gayanus)–bushfire nexus in northern Australia. Land Use Policy 2015, 47, 225–234. [Google Scholar] [CrossRef]










| BF01-05 | BF01 | BF02 | BF03 | BF04 | BF05 |
|---|---|---|---|---|---|
| Scientific name | Digitaria milanjiana | Brachiaria humidicola | Brachiaria humidicola | Digitariamilanjiana | Digitaria eriantha |
| Common name | cv Jarra Finger grass | Tully grass | Tully grass | cv Arnhem Finger grass | Pangola Grass |
| Photo (~10 cm across) |  |  |  |  |  |
| Season | Digitaria milanjiana (BF01 and BF04) | Digitaria eriantha (BF05) | Brachiaria humidicola (BF02 and BF03) |
|---|---|---|---|
| November —very late dry season prior to wet season rains but after rain showers | Differs from the other species by showing chlorophyll absorption and deep-water absorption as well as higher reflectance magnitude in the SWIR. | ||
| April–May —late wet season after the monsoon | Higher overall reflectance magnitude. | Decrease in chlorophyll and overall reflectance magnitude and loss of water absorption intensity, although similar spectral response. | More intense absorption around 1170 nm. Subtle difference. |
| July–August —dry season | The absorptions in the leaf structure region of the spectrum (750–1300 nm) are less for D. milanjiana (BF04) when compared to B. humidicola (BF03). D. milanjiana showed stronger chlorophyll absorption than B. humidicola. | Lowest reflectance magnitude across the VNIR. | The absorptions in the leaf structure region of the spectrum (750–1300 nm) are greater for B. humidicola (BF03) compared to D. milanjiana (BF04). Absorptions centred at 973 nm and 1163 nm were strongest in B. humidicola, but present and less intense in D. milanjiana. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfitzner, K.; Bartolo, R.; Whiteside, T.; Loewensteiner, D.; Esparon, A. Hyperspectral Monitoring of Non-Native Tropical Grasses over Phenological Seasons. Remote Sens. 2021, 13, 738. https://doi.org/10.3390/rs13040738
Pfitzner K, Bartolo R, Whiteside T, Loewensteiner D, Esparon A. Hyperspectral Monitoring of Non-Native Tropical Grasses over Phenological Seasons. Remote Sensing. 2021; 13(4):738. https://doi.org/10.3390/rs13040738
Chicago/Turabian StylePfitzner, Kirrilly, Renee Bartolo, Tim Whiteside, David Loewensteiner, and Andrew Esparon. 2021. "Hyperspectral Monitoring of Non-Native Tropical Grasses over Phenological Seasons" Remote Sensing 13, no. 4: 738. https://doi.org/10.3390/rs13040738
APA StylePfitzner, K., Bartolo, R., Whiteside, T., Loewensteiner, D., & Esparon, A. (2021). Hyperspectral Monitoring of Non-Native Tropical Grasses over Phenological Seasons. Remote Sensing, 13(4), 738. https://doi.org/10.3390/rs13040738