A New Approach for Nitrogen Status Monitoring in Potato Plants by Combining RGB Images and SPAD Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Crop Management
2.2. Experimental Design
2.3. Measurements
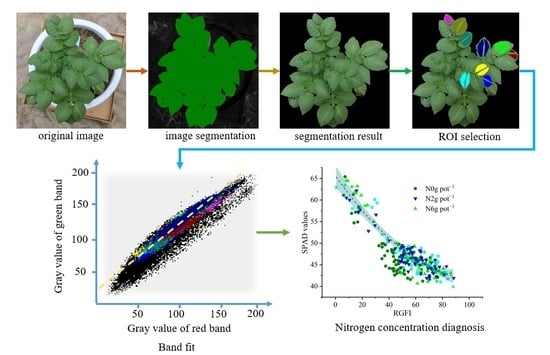
2.4. RGB Image Processing
2.4.1. The Extraction of Potato Plant Characteristics
2.4.2. Feature Construction
2.4.3. Feature Selection
2.5. Establishment of CNDC for Potato Plants
2.6. Statistical Analysis
3. Results
3.1. Tuber Yield and SPAD Value Response to N Rate
3.2. The Results of Feature Selection
3.3. Construction and Validation of a CNCD for Ppotato Plants
4. Discussion
4.1. The Response of Yield and SPAD Values to the N ApplicationRate
4.2. Application of the RGFI for CNDC Construction in Potato Plants
4.3. RGFI/SPAD Value-reference Curve Method
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boivin, M.; Bourdeau, N.; Barnabé, S.; Desgagné-Penix, I. Sprout Suppressive Molecules Effective on Potato (Solanum tuberosum) Tubers during Storage: A Review. Am. J. Potato Res. 2020, 97, 451–463. [Google Scholar] [CrossRef]
- Tawa, S.; Nedunchezhiyan, M. Global Status of Sweet Potato Cultivation. Fruit Veg. Cereal Sci. Biotechnol. 2012, 6, 143–147. [Google Scholar]
- Jia, L.; Qin, Y.; Chen, Y.; Fan, M. Fertigation improves potato production in Inner Mongolia (China). J. Crop Improv. 2018, 32, 648–656. [Google Scholar] [CrossRef]
- Wang, X.; Fan, J.; Xing, Y.; Xu, G.; Wang, H.; Jian, D.; Wang, Y.; Zhang, F.; Li, P.; Li, Z. The Effects of Mulch and Nitrogen Fertilizer on the Soil Environment of Crop Plants. Adv. Agron. 2019, 153, 121–173. [Google Scholar]
- Ata-Ul-Karim, S.T.; Liu, X.; Lu, Z.; Zheng, H.; Cao, W.; Zhu, Y. Estimation of nitrogen fertilizer requirement for rice crop using critical nitrogen dilution curve. Field Crops Res. 2017, 201, 32–40. [Google Scholar] [CrossRef]
- Ayyub, C.M.; Haidar, M.W.; Zulfiqar, F.; Abideen, Z.; Wright, S.R. Potato tuber yield and quality in response to different nitrogen fertilizer application rates under two split doses in an irrigated sandy loam soil. J. Plant Nutr. 2019, 42, 1850–1860. [Google Scholar] [CrossRef]
- Qiang, S.C.; Zhang, F.C.; Dyck, M.; Zhang, Y.; Xiang, Y.Z.; Fan, J.L. Determination of critical nitrogen dilution curve based on leaf area index for winter wheat in the Guanzhong Plain, Northwest China. J. Integr. Agric. 2019, 18, 2369–2380. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, H.; Zhao, W. Plastic film mulching affects the critical nitrogen dilution curve of drip-irrigated maize. Field Crops Res. 2021, 263, 108055. [Google Scholar] [CrossRef]
- Scott, J.T.; Lambie, S.M.; Stevenson, B.A.; LSchipper, A.; Parfitt, R.L.; McGill, A.C. Carbon and nitrogen leaching under high and low phosphate fertility pasture with increasing nitrogen inputs. Agric. Ecosyst. Environ. 2015, 202, 139–147. [Google Scholar] [CrossRef]
- Muttucumaru, N.; Powers, S.J.; Elmore, J.S.; Mottram, D.S.; Halford, N.G. Effects of nitrogen and sulfur fertilization on free amino acids, sugars, and acrylamide-forming potential in potato. J. Agric. Food Chem. 2013, 61, 6734–6742. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Branquinho, C.; Mcdowell, W.; Mitchell, M.; Monteith, D.; Tang, J.; Arvola, L.; Cruz, C.; Cusack, D.; Halada, L. Consequence of altered nitrogen cycles in the coupled human and ecological system under changing climate: The need for long-term and site-based research. Ambio 2015, 44, 178–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, B.; Thorling, L.; Kim, H.; Blicher-Mathiesen, G. Long-term nitrate response in shallow groundwater to agricultural N regulations in Denmark. J. Environ. Manag. 2019, 240, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, H.; Hou, L.; Zhu, Y.; Zhang, Q.; Chen, L.; Mao, P. Development of a critical nitrogen dilution curve of Siberian wildrye for seed production. Field Crops Res. 2018, 219, 250–255. [Google Scholar] [CrossRef]
- He, Z.; Qiu, X.; Tahir, A.; Li, Y.; Liu, X.; Qiang, C.; Zhu, Y.; Cao, W.; Liang, T. Development of a Critical Nitrogen Dilution Curve of Double Cropping Rice in South China. Front. Plant Sci. 2017, 8, 638. [Google Scholar] [CrossRef] [PubMed]
- Giletto, C.M.; heverría, H.E. Critical Nitrogen Dilution Curve for Processing Potato in Argentinean Humid Pampas. Am. J. Potato Res. 2012, 89, 102–110. [Google Scholar] [CrossRef]
- Xue, X.P.; Zhou, Z.G.; Zhang, L.J.; Wang, Y.L.; Guo, W.Q.; Chen, B.L. Development and application of critical nitrogen concentration Dilution Model for cotton after flowering. Acta Ecol. Sin. 2006, 26, 1781–1791. [Google Scholar]
- Li, Z.; Song, M.; Feng, H. Development and validation of critical nitrogen content curve for maize in Guanzhong area. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2015, 31, 135–141. [Google Scholar]
- Ata-Ul-Karim, S.T.; Yao, X.; Liu, X.; Cao, W.; Zhu, Y. Development of critical nitrogen dilution curve of Japonica rice in Yangtze River Reaches. Field Crops Res. 2013, 149, 149–158. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, B.; Tian, Y.C.; Xiao, J.L.; Yan, Z. Using leaf dry matter to quantify the critical nitrogen dilution curve for winter wheat cultivated in eastern China. Field Crops Res. 2014, 159, 33–42. [Google Scholar]
- Zhou, Z.; Jabloun, M.; Plauborg, F.; Andersen, M.N. Using ground-based spectral reflectance sensors and photography to estimate shoot N concentration and dry matter of potato. Comput. Electron. Agric. 2018, 144, 154–163. [Google Scholar] [CrossRef]
- Dang, R.J.; Shi-Qing, L.I.; Xiao-Hui, M.U.; Sheng-Xiu, L.I. Effect of nitrogen on vertical distribution of canopy nitrogen and chlorophyll relative value (SPAD value) of summer maize in sub-humid areas. Chin. J. Eco-Agric. 2009, 17, 54–59. [Google Scholar] [CrossRef]
- Padilla, F.M.; Marisa, G.; Teresa, P.; Romina, D.S.; Thompson, R.B. Proximal Optical Sensors for Nitrogen Management of Vegetable Crops: A Review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef] [PubMed]
- Padilla, F.M.; Pea-Fleitas, M.T.; Gallardo, M.; Giménez, C.; Thompson, R.B. Derivation of sufficiency values of a chlorophyll meter to estimate cucumber nitrogen status and yield. Comput. Electron. Agric. 2017, 141, 54–64. [Google Scholar] [CrossRef]
- Fernandes, F.M.; Soratto, R.P.; Fernandes, A.M.; Souza, E. Chlorophyll meter-based leaf nitrogen status to manage nitrogen in tropical potato production. Agron. J. 2021, 113, 1733–1746. [Google Scholar] [CrossRef]
- Padilla, F.M.; De Souza, R.; Peña-Fleitas, M.; Grasso, R.; Gallardo, M.; Thompson, R.B. Influence of time of day on measurement with chlorophyll meters and canopy reflectance sensors of different crop N status. Precis. Agric. 2019, 20, 1087–1106. [Google Scholar] [CrossRef]
- Gupta, S.D.; Ibaraki, Y.; Pattanayak, A.K. Development of a digital image analysis method for real-time estimation of chlorophyll content in micropropagated potato plants. Plant Biotechnol. Rep. 2012, 7, 91–97. [Google Scholar] [CrossRef]
- Zheng, H.L.; Liu, Y.C.; Qin, Y.L.; Chen, Y.; Fan, M.S. Establishing dynamic thresholds for potato nitrogen status diagnosis with the SPAD chlorophyll meter. J. Integr. Agric. 2015, 14, 190–195. [Google Scholar] [CrossRef]
- Rigon, J.P.G.; Capuani, S.; Fernandes, D.M.; Guimarães, T.M. A novel method for the estimation of soybean chlorophyll content using a smartphone and image analysis. Photosynthetica 2016, 54, 559–566. [Google Scholar] [CrossRef]
- Giletto, C.M.; Echeverría, H. Chlorophyll Meter for the Evaluation of Potato N Status. Am. J. Potato Res. 2013, 90, 313–323. [Google Scholar] [CrossRef]
- Sun, G.; Wang, X.; Sun, Y.; Ding, Y.; Lu, W. Measurement Method Based on Multispectral Three-Dimensional Imaging for the Chlorophyll Contents of Greenhouse Tomato Plants. Sensors 2019, 19, 3345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, G.; Lang, K.; Su, B.; Chen, X.; Xi, X.; Zhang, H. Integrated Satellite, Unmanned Aerial Vehicle (UAV) and Ground Inversion of the SPAD of Winter Wheat in the Reviving Stage. Sensors 2019, 19, 1485. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Lee, B.W. Estimation of rice growth and nitrogen nutrition status using color digital camera image analysis. Eur. J. Agron. 2013, 48, 57–65. [Google Scholar] [CrossRef]
- Riccardi, M.; Mele, G.; Pulvento, C.; Lavini, A.; Jacobsen, S.E. Non-destructive evaluation of chlorophyll content in quinoa and amaranth leaves by simple and multiple regression analysis of RGB image components. Photosynth. Res. 2014, 120, 263–272. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Fan, M. Determination of Plants Number for Evaluating Potato N Status Using Chlorophyll Meter SPAD-502. Crops 2015, 135–137. [Google Scholar] [CrossRef]
- Shibaeva, T.G.; Mamaev, A.V.; Sherudilo, E.G. Evaluation of a SPAD-502 Plus Chlorophyll Meter to Estimate Chlorophyll Content in Leaves with Interveinal Chlorosis. Russ. J. Plant Physiol. 2020, 67, 690–696. [Google Scholar] [CrossRef]
- Meyer, G.E.; Neto, J.O.C. Verification of color vegetation indices for automated crop imaging applications. Comput. Electron. Agric. 2008, 63, 282–293. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Han, W.; Niu, Y.; Ma, W. The mean value of gaussian distribution of excess green index: A new crop water stress indicator. Agric. Water Manag. 2021, 251, 106866. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Qi, Y.; Deng, L.; Wang, X.; Yang, S. New research methods for vegetation information extraction based on visible light remote sensing images from an unmanned aerial vehicle (UAV). Int. J. Appl. Earth Obs. Geoinf. 2019, 78, 215–226. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.C.; Li, J.F.; Wang, Y.Z.; Qi, W.C. Study on GLI values of Polygonatum odoratum base on multi-temporal of unmanned aerial vehicle remote sensing. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2020, 45, 5663–5668. [Google Scholar]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. Stud. Media Commun. 1973, 610–621. [Google Scholar] [CrossRef]
- Yadav, S.P.; Ibaraki, Y.; Gupta, S.D. Estimation of the chlorophyll content of micropropagated potato plants using RGB based image analysis. Plant Cell Tissue Organ Cult. 2010, 100, 183–188. [Google Scholar] [CrossRef]
- Gong, A.; Yu, J.; He, Y.; Qiu, Z. Citrus yield estimation based on images processed by an Android mobile phone. Biosyst. Eng. 2013, 115, 162–170. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, J.; Lan, Y.; Lu, L.; Li, Z. Fraction vegetation cover extraction of winter wheat based on RGB image obtained by UAV. Int. J. Precis. Agric. Aviat. 2018, 1, 54–61. [Google Scholar] [CrossRef]
- Liang, H.; Yang, G.; Dai, H.; Bo, X.; Yang, X. Modeling maize above-ground biomass based on machine learning approaches using UAV remote-sensing data. Plant Methods 2019, 15, 10. [Google Scholar]
- Ge, H.; Xiang, H.; Ma, F.; Li, Z.; Du, C. Estimating Plant Nitrogen Concentration of Rice through Fusing Vegetation Indices and Color Moments Derived from UAV-RGB Images. Remote Sens. 2021, 13, 1620. [Google Scholar] [CrossRef]
- Zhou, Z.; Plauborg, F.; Thomsen, A.G.; Andersen, M.N. A RVI/LAI-reference curve to detect N stress and guide N fertigation using combined information from spectral reflectance and leaf area measurements in potato. Eur. J. Agron. 2017, 87, 1–7. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Zhu, Y.; Cao, Q.; Rehmani, M.; Cao, W.; Tang, L. In-season assessment of grain protein and amylose content in rice using critical nitrogen dilution curve. Eur. J. Agron. 2017, 90, 139–151. [Google Scholar] [CrossRef]
- Zamora-Re, M.I.; Dukes, M.; Hensley, D.; Rowland, D.; Graham, W. The effect of irrigation strategies and nitrogen fertilizer rates on maize growth and grain yield. Irrig. Sci. 2020, 38, 461–478. [Google Scholar] [CrossRef]
- Liu, N.; Qiao, L.; Xing, Z.; Li, M.; Sun, H.; Zhang, J.; Zhang, Y. Detection of chlorophyll content in growth potato based on spectral variable analysis. Spectrosc. Lett. 2020, 53, 476–488. [Google Scholar] [CrossRef]
- Liu, N.; Xing, Z.; Zhao, R.; Qiao, L.; Li, M.; Liu, G.; Sun, H. Analysis of chlorophyll concentration in potato crop by coupling continuous wavelet transform and spectral variable optimization. Remote Sens. 2020, 12, 2826. [Google Scholar] [CrossRef]
- Khan, F.; Khan, S.; Fahad, S.; Faisal, S.; Hussain, S.; Ali, S.; Ali, A. Effect of Different Levels of Nitrogen and Phosphorus on the Phenology and Yield of Maize Varieties. Am. J. Plant Sci. 2014, 5, 2582–2590. [Google Scholar] [CrossRef]
- Wei, W.; Yang, M.; Liu, Y.; Huang, H.; Ye, C.; Zheng, J.; Guo, C.; Hao, M.; He, X.; Zhu, S. Fertilizer N application rate impacts plant-soil feedback in a sanqi production system. Sci. Total Environ. 2018, 633, 796–807. [Google Scholar] [CrossRef]
- Correndo, A.A.; Rotundo, J.L.; Tremblay, N.; Archontoulis, S.; Ciampitti, I.A. Assessing the uncertainty of maize yield without nitrogen fertilization. Field Crops Res. 2021, 260, 107985. [Google Scholar] [CrossRef]
- Gáborčík, N. Relationship between Contents of Chlorophyll (a + b) (SPAD values) and Nitrogen of Some Temperate Grasses. Photosynthetica 2003, 41, 285–287. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Yang, J.; Hua, W.; Zou, J.; He, J.; Hui, D. Effects of Nitrogen Application Rate and Leaf Age on the Distribution Pattern of Leaf SPAD Readings in the Rice Canopy. PLoS ONE 2014, 9, e88421. [Google Scholar] [CrossRef]
- Li, R.; Chen, J.; Qin, Y.; Fan, M. Possibility of using a SPAD chlorophyll meter to establish a normalized threshold index of nitrogen status in different potato cultivars. J. Plant Nutr. 2019, 42, 834–841. [Google Scholar] [CrossRef]
- Sonobe, R.; Sano, T.; Horie, H. Using spectral reflectance to estimate leaf chlorophyll content of tea with shading treatments. Biosyst. Eng. 2018, 175, 168–182. [Google Scholar] [CrossRef]
- Yu, K.Q.; Zhao, Y.R.; Zhu, F.L.; Li, X.L.; He, Y. Mapping of Chlorophyll and SPAD Distribution in Pepper Leaves During Leaf Senescence Using Visible and Near-Infrared Hyperspectral Imaging. Trans. Asabe 2016, 59, 13–24. [Google Scholar]
- Zhang, J.; Han, W.; Huang, L.; Zhang, Z.; Ma, Y.; Hu, Y. Leaf Chlorophyll Content Estimation of Winter Wheat Based on Visible and Near-Infrared Sensors. Sensors 2016, 16, 437. [Google Scholar] [CrossRef]
- Qin, C.; Huang, J.; Wang, H.; Sun, H.; Zhang, Y.; Yingying, L.I. Polarized hyperspectral characteristics of smooth leaves and its relationship with chlorophyll content. J. Meteorol. Sci. 2019, 39, 421–426. [Google Scholar]
- Yang, Z.; Hu, J.; Duan, T.; Wang, S.; Wu, J.; Su, W.; Zhang, Y.; University, H.A. Research Progress of Nondestructive Diagnostic Technique of Chlorophyll in Plants. Chin. Agric. Sci. Bull. 2019, 35, 139–144. [Google Scholar]
- He, Y.; Peng, J.; Liu, F.; Zhang, C.; Kong, W. Critical review of fast detection of crop nutrient and physiological information with spectral and imaging technology. Trans. Chin. Soc. Agric. Eng. 2015, 31, 174–189. [Google Scholar]
- Yong, H.E.; Zhao, C.J.; Di, W.U.; Nie, P.C. Fast detection technique and sensor instruments for crop-environment information: A review. Entia Sinica 2010, 40, 1–20. [Google Scholar]
- Zhao, B.; Niu, X.; Ata-Ul-Karim, S.T.; Wang, L.; Lemaire, G. Determination of the post-anthesis nitrogen status using ear critical nitrogen dilution curve and its implications for nitrogen management in maize and wheat. Eur. J. Agron. 2020, 113, 125967. [Google Scholar] [CrossRef]
- Rahimikhoob, H.; Sohrabi, T.; Delshad, M. Development of a Critical Nitrogen Dilution Curve for Basil (Ocimum basilicum L.) Under Greenhouse Conditions. J. Soil Sci. Plant Nutr. 2020, 20, 881–891. [Google Scholar] [CrossRef]








| Type | Name | Formulas |
|---|---|---|
| Vegetation indices | Excess green index (EXG) | |
| Normalized green–blue difference index (NGBDI) | ||
| Green–red vegetation index (GRVI) | ||
| Green leaf index (GLI) | ||
| Texture features | Mean (mean) | |
| Variance (var) | ||
| Homogeneity (hom) | ||
| Contrast (con) | ||
| Dissimilarity (dis) | ||
| Entropy (ent) | ||
| Second moment (sm) | ||
| Correlation (corr) |
| Feature Name | Importance |
|---|---|
| RGFI | 0.3068 |
| EXG | 0.2798 |
| GLI | 0.1673 |
| GRVI | 0.1261 |
| NGBDI | 0.0204 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Hu, Y.; Zheng, Z.; Qiao, Y.; Hou, B.; Chen, J. A New Approach for Nitrogen Status Monitoring in Potato Plants by Combining RGB Images and SPAD Measurements. Remote Sens. 2022, 14, 4814. https://doi.org/10.3390/rs14194814
Yang H, Hu Y, Zheng Z, Qiao Y, Hou B, Chen J. A New Approach for Nitrogen Status Monitoring in Potato Plants by Combining RGB Images and SPAD Measurements. Remote Sensing. 2022; 14(19):4814. https://doi.org/10.3390/rs14194814
Chicago/Turabian StyleYang, Huanbo, Yaohua Hu, Zhouzhou Zheng, Yichen Qiao, Bingru Hou, and Jun Chen. 2022. "A New Approach for Nitrogen Status Monitoring in Potato Plants by Combining RGB Images and SPAD Measurements" Remote Sensing 14, no. 19: 4814. https://doi.org/10.3390/rs14194814
APA StyleYang, H., Hu, Y., Zheng, Z., Qiao, Y., Hou, B., & Chen, J. (2022). A New Approach for Nitrogen Status Monitoring in Potato Plants by Combining RGB Images and SPAD Measurements. Remote Sensing, 14(19), 4814. https://doi.org/10.3390/rs14194814