Modelling Species Richness and Functional Diversity in Tropical Dry Forests Using Multispectral Remotely Sensed and Topographic Data
Abstract
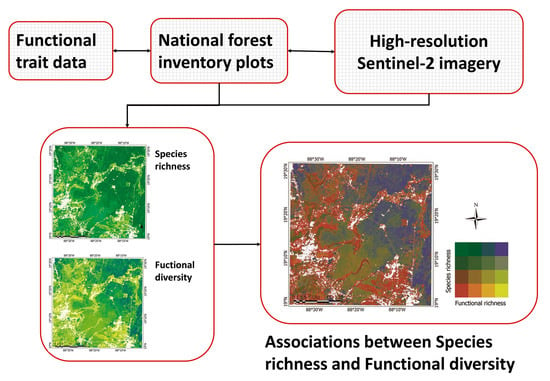
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Data and Calculations of Tree Species Richness and Functional Diversity
2.3. Satellite Image Processing
2.4. Estimating Species Richness and Functional Richness
2.5. Species Richness, Functional Diversity, and Their Associations
3. Results
3.1. Species Richness and Functional Diversity
3.2. Performance of Models Predicting Species Richness and Functional Diversity
3.3. Relationships between Species Richness and Functional Richness
4. Discussion
4.1. Evaluation of Species Richness and Functional Richness Maps
4.2. Relationships between Tree Species Richness and Functional Richness
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powers, J.S.; Feng, X.; Sanchez-Azofeifa, A.; Medvigy, D. Focus on tropical dry forest ecosystems and ecosystem services in the face of global change. Environ. Res. Lett. 2018, 13, 090201. [Google Scholar] [CrossRef]
- Portillo-Quintero, C.A.; Sánchez-Azofeifa, G.A. Extent and conservation of tropical dry forests in the Americas. Biol. Conserv. 2010, 143, 144–155. [Google Scholar] [CrossRef]
- Portillo-Quintero, C.; Sanchez-Azofeifa, A.; Calvo-Alvarado, J.; Quesada, M.; do Espirito Santo, M.M. The role of tropical dry forests for biodiversity, carbon and water conservation in the neotropics: Lessons learned and opportunities for its sustainable management. Reg. Environ. Chang. 2015, 15, 1039–1049. [Google Scholar] [CrossRef]
- Van der Plas, F. Biodiversity and ecosystem functioning in naturally assembled communities. Biol. Rev. 2019, 94, 1220–1245. [Google Scholar] [CrossRef]
- Stuart-Smith, R.D.; Bates, A.E.; Lefcheck, J.S.; Duffy, J.E.; Baker, S.C.; Thomson, R.J.; Stuart-Smith, J.F.; Hill, N.A.; Kininmonth, S.J.; Airoldi, L.; et al. Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature 2013, 501, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Díaz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2002, 16, 646–655. [Google Scholar] [CrossRef]
- Schweiger, A.K.; Cavender-Bares, J.; Townsend, P.A.; Hobbie, S.E.; Madritch, M.D.; Wang, R.; Tilman, D.; Gamon, J.A. Plant spectral diversity integrates functional and phylogenetic components of biodiversity and predicts ecosystem function. Nat. Ecol. Evol. 2018, 2, 976–982. [Google Scholar] [CrossRef]
- Ma, X.; Mahecha, M.D.; Migliavacca, M.; Van Der Plas, F.; Benavides, R.; Ratcliffe, S.; Kattge, J.; Richter, R.; Musavi, T.; Baeten, L.; et al. Inferring plant functional diversity from space: The potential of Sentinel-2. Remote Sens. Environ. 2019, 233, 111368. [Google Scholar] [CrossRef]
- Correa, J.B.; Torres, J.P. Functional diversity: A key aspect in the provision of ecosystem services. Rev. Colomb. Cienc. Anim.-RECIA 2016, 8, 94–111. [Google Scholar] [CrossRef] [Green Version]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Sanaphre-Villanueva, L.; Dupuy, J.M.; Andrade, J.L.; Reyes-García, C.; Paz, H.; Jackson, P.C. Functional diversity of small and large trees along secondary succession in a tropical dry forest. Forests 2016, 7, 163. [Google Scholar] [CrossRef] [Green Version]
- Mason, N.W.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [Green Version]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Mouillot, D.; Villéger, S.; Scherer-Lorenzen, M.; Mason, N.W. Functional structure of biological communities predicts ecosystem multifunctionality. PLoS ONE 2011, 6, e17476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Su, J.; Li, S.; Liu, W.; Lang, X. Functional diversity drives ecosystem multifunctionality in a Pinus yunnanensis natural secondary forest. Sci. Rep. 2019, 9, 6979. [Google Scholar] [CrossRef] [Green Version]
- Durán, S.M.; Martin, R.E.; Díaz, S.; Maitner, B.S.; Malhi, Y.; Salinas, N.; Shenkin, A.; Silman, M.R.; Wieczynski, D.J.; Asner, G.P.; et al. Informing trait-based ecology by assessing remotely sensed functional diversity across a broad tropical temperature gradient. Sci. Adv. 2019, 5, eaaw8114. [Google Scholar] [CrossRef] [Green Version]
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Rödenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Gamon, J.A. Remote sensing of terrestrial plant biodiversity. Remote Sens. Environ. 2019, 231, 111218. [Google Scholar] [CrossRef]
- Kier, G.; Mutke, J.; Dinerstein, E.; Ricketts, T.H.; Küper, W.; Kreft, H.; Barthlott, W. Global patterns of plant diversity and floristic knowledge. J. Biogeogr. 2005, 32, 1107–1116. [Google Scholar] [CrossRef]
- Kreft, H.; Jetz, W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 5925–5930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Stefanoni, J.L.; Castillo-Santiago, M.Á.; Andres-Mauricio, J.; Portillo-Quintero, C.A.; Tun-Dzul, F.; Dupuy, J.M. Carbon Stocks, Species Diversity and Their Spatial Relationships in the Yucatán Peninsula, Mexico. Remote Sens. 2021, 13, 3179. [Google Scholar] [CrossRef]
- George-Chacon, S.P.; Dupuy, J.M.; Peduzzi, A.; Hernandez-Stefanoni, J.L. Combining high resolution satellite imagery and lidar data to model woody species diversity of tropical dry forests. Ecol. Indic. 2019, 101, 975–984. [Google Scholar] [CrossRef]
- Vieira, I.C.G.; de Almeida, A.S.; Davidson, E.A.; Stone, T.A.; de Carvalho, C.J.R.; Guerrero, J.B. Classifying successional forests using Landsat spectral properties and ecological characteristics in eastern Amazonia. Remote Sens. Environ. 2003, 87, 470–481. [Google Scholar] [CrossRef]
- Oehri, J.; Schmid, B.; Schaepman-Strub, G.; Niklaus, P.A. Biodiversity promotes primary productivity and growing season lengthening at the landscape scale. Proc. Natl. Acad. Sci. USA 2017, 114, 10160–10165. [Google Scholar] [CrossRef] [Green Version]
- Rocchini, D.; Balkenhol, N.; Carter, G.A.; Foody, G.M.; Gillespie, T.W.; He, K.S.; Kark, S.; Levin, N.; Lucas, K.; Luoto, M.; et al. Remotely sensed spectral heterogeneity as a proxy of species diversity: Recent advances and open challenges. Ecol. Inform. 2010, 5, 318–329. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural features for image classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Martínez, Á.; Camps-Valls, G.; Kattge, J.; Robinson, N.; Reichstein, M.; van Bodegom, P.; Kramer, K.; Cornelissen, J.H.C.; Reich, P.; Bahn, M.; et al. A methodology to derive global maps of leaf traits using remote sensing and climate data. Remote Sens. Environ. 2018, 218, 69–88. [Google Scholar] [CrossRef] [Green Version]
- Cavender-Bares, J.; Meireles, J.E.; Couture, J.J.; Kaproth, M.A.; Kingdon, C.C.; Singh, A.; Serbin, S.P.; Center, A.; Zuniga, E.; Pilz, G.; et al. Associations of leaf spectra with genetic and phylogenetic variation in oaks: Prospects for remote detection of biodiversity. Remote Sens. 2016, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Zeng, Y.; Schneider, F.D.; Zhao, Y.; Zhao, D.; Schmid, B.; Schaepman, M.E.; Morsdorf, F. Mapping functional diversity using individual tree-based morphological and physiological traits in a subtropical forest. Remote Sens. Environ. 2021, 252, 112170. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Knapp, D.E.; Tupayachi, R.; Anderson, C.B.; Sinca, F.; Vaughn, N.R.; Llactayo, W. Airborne laser-guided imaging spectroscopy to map forest trait diversity and guide conservation. Science 2017, 355, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.D.; Morsdorf, F.; Schmid, B.; Petchey, O.L.; Hueni, A.; Schimel, D.S.; Schaepman, M.E. Mapping functional diversity from remotely sensed morphological and physiological forest traits. Nat. Commun. 2017, 8, 1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre-Gutiérrez, J.; Rifai, S.; Shenkin, A.; Oliveras, I.; Bentley, L.P.; Svátek, M.; Girardin, C.A.; Both, S.; Riutta, T.; Berenguer, E.; et al. Pantropical modelling of canopy functional traits using Sentinel-2 remote sensing data. Remote Sens. Environ. 2021, 252, 112122. [Google Scholar] [CrossRef]
- Wallis, C.I.; Homeier, J.; Peña, J.; Brandl, R.; Farwig, N.; Bendix, J. Modeling tropical montane forest biomass, productivity and canopy traits with multispectral remote sensing data. Remote Sens. Environ. 2019, 225, 77–92. [Google Scholar] [CrossRef]
- Fischer, R.; Knapp, N.; Bohn, F.; Shugart, H.H.; Huth, A. The relevance of forest structure for biomass and productivity in temperate forests: New perspectives for remote sensing. Surv. Geophys. 2019, 40, 709–734. [Google Scholar] [CrossRef]
- Karadimou, E.K.; Kallimanis, A.S.; Tsiripidis, I.; Dimopoulos, P. Functional diversity exhibits a diverse relationship with area, even a decreasing one. Sci. Rep. 2016, 6, 35420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roscher, C.; Schumacher, J.; Gerighausen, U.; Schmid, B. Different assembly processes drive shifts in species and functional composition in experimental grasslands varying in sown diversity and community history. PLoS ONE 2014, 9, e101928. [Google Scholar] [CrossRef] [Green Version]
- Pakeman, R.J. Functional diversity indices reveal the impacts of land use intensification on plant community assembly. J. Ecol. 2011, 99, 1143–1151. [Google Scholar] [CrossRef]
- Miranda, F.; Hernández, X.E. Los Tipos de Vegetación de México y su Clasificación; Escuela Nacional de Agricultura, Colegio de Postgraduados: Texcoco, Mexico, 1963; p. 151. [Google Scholar]
- Orellana, R.; Islebe, G.A.; González-Iturbe, J.A. Presente, Pasado y Futuro de los Climas de la Península de Yucatán. En Naturaleza y Sociedad en el área Maya; Marín, P.C.-G., Saavedra, A.L., Eds.; Academia Mexicana de Ciencias, Centro de Investigación Científica de Yucatán: Mérida, México, 2003; pp. 37–52. [Google Scholar]
- Hernández-Stefanoni, J.L.; Dupuy, J.M.; Jhonson, K.J.; Birdsey, R.; Tun-Dzul, F.; Peduzzi, A.; Camal-Sosa, J.P.; Sánchez-Santos, G.; López-Merlín, D. Improving Species Diversity and Biomass Estimates of Tropical Dry Forest Using Airbone LiDAR. Remote Sens. 2014, 6, 4741–4763. [Google Scholar] [CrossRef] [Green Version]
- Huechacona-Ruiz, A.H.; Dupuy, J.M.; Schwartz, N.B.; Powers, J.S.; Reyes-García, C.; Tun-Dzul, F.; Hernández-Stefanoni, J.L. Mapping tree species deciduousness of tropical dry forests combining reflectance, spectral unmixing, and texture data from high-resolution imagery. Forests 2020, 11, 1234. [Google Scholar] [CrossRef]
- Hernández-Stefanoni, J.L.; Castillo-Santiago, M.; Mas, J.F.; Wheeler, C.E.; Andres-Mauricio, J.; Tun-Dzul, F.; George-Chacón, S.P.; Reyes-Palomeque, G.; Castellanos-Basto, B.; Vaca, R.; et al. Improving aboveground biomass maps of tropical dry forests by integrating LiDAR, ALOS PALSAR, climate and field data. Carbon Balance Manag. 2020, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database—Enhanced coverage and open access. Glob Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanaphre-Villanueva, L.; Dupuy, J.M.; Andrade, J.L.; Reyes-García, C.; Jackson, P.C.; Paz, H. Patterns of plant functional variation and specialization along secondary succession and topography in a tropical dry forest. Environ. Res. Lett. 2017, 12, 055004. [Google Scholar] [CrossRef]
- Letcher, S.G.; Lasky, J.R.; Chazdon, R.L.; Norden, N.; Wright, S.J.; Meave, J.A.; Pérez-García, E.A.; Muñoz, R.; Romero-Pérez, E.; Andrade, A.; et al. Environmental gradients and the evolution of successional habitat specialization: A test case with 14 Neotropical forest sites. J. Ecol. 2015, 103, 1276–1290. [Google Scholar] [CrossRef] [Green Version]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Swenson, N.G.; Weiser, M.D.; Mao, L.; Araújo, M.B.; Diniz-Filho, J.A.F.; Kollmann, J.; Nogués-Bravo, D.; Normand, S.; Rodríguez, M.A.; García-Valdés, R.; et al. Phylogeny and the prediction of tree functional diversity across novel continental settings. Glob. Ecol. Biogeogr. 2017, 26, 553–562. [Google Scholar] [CrossRef]
- Rousel, J.; Haas, R.; Schell, J.; Deering, D. Monitoring vegetation systems in the great plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite—1 Symposium, NASA SP-351, Washington, DC, USA, 10–15 December 1974; pp. 309–317. [Google Scholar]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Zvoleff, A. Package ‘glcm’. Calculate Textures from Grey-Level Co-Occurence Matrices (GLCMs). Available online: https://cran.r-project.org/web/packages/glcm/index.html (accessed on 25 October 2022).
- Dupuy, J.M.; Hernandez-Stefanoni, J.L.; Hernández-Juárez, R.A.; Tetetla-Rangel, E.; López-Martínez, J.O.; Leyequién-Abarca, E.; Tun-Dzul, F.J.; May-Pat, F. Patterns and correlates of tropical dry forest structure and composition in a highly replicated chronosequence in Yucatan, Mexico. Biotropica 2012, 44, 151–162. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Freeman, E.A.; Frescino, T.S.; Moisen, G.G. ModelMap: And R Package for Model Creation and Map Production. Available online: https://cran.r-project.org/web/packages/ModelMap/vignettes/VModelMap.pdf (accessed on 18 October 2022).
- Andres-Mauricio, J.; Valdez-Lazalde, J.R.; George-Chacón, S.P.; Hernández-Stefanoni, J.L. Mapping structural attributes of tropical dry forests by combining Synthetic Aperture Radar and high-resolution satellite imagery data. Appl. Veg. Sci. 2021, 24, e12580. [Google Scholar] [CrossRef]
- Hauser, L.T.; Féret, J.-B.; Binh, N.A.; van der Windt, N.; Sil, F.; Timmermans, J.; Soudzilovskaia, N.A.; van Bodegom, P.M. Towards scalable estimation of plant functional diversity from Sentinel-2: In-situ validation in a heterogeneous (semi-)natural landscape. Remote Sens. Environ. 2021, 262, 112505. [Google Scholar] [CrossRef]
- Warren, S.D.; Alt, M.; Olson, K.D.; Irl, S.D.; Steinbauer, M.J.; Jentsch, A. The relationship between the spectral diversity of satellite imagery, habitat heterogeneity, and plant species richness. Ecol. Inform. 2014, 24, 160–168. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Bergholz, K.; May, F.; Giladi, I.; Ristow, M.; Ziv, Y.; Jeltsch, F. Environmental heterogeneity drives fine-scale species assembly and functional diversity of annual plants in a semi-arid environment. Perspect. Plant Ecol. Evol. Syst. 2017, 24, 138–146. [Google Scholar] [CrossRef]
- Schmidtlein, S.; Fassnacht, F.E. The spectral variability hypothesis does not hold across landscapes. Remote Sens. Environ. 2017, 192, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Conroy, M.J.; Noon, B.R. Mapping of species richness for conservation of biological diversity: Conceptual and methodological issues. Ecol. Appl. 1996, 6, 763–773. [Google Scholar] [CrossRef]
- Suárez-Castro, A.F.; Raymundo, M.; Bimler, M.; Mayfield, M.M. Using multi-scale spatially explicit frameworks to understand the relationship between functional diversity and species richness. Ecography 2022, 22, e05844. [Google Scholar] [CrossRef]






| Type of Variable | Variable | DESCRIPTION |
|---|---|---|
| Sentinel-2 | Blue | Reflectance of blue band |
| Green Red | Reflectance of green band Reflectance of red band | |
| NDVI SAVI | The normalized difference vegetation index [49] The Soil-Adjusted Vegetation Index [50] | |
| Texture of Blue, Green, Red, NDVI and SAVI | The second-order texture measures used in this study are homogeneity (hom), contrast (cont), dissimilarity (dis), entropy (ent), angular second moment (asm), mean (mean), variance (var), and correlation (cor). See Haralick et al. [27] for details and formulas. | |
| Topography | DEM | Digital elevation models (DEM) obtained from the ALOS PALSAR synthetic aperture radar. |
| Variable | Type of Data | Number of Plots | R2 | RMSE | %RMSE |
|---|---|---|---|---|---|
| Functional Richness | Calibration | 174 | 0.36 | 21.0 | 65.5 |
| Validation | 58 | 0.50 | 18.6 | 48.8 | |
| Species Richness | Calibration | 174 | 0.32 | 9.0 | 33.9 |
| Validation | 58 | 0.44 | 7.4 | 25.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Lara, V.A.; Dupuy, J.M.; Reyes-Garcia, C.; Sanaphre-Villanueva, L.; Portillo-Quintero, C.A.; Hernández-Stefanoni, J.L. Modelling Species Richness and Functional Diversity in Tropical Dry Forests Using Multispectral Remotely Sensed and Topographic Data. Remote Sens. 2022, 14, 5919. https://doi.org/10.3390/rs14235919
Peña-Lara VA, Dupuy JM, Reyes-Garcia C, Sanaphre-Villanueva L, Portillo-Quintero CA, Hernández-Stefanoni JL. Modelling Species Richness and Functional Diversity in Tropical Dry Forests Using Multispectral Remotely Sensed and Topographic Data. Remote Sensing. 2022; 14(23):5919. https://doi.org/10.3390/rs14235919
Chicago/Turabian StylePeña-Lara, Víctor Alexis, Juan Manuel Dupuy, Casandra Reyes-Garcia, Lucia Sanaphre-Villanueva, Carlos A. Portillo-Quintero, and José Luis Hernández-Stefanoni. 2022. "Modelling Species Richness and Functional Diversity in Tropical Dry Forests Using Multispectral Remotely Sensed and Topographic Data" Remote Sensing 14, no. 23: 5919. https://doi.org/10.3390/rs14235919
APA StylePeña-Lara, V. A., Dupuy, J. M., Reyes-Garcia, C., Sanaphre-Villanueva, L., Portillo-Quintero, C. A., & Hernández-Stefanoni, J. L. (2022). Modelling Species Richness and Functional Diversity in Tropical Dry Forests Using Multispectral Remotely Sensed and Topographic Data. Remote Sensing, 14(23), 5919. https://doi.org/10.3390/rs14235919