1. Introduction
With rapid urbanization, energy-intensive industries and factories are increasing, resulting in a larger consumption of coal than that in the past [
1]. Car ownership is growing, resulting in increasing amounts of nitrogen oxides and sulfur dioxide emitted into the atmosphere. Sulfur dioxide (SO
2) is a colorless and pungent pollutant that is widely distributed in the atmosphere [
2]. It has a significant impact on air conditions and is responsible for increasing the acidity of rainfall. SO
2 also has a serious impact on the growth of plants in cities [
1,
3,
4,
5], it hinders the development of plants, long-term exposure to sulfur dioxide will damage the epidermis of plants, weaken photosynthesis and cause injury spots on the foliage, which will gradually wither until death.
In polluted environments, sensitive plant species can act as bio-indicators of air pollution, whereas tolerant plant species can act as air pollutant sinks [
6,
7]. A major challenge for future research is understanding the resistance and resilience of tropical arid forests to alternating severe droughts and major storms [
8]. Resistance indicators play an important role as standards in the evaluation of plant resistance to air pollution and the selection of resistant tree species. This has long been a target of research and substantial results have been achieved in this field. Currently, indicators for evaluating the resistance of plants to air pollution include leaf anatomical structure, changes in leaf fluid values and chlorophyll content, changes in cell membrane permeability, activity, and content, measures of the acute injury threshold and tolerance formula, and comprehensive evaluations of plant resistance [
9,
10,
11,
12,
13,
14,
15,
16,
17,
18]. These indicators can reflect the resistance and sensitivity of plants to SO
2 to a certain extent. The resistance of plants to environmental stress can be affected by many factors; however, the effects of multiple factors are different [
19,
20]. Many researchers have comprehensively evaluated many factors to evaluate plant resistance. Currently, the comprehensive evaluation methods for plant stress resistance mainly include the membership function method, coordinated comprehensive evaluation method, and analytic hierarchy process [
20,
21]. These methods use statistical approaches to analyze several indices related to plant resistance and to sort the indices to determine plant resistance. A comprehensive evaluation method can accurately reflect the strength of plant resistance [
19].
As an important indicator of the continuous life process of leaves from spreading through to maturation, aging, and withering, leaf age reflects the development process of plant leaves and is the scale of the length of leaf life activities. The resistance of different plants to SO
2 varies greatly, as do plant species, varieties, ontogeny, tree parts, and leaf age [
15,
16,
17,
18]. Among them, plant ontogeny may be crucial and is well known in the so-called age-related resistance (ARR) [
22]. ARR, also known as ontogenetic resistance, describes the ability of an entire plant or plant part to resist or tolerate diseases as it ages and matures. ARR occurs in many plant species and is usually broad-spectral [
23]. Farber and Mundt [
24] showed that the disease severity in young plants was significantly higher than that in old plants. The average disease severity of inoculated plants was 50.4%, 30.1%, and 12.9% for 3-week-old, 4-week-old, and 5-week-old plants, respectively. The disease severity decreased from the upper young leaves to the older lower leaves of wheat plants. Photosynthetic capacity decreases with increasing leaf age and is accompanied by an increase in leaf dry mass per unit area and a decrease in N, P, K, and Mg contents [
25,
26]. Gilmore [
27] showed that as leaf age increased in
Abies balsamea, the number of rows of viable cells in the primordia decreased, thus gradually reducing the ability of the needles to export photosynthetic products. Rapid changes in the age-class structure of boreal forests due to intensified forest use and, in some areas, due to increased natural disturbance, the proportion of old-growth forests has declined significantly, whereas that of young post-harvest and post-natural disturbance forests has increased [
28].
Resistance to tropical storms and the restoration of tropical dry forests after disturbance also depends on other structural and functional characteristics [
8]. Plant functional traits are a research hotspot in plant physiological and community ecology and are frequently used to predict species distributions, dynamics, and responses to environmental change [
29]. Chen et al. [
30] investigated a series of functional traits of the leaves and branches of 20 drought-tolerant broadleaf species planted in an arid limestone habitat in northern China. Plant ecologists have long been interested in elucidating the relationships between plant functional traits and the environment. Plant functional trait-environment relationships are the result of a combination of climatic, disturbance, and biotic conditions [
31], and plants develop adaptive strategies under different environmental gradients by changing their functional traits. In addition, environmental conditions vary in communities at different successional stages, and changing environmental factors drive changes in the functional traits and ecological responses of species to adapt to the environment at the community level.
With the development of new techniques, such as infrared thermography and ground-based spectroscopy, these physiological changes can now be directly and non-destructively detected before the appearance of obvious signs of injury. Thermal infrared imaging is a promising option [
32] and is based on the principle that water evapotranspiration cools and stomata close to warm leaves. Nilsson [
33] predicted that the dynamic process of temperature change is important for identifying different levels of biotic or abiotic stresses. Hyperspectral remote sensing plays an important role in quantitative vegetation monitoring because of its rapid, nondestructive, and high spectral resolution [
34,
35]. This avoids blade damage and tedious workloads.
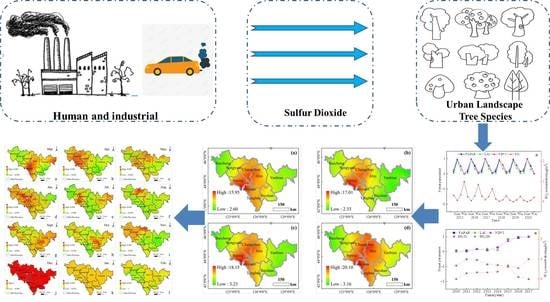
Therefore, this study combined biophysical methods such as spectroscopy, thermography with biochemical studies, and indoor simulated infiltration experiments to investigate variations in leaf functional traits at different ontogenies under SO2 stress and the resistance to SO2 stress. The specific objectives were as follows: (1) to investigate the changes in plant analysis development Chlorophyll content SPAD values, leaf temperature, spectral characteristics, and chlorophyll fluorescence characteristics of different tree species and ontogeny (10 days and 40 days) under SO2 stress, (2) to comprehensively evaluate the resistance of the three green tree species to SO2 at different ontogenies, and (3) to explore the resistance mechanisms of broadleaf trees to different SO2 concentration classes in different seasons on a large scale using the spatial products of fraction of photosynthetically active radiation (FPAR), gross primary productivity (GPP), and leaf area index (LAI), and normalized difference vegetation index (NDVI). Examining variations in functional traits of urban greening trees and environmental factors, as well as the response of the functional traits to environmental changes will provide a basis for the scientific guidance of artificial plant community construction and prevention of future vegetation degradation.
4. Discussion
As an important physiological indicator of plants, variations in chlorophyll SPAD, leaf temperature, green-peak and red-valley reflectance, and Fv/Fm are closely related to changes in stomatal aperture, leaf water content, photosynthetic activity, and enzyme activity [
49,
53]. As plants are exposed to SO
2, photosynthesis is inhibited and chlorophyll is converted to demagnetized chlorophyll [
54]. The limitation of photosynthesis can be divided into stomatal and non-stomatal factors [
55,
56], with the stomatal factor showing a decrease in Gs (stomatal conductance) and insufficient CO
2 supply and the non-stomatal factor, demonstrating a reduction in the activity of key enzymes for photosynthesis. If Pn decreases along with Gs and Ci, stomatal factors are likely responsible, and if Pn decreases as Ci increases, the main limiting factors for photosynthesis seem to be non-stomatal factors. The chlorophyll content, leaf temperature, green-peak reflectance, and Fv/Fm at 10 days were significantly lower than those at 40 days, regardless of the sampling date or SO
2 concentration. The SO
2 resistance for the 10-day leaves was consistently lower than for the 40-day leaves.
Juveniles of most plants are more sensitive to environmental changes and extremes, even in resilient species. This is because they require a more suitable environment to re-establish a fully independent SPAC system from a dormant state that is isolated from the soil and atmosphere. Some plants are salt-tolerant to the point of being salt-loving, and moderate salinity is beneficial to their growth [
18]. However, the seed germination of these plants is sensitive to salt, and rainfall and other salinity-eluting environments are more conducive to seed germination. The fragile tissue structure and low lignification of seedlings of some tree species makes them vulnerable to adverse effects, such as extreme low temperatures and summer droughts.
The stomatal opening of red-leaved
P. cerasifera was the largest and the least vulnerable to SO
2 damage. Omasa [
57] also found that the stomata of the lightly damaged parts of leaves were more open during the examination of the damage caused by SO
2 on plants. This study also concluded that red leaves of
P. cerasifera were slightly damaged, followed by
S. oblate with green leaves, while
U. pumila with yellow leaves were the most damaged. Significant differences in the stomatal aperture values were also observed. Red-leaved tree species with large stomatal aperture values have a stable metabolic balance of water and energy, excellent growth conditions, resistance to adversity, and ability to resist atmospheric pollution.
4.1. Effect of SO2 Stress on Vegetation Characteristics
SO
2 concentrations in summer, spring (autumn), and winter were differently regarded as T1, T2, and T3, respectively. As the SO
2 treatment concentration increased, the values of the vegetation parameters first increased and then decreased. That is, the highest SO
2 concentration and the lowest values for the vegetation parameters were observed in the winter, the lowest SO
2 concentration and the highest FPAR, LAI, and NDVI values were observed in the summer, and the SO
2 concentration and FPAR, LAI, and NDVI values were in the middle of summer and winter in spring and autumn. The SO
2 concentration in autumn is higher than that in spring, and the values of the vegetation parameters are also higher, which may be related to the vegetation characteristics during the growing season. These coincide with the results previously obtained from this study. The results in
Table 3 and
Table 5 suggest that a medium dose (T2) of SO
2 had a positive effect on the studied trees; the reduction in SO
2 concentration resulted in an increased reduction in SO
2 emissions. This resulted in an increased frequency of S deficiency in the crops and thus increased S fertilization. It is clear that excessive emissions of SO
2 (T3) result in the destruction of pine forests and acid rain, but the complete reduction of these emissions has certain disadvantages [
58].
4.2. Broadleaf Tree Resistance to SO2 Stress in Different Seasons
According to results of laboratory study, the 10-day leaf resistance performance of the three tree species under different SO
2 stress concentrations was as follows:
P. cerasifera > S. oblate > U. pumila, on 9 September. As leaf age increased, chlorophyll content and the net photosynthetic rate of the leaf gradually increased, which is closely related to the physiological changes that occur in the leaf during development [
59]. Different leaf growth conditions lead to differences in leaf pigment, moisture content, nitrogen, phosphorus, potassium, and other trace elements, as well as differences in cell structure and function. These changes affect the absorption and reflection of light, and ultimately lead to greater differences in the internal chemical composition and tolerance at different locations on the same leaf [
60,
61,
62,
63]. In addition to the phenological variations between the upper and lower plant parts, phenological differences between leaves in sun and those in shade have also been found in the canopy or branches [
13,
64,
65].
The resistance of different plants to SO
2 is known to vary significantly [
18]. In general, herbaceous plants are more sensitive than woody plants, coniferous trees are more sensitive than broad-leaved trees, and deciduous broad-leaved trees are more sensitive than evergreen trees. In addition, previous studies have investigated the resistance and purification ability of evergreen and deciduous plants [
66,
67,
68]. In this study, broadleaf trees differed in their resistance to different concentrations of SO
2.
Figure 8 shows broadleaf tree resistance to SO
2 stress during different seasons. Resistance decreased with increasing ρ(SO
2) class in different seasons. In the same ρ(SO
2) class, the resistance in different seasons was in the following order: summer > autumn > spring > winter. The resistance of different tree species to SO
2 is affected by season. In this study, the order of ρ(SO
2) in different seasons was winter > autumn > summer > spring; however, the difference was not significant (
Figure 9). When SO
2 is absorbed by a plant leaf, it can form sulfites, which are then oxidized to sulfates and turned into nutrients useful for plant growth. As the leaves age and wither, they can then continuously transfer sulfur dioxide from the air to the soil, creating a cycle that allows the air to be continuously purified [
69]. The leaves renew in the spring, which results in a reduction in sulfur dioxide emissions in the spring. Notably, although the amount of FP (fine particle) collected by vegetation is affected by the season [
18], the current study has determined that the seasonal differences was not statistically significant. One study conducted in a high-traffic area in Nanjing, China, showed that the order of different seasons in which dust was retained by trees could be ranked as winter > autumn > summer > spring [
70]. Another study conducted in Qingdao, China, reported that the dust retention capacity of ground cover plants showed seasonal variation in the order of winter > spring > autumn > summer [
18]. This may be due to the dry winter climate, a greater FP content in the air, and a lesser effect of rainfall. In the summer rainfall, a high relative humidity of the air, a lesser FP content in the air, and SO
2 pollution is also the same. Song et al. [
71] found that the season with the highest concentration of PM 2.5 in 2013 was winter (112.30 mg/m
3), while the cleanest season was summer (44.63 mg/m
3).
4.3. Correlation between GPP and ρ(SO2) in Different Vegetation Types
The resistance and resilience of deciduous broad-leaf forest and rational needleleaf forests differ under different continuous drought events [
72]. Changes in vegetation GPP can be affected by the concentration of air pollutants. Therefore, by analyzing the relationship between GPP and SO
2 concentration at the time corresponding to the experiment, we can better reveal the relationship between GPP changes and SO
2 concentrations. In this study, a correlation analysis was performed between the GPP of different vegetation types and their corresponding urban air SO
2 concentrations for the previous 30 days.
Figure 10 shows a plot of the correlation coefficients between vegetation GPP and urban air SO
2 concentrations from that day to the previous 30 days in 2019. The correlation coefficients were found to be more significant with the SO
2 concentrations in the previous four days, and the correlations between GPP and SO
2 concentrations on other dates were unstable, with highly significant negative correlations with the SO
2 concentrations in the previous four days. The relationship between the change in GPP and the change in SO
2 concentration from the current day to the previous 30 days was compared to reveal the influence of SO
2 concentration on GPP rhythm. From the results, the GPP change was consistent with the SO
2 concentration change in the previous four days, indicating a lag in GPP change in SO
2 in the previous four days. The correlation between different vegetation types, GPP, and SO
2 concentrations in the previous four days could be ranked in the following order: temperate deciduous shrub > temperate deciduous broadleaf forest > temperate leaflet deciduous forest > temperate and cold temperate mountain coniferous forest > temperate coniferous and broadleaf mixed forest > temperate coniferous forests.
The high concentration of SO
2 inhibits the growth of vegetation, which can be explained by the effect of SO
2 pollution on plants, which is macroscopically manifested as interference with the normal growth and development of vegetation, leading to dwarfing, a smaller leaf area, a lower pollination rate and a lower yield, which results in lower plant GPP. Winter is affected by the seasonal effect, which leads to a more abnormal correlation coefficient. Urban development boundaries and industrial parks mostly showed negative correlations, indicating that human activities have an impact on urban green tree growth. Some study areas, including primary forests in the northeast and low vegetation cover areas in the northwest and south, showed positive correlations because primary forests have a stable vegetation cover [
73] and stronger ecological functions, while low vegetation cover areas contain more building sites and lower SO
2 concentrations. Based on this, it is difficult for low SO
2 concentrations to affect vegetation GPP. The amount of precipitation in an area affects the type and growth state of vegetation, which in turn affects its ability to resist SO
2 pollution.