Spatiotemporal Protein Variations Based on VIIRS-Derived Regional Protein Algorithm in the Northern East China Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Situ Data Collection
2.2. Development of the Regional PRT Algorithm
2.3. Satellite Ocean Color Data
2.4. Regional Chla Algorithm for the NECS
2.5. Regional PON Algorithm for the NECS
2.6. Statistical Analysis and Data Processing
3. Results
3.1. In Situ Data in the NECS
3.2. Algorithm Development
3.2.1. Validation of PRT Algorithm for the NECS
3.2.2. Satellite Validation of Parameters for the Regional PRT Algorithm
3.2.3. Comparison of Satellite-Estimated PRT Concentration with In Situ Data
3.3. Correlation Results
3.4. Spatial and Seasonal PRT Concentration Variations in the NECS
4. Discussion
4.1. Evaluation of Regional Algorithms
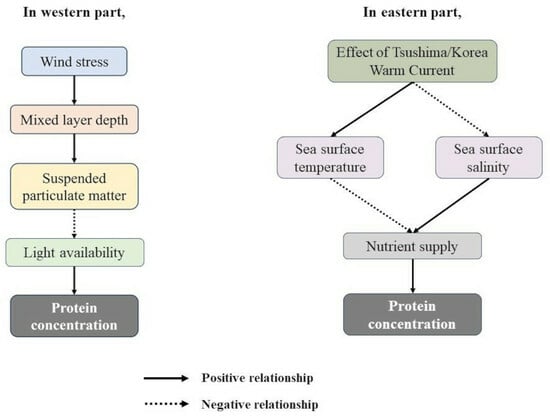
4.2. Major Environmental Controlling Factors for PRT of Each Part
4.3. Spatial and Temporal Variation for PRT in the NECS
5. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Q.; Wang, S.; Sukigara, C.; Goes, J.I.; Gomes, H.d.R.; Matsuno, T.; Zhu, Y.; Xu, Y.; Luang-on, J.; Watanabe, Y. High-resolution vertical observations of phytoplankton groups derived from an in-situ fluorometer in the East China Sea and Tsushima Strait. Front. Mar. Sci. 2022, 8, 756180. [Google Scholar] [CrossRef]
- Hao, J.; Yuan, D.; He, L.; Yuan, H.; Su, J.; Pohlmann, T.; Ran, X. Cross-Shelf Carbon Transport in the East China Sea and Its Future Trend Under Global Warming. J. Geophys. Res. Oceans. 2024, 129, e2022JC019403. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, Y.S.; Oh, H.J.; Suh, Y.S.; Hwang, J.D. Spatial distribution of early life stages of anchovy (Engraulis japonicus) and hairtail (Trichiurus lepturus) and their relationship with oceanographic features of the East China Sea during the 1997–1998 El Niño Event. Estuar. Coast. Shelf. Sci. 2005, 63, 13–21. [Google Scholar] [CrossRef]
- Bao, B.; Ren, G. Climatological characteristics and long-term change of SST over the marginal seas of China. Cont. Shelf Res. 2014, 77, 96–106. [Google Scholar] [CrossRef]
- Whyte, J.N.C. Biochemical composition and energy content of 6 species of phytoplankton used in mariculture of bivalves. Aquaculture 1987, 60, 231–241. [Google Scholar] [CrossRef]
- Kleppel, G.; Burkart, C. Egg production and the nutritional environment of Acartia tonsa: The role of food quality in copepod nutrition. ICES J. Mar. Sci. 1995, 52, 297–304. [Google Scholar] [CrossRef]
- Kang, J.J.; Joo, H.T.; Lee, J.H.; Lee, J.H.; Lee, H.W.; Lee, D.; Kang, C.K.; Yun, M.S.; Lee, S.H. Comparison of biochemical compositions of phytoplankton during spring and fall seasons in the northern East/Japan Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2017, 143, 73–81. [Google Scholar] [CrossRef]
- Kang, J.J.; Jang, H.K.; Lim, J.-H.; Lee, D.; Lee, J.H.; Bae, H. Characteristics of different size phytoplankton for primary pro-duction and biochemical compositions in the Western East/Japan Sea. Front. Microbiol. 2020, 11, 3306. [Google Scholar] [CrossRef]
- Mayzaud, P.; Chanut, J.P.; Ackman, R.G. Seasonal changes of the biochemical composition of marine particulate matter with special reference to fatty acids and sterols. Mar. Ecol. Prog. Ser. 1989, 56, 189–204. [Google Scholar] [CrossRef]
- Duncan, R.J.; Petrou, K. Biomolecular composition of sea ice microalgae and its influence on marine biogeochemical cycling and carbon Transfer through polar marine food webs. Geoscience 2022, 12, 38. [Google Scholar] [CrossRef]
- Scott, J. Effect of growth rate of the food alga on the growth/ingestion efficiency of a marine herbivore. J. Mar. Biol. Assoc. UK 1980, 60, 681–702. [Google Scholar] [CrossRef]
- Lindqvist, K.; Lignell, R. Intracellular partitioning of 14CO2 in phytoplankton during a growth season in the northern Baltic. Mar. Ecol. Prog. Ser. 1997, 152, 41–50. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic diversity in the macromolecular composition of microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef]
- Geider, R.J.; MacIntyre, H.L.; Kana, T.M. A dynamic model of photoadaptation in phytoplankton. Limnol. Oceanogr. 1996, 41, 1–15. [Google Scholar] [CrossRef]
- Morris, I. Photosynthetic products, physiological state, and phytoplankton growth. Can. Bull. Fish. Aquat. Sci. 1981, 210, 83–102. [Google Scholar]
- Ríos, A.F.; Fraga, F.; Pérez, F.F.; Figueiras, F.G. Chemical composition of phytoplankton and particulate organic matter in the Ría de Vigo (NW Spain). Sci. Mar. 1998, 62, 257–271. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.J.; Whitledge, T.E. High incorporation of carbon into proteins by the phytoplankton of the Bering Strait and Chukchi Sea. Cont. Shelf Res. 2009, 29, 1689–1696. [Google Scholar] [CrossRef]
- Bhavya, P.S.; Kim, B.K.; Jo, N.; Kim, K.; Kang, J.J.; Lee, J.H.; Lee, D.; Lee, J.H.; Joo, H.T.; Ahn, S.H.; et al. A review on the macromolecular compositions of phytoplankton and the implications for aquatic biogeochemistry. Ocean Sci. J. 2019, 54, 1–14. [Google Scholar] [CrossRef]
- Bae, H.; Lee, D.; Kang, J.J.; Lee, J.H.; Jo, N.; Kim, K.; Jang, H.K.; Kim, M.J.; Kim, Y.; Kwon, J.-I. Satellite-derived protein concentration of phytoplankton in the Southwestern East/Japan Sea. J. Mar. Sci. Eng. 2021, 9, 189. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A manual of chemical & biological methods for seawater analysis. Mar. Pollut. Bull. 1984, 15, 419–420. [Google Scholar]
- Lowry, O.H.; Roserough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Uyanık, G.K.; Güler, N. A study on multiple linear regression analysis. Procedia Soc. Behav. Sci. 2013, 106, 234–240. [Google Scholar] [CrossRef]
- Akinwande, M.O.; Dikko, H.G.; Samson, A. Variance inflation factor: As a condition for the inclusion of suppressor variables in regression analysis. Open J. Stat. 2015, 5, 754–767. [Google Scholar] [CrossRef]
- Shrestha, N. Detecting multicollinearity in regression analysis. Am. J. Appl. Math. Stat. 2020, 8, 39–42. [Google Scholar] [CrossRef]
- He, S.; Huang, D.; Zeng, D. Double SST fronts observed from MODIS data in the East China Sea off the Zhejiang–Fujian coast, China. J. Mar. Syst. 2016, 154, 93–102. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, J.; Wu, H.; Li, X. Dynamics of the sediment plume over the Yangtze Bank in the Yellow and East China Seas. J. Geophys. Res. Oceans. 2017, 122, 10073–10090. [Google Scholar] [CrossRef]
- Siswanto, E.; Tang, J.; Yamaguchi, H.; Ahn, Y.-H.; Ishizaka, J.; Yoo, S.; Kim, S.-W.; Kiyomoto, Y.; Yamada, K.; Chiang, C. Empirical ocean-color algorithms to retrieve chlorophyll-a, total suspended matter, and colored dissolved organic matter absorption coefficient in the Yellow and East China Seas. J. Oceanol. 2011, 67, 627–650. [Google Scholar] [CrossRef]
- Son, S.; Wang, M. Ice detection for satellite ocean color data processing in the Great Lakes. IEEE Trans. Geosci. Remote Sens. 2017, 55, 6793–6804. [Google Scholar] [CrossRef]
- Yoon, J.-E.; Son, S.; Kim, I.-N. Capture of decline in spring phytoplankton biomass derived from COVID-19 lockdown effect in the Yellow Sea offshore waters. Mar. Pollut. Bull. 2022, 174, 113175. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Maritorena, S.; Mitchell, B.G.; Siegel, D.A.; Carder, K.L.; Garver, S.A.; Kahru, M.; McClain, C. Ocean color chlorophyll algorithms for SeaWiFS. J. Geophys. Res. Oceans. 1998, 103, 24937–24953. [Google Scholar] [CrossRef]
- Tassan, S. Local algorithms using SeaWiFS data for the retrieval of phytoplankton, pigments, suspended sediment, and yellow substance in coastal waters. Appl. Opt. 1994, 33, 2369–2378. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wu, G. Satellite retrieval of oceanic particulate organic nitrogen concentration. Front. Mar. Sci. 2022, 9, 943867. [Google Scholar] [CrossRef]
- Hu, C.; Lee, Z.; Franz, B. Chlorophyll a algorithms for oligotrophic oceans: A novel approach based on three-band reflectance difference. J. Geophys. Res. Oceans. 2012, 117. [Google Scholar] [CrossRef]
- Werdell, P.J.; Franz, B.A.; Bailey, S.W.; Feldman, G.C.; Vega-Rodriguez, M.; Guild, L.S.; De La Cour, J.L.; Boss, E.; Brando, V.E.; Dowell, M.; et al. Generalized ocean color inversion model for retrieving marine inherent optical properties. Appl. Opt. 2013, 52, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Lin, C.; Su, J.; Liu, C.; Hao, Q.; Le, F. Long-term changes of dissolved oxygen, hypoxia, and the responses of the ecosystems in the East China Sea from 1975 to 1995. J. Oceanol. 2011, 67, 59–75. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, G.; Zhu, Z.; Huang, D.; Han, Y.; Wang, L.; Sun, M. Methane distribution and sea-to-air flux in the East China Sea during the summer of 2013: Impact of hypoxia. Deep Res. Part II Top. Stud. Oceanogr. 2016, 124, 74–83. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Ishizaka, J.; Siswanto, E.; Son, Y.B.; Yoo, S.; Kiyomoto, Y. Seasonal and spring interannual variations in satellite-observed chlorophyll-a in the Yellow and East China Seas: New datasets with reduced interference from high concentration of resuspended sediment. Cont. Shelf Res. 2013, 59, 1–9. [Google Scholar] [CrossRef]
- Yoo, S.; Kong, C.E.; Son, Y.B.; Ishizaka, J. A critical re-assessment of the primary productivity of the Yellow Sea, East China Sea and sea of Japan/East Sea large marine ecosystems. Deep Res. Part II Top. Stud. Oceanogr. 2019, 163, 6–15. [Google Scholar] [CrossRef]
- Liu, H.; Hu, S.; Zhou, Q.; Li, Q.; Wu, G. Revisiting effectiveness of turbidity index for the switching scheme of NIR-SWIR combined ocean color atmospheric correction algorithm. Int. J. Appl. Earth Observ. Geoinf. 2019, 76, 1–9. [Google Scholar] [CrossRef]
- Jang, H.-K.; Youn, S.-H.; Joo, H.; Kang, J.-J.; Lee, J.-H.; Lee, D.; Jo, N.; Kim, Y.; Kim, K.; Kim, M.-J. First Estimation of the annual biosynthetic calorie production by phytoplankton in the Yellow Sea, South Sea of Korea, East China Sea, and East Sea. Water 2023, 15, 2489. [Google Scholar] [CrossRef]
- Roy, S. Distributions of phytoplankton carbohydrate, protein and lipid in the world oceans from satellite ocean colour. ISME J. 2018, 12, 1457–1472. [Google Scholar] [CrossRef]
- Zhang, C.-I.; Seo, Y.-I.; Kang, H.-J.; Lim, J.-H. Exploitable carrying capacity and potential biomass yield of sectors in the East China Sea, Yellow Sea, and East Sea/Sea of Japan large marine ecosystems. Deep Res. Part II Top. Stud. Oceanogr. 2019, 163, 16–28. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Y. Influences of environmental factors on the spawning stock-recruitment relationship of Portunus trituberculatus in the northern East China Sea. Acta Oceanol. Sin. 2021, 40, 145–159. [Google Scholar] [CrossRef]
- Jo, N.; Kang, J.J.; Park, W.G.; Lee, B.R.; Yun, M.S.; Lee, J.H.; Kim, S.M.; Lee, D.; Joo, H.T.; Lee, J.H.; et al. Seasonal variation in the biochemical compositions of phytoplankton and zooplankton communities in the southwestern East/Japan Sea. Deep Res. Part II Top. Stud. Oceanogr. 2017, 143, 82–90. [Google Scholar] [CrossRef]
- Chang, K.-I.; Zhang, C.-I.; Park, C.; Kang, D.-J.; Ju, S.-J.; Lee, S.-H.; Wimbush, M. (Eds.) Oceanography of the East Sea (Japan Sea); Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-22719-1. [Google Scholar]
- Longhurst, A. Seasonal cycles of pelagic production and consumption. Prog. Oceanogr. 1995, 36, 77–167. [Google Scholar] [CrossRef]
- Morris, I.; Glover, H.E.; Yentsch, C.S. Products of photosynthesis by marine phytoplankton: The effect of environmental factors on the relative rates of protein synthesis. Mar. Biol. 1974, 27, 1–9. [Google Scholar] [CrossRef]
- Kilham, S.S.; Kreeger, D.A.; Goulden, C.E.; Lynn, S.G. Effects of nutrient limitation on biochemical constituents of An-kistrodesmus falcatus. Freshw. Biol. 1997, 38, 591–596. [Google Scholar] [CrossRef]
- Smith, A.; Morris, I. Pathways of carbon assimilation in phytoplankton from the Antarctic Ocean 1. Limnol. Oceanogr. 1980, 25, 865–872. [Google Scholar] [CrossRef]
- Pirt, S.J. Principles of Microbe and cell cultivation, 1st ed.; Blackwell Scientific Publications: London, UK, 1975; pp. 115–117. [Google Scholar]
- Lee, J.H.; Lee, D.; Kang, J.J.; Joo, H.T.; Lee, J.H.; Lee, H.W.; Ahn, S.H.; Kang, C.K.; Lee, S.H. The effects of different environmental factors on the biochemical composition of particulate organic matter in Gwangyang Bay, South Korea. Biogeosciences 2017, 14, 1903–1917. [Google Scholar] [CrossRef]
- Rivkin, R.B.; Voytek, M.A. Photoadaptations of photosynthesis and carbon metabolism by phytoplankton from McMurdo Sound, Antarctica. 1. Species-specific and community responses to reduced irradiances. Limnol. Oceanogr. 1987, 32, 249–259. [Google Scholar] [CrossRef]
- Wear, E.K.; Carlson, C.A.; Windecker, L.A.; Brzezinski, M.A. Roles of diatom nutrient stress and species identity in determining the short-and long-term bioavailability of diatom exudates to bacterioplankton. Mar. Chem. 2015, 177, 335–348. [Google Scholar] [CrossRef]
- Suárez, I.; Marañón, E. Photosynthate allocation in a temperate sea over an annual cycle: The relationship between protein synthesis and phytoplankton physiological state. J. Sea Res. 2003, 50, 285–299. [Google Scholar] [CrossRef]
- McConville, M.; Mitchell, C.; Wetherbee, R. Patterns of carbon assimilation in a microalgal community from annual sea ice, East Antarctica. Polar Biol. 1985, 4, 135–141. [Google Scholar] [CrossRef]
- Smith, R.E.; Clement, P.; Cota, G.F.; Li, W.K. Intracellular photosynthate allocation and the control of arctic marine ice algal production 1. J. Phycol. 1987, 23, 124–132. [Google Scholar] [CrossRef]
- Madariaga, I.; Fernández, E. Photosynthetic carbon metabolism of size-fractionated phytoplankton during an experimental bloom in marine microcosms. J. Mar. Biol. Assoc. UK 1990, 70, 531–543. [Google Scholar] [CrossRef]
- Liu, K.-K.; Chao, S.-Y.; Lee, H.-J.; Gong, G.-C.; Teng, Y.-C. Seasonal variation of primary productivity in the East China Sea: A numerical study based on coupled physical-biogeochemical model. Deep Res. Part II Top. Stud. Oceanogr. 2010, 57, 1762–1782. [Google Scholar] [CrossRef]
- Shi, W.; Wang, M. Satellite observations of the seasonal sediment plume in central East China Sea. J. Mar. Syst. 2010, 82, 280–285. [Google Scholar] [CrossRef]
- Bian, C.; Jiang, W.; Quan, Q.; Wang, T.; Greatbatch, R.J.; Li, W. Distributions of suspended sediment concentration in the Yellow Sea and the East China Sea based on field surveys during the four seasons of 2011. J. Mar. Syst. 2013, 121, 24–35. [Google Scholar] [CrossRef]
- Qiao, L.; Zhong, Y.; Wang, N.; Zhao, K.; Huang, L.; Wang, Z. Seasonal transportation and deposition of the suspended sediments in the Bohai Sea and Yellow Sea and the related mechanisms. Ocean Dyn. 2016, 66, 751–766. [Google Scholar] [CrossRef]
- Lu, Z.; Gan, J.; Dai, M.; Cheung, A.Y. The influence of coastal upwelling and a river plume on the subsurface chlorophyll maximum over the shelf of the northeastern South China Sea. J. Mar. Syst. 2010, 82, 35–46. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X. Influence of cross-shelf water transport on nutrients and phytoplankton in the East China Sea: A model study. Ocean Sci. 2011, 7, 27–43. [Google Scholar] [CrossRef]
- Jiang, L.; Guo, X.; Wang, L.; Sathyendranath, S.; Evers-King, H.; Chen, Y.; Li, B. Validation of MODIS ocean-colour products in the coastal waters of the Yellow Sea and East China Sea. Acta Oceanol. Sin. 2020, 39, 91–101. [Google Scholar] [CrossRef]
- Chen, Y.-l.L.; Chen, H.-Y.; Lee, W.-H.; Hung, C.-C.; Wong, G.T.; Kanda, J. New production in the East China Sea, comparison between well-mixed winter and stratified summer conditions. Cont. Shelf Res. 2001, 21, 751–764. [Google Scholar] [CrossRef]
- Kim, D.; Choi, S.H.; Kim, K.H.; Shim, J.; Yoo, S.; Kim, C.H. Spatial and temporal variations in nutrient and chlorophyll-a concentrations in the northern East China Sea surrounding Cheju Island. Cont. Shelf Res. 2009, 29, 1426–1436. [Google Scholar] [CrossRef]
- Lie, H.J.; Cho, C.H. On the origin of the Tsushima Warm Current. J. Geophys. Res. Oceans 1994, 99, 25081–25091. [Google Scholar] [CrossRef]
- Gong, G.-C.; Chen, Y.-L.L.; Liu, K.-K. Chemical hydrography and chlorophyll a distribution in the East China Sea in summer: Implications in nutrient dynamics. Cont. Shelf Res. 1996, 16, 1561–1590. [Google Scholar] [CrossRef]
- Shim, J.; Kim, D.; Kang, Y.C.; Lee, J.H.; Jang, S.-T.; Kim, C.-H. Seasonal variations in pCO2 and its controlling factors in surface seawater of the northern East China Sea. Cont. Shelf Res. 2007, 27, 2623–2636. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Ren, J.; Wu, Y.; Zhang, G. Nutrient gradients from the eutrophic Changjiang (Yangtze River) Estuary to the oligotrophic Kuroshio waters and re-evaluation of budgets for the East China Sea Shelf. Prog. Oceanogr. 2007, 74, 449–478. [Google Scholar] [CrossRef]
- Tomaselli, L.; Giovannetti, L.; Sacchi, A.; Bocci, F. Effects of temperature on growth and biochemical composition in Spirulina platensis strain M2. In Algal Biotechnology; Stadler, T., Mellion, J., Verdus, M., Karamanos, Y., Morvan, H., Christiaen, D., Eds.; Elsevier Applied Science: London, UK, 1988; pp. 303–314. [Google Scholar]
- Oliveira, M.; Monteiro, M.; Robbs, P.; Leite, S. Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquac. Int. 1999, 7, 261–275. [Google Scholar] [CrossRef]
- Jiao, N.; Yang, Y.; Hong, N.; Ma, Y.; Harada, S.; Koshikawa, H.; Watanabe, M. Dynamics of autotrophic picoplankton and heterotrophic bacteria in the East China Sea. Cont. Shelf Res. 2005, 25, 1265–1279. [Google Scholar] [CrossRef]
- Chen, C.-T.A. Chemical and physical fronts in the Bohai, Yellow and East China seas. J. Mar. Syst. 2009, 78, 394–410. [Google Scholar] [CrossRef]
- Teague, W.J.; Jacobs, G.A.; Perkins, H.T.; Book, J.W.; Chang, K.I.; Suk, M.S. Low-frequency current observation in the Korea/Tsushima Strait. J. Phys. Oceanogr. 2002, 32, 1621–1641. [Google Scholar] [CrossRef]
- Ma, C.; Wu, D.; Lin, X.; Yang, J.; Ju, X. On the mechanism of seasonal variation of the Tsushima Warm Current. Cont. Shelf Res. 2012, 48, 1–7. [Google Scholar] [CrossRef]
- Seung, Y.H. Some dynamical issues about the Tsushima Warm Current based on bibliographical review. Sea J. Korean Soc. Oceanogr. 2019, 24, 439–447. [Google Scholar]
- Ichikawa, H.; Chaen, M. Seasonal variation of heat and freshwater transports by the Kuroshio in the East China Sea. J. Mar. Syst. 2000, 24, 119–129. [Google Scholar] [CrossRef]
- Chu, P.; Chen, Y.C.; Kununaka, A. Seasonal variability of the Yellow Sea/East China Sea surface fluxes and thermohaline structure. Adv. Atmos. Sci. 2005, 22, 1–20. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, J.H.; de Matthaeis, P.; Yueh, S.; Hong, C.S.; Lee, J.H.; Lagerloef, G. Sea surface salinity variability in the East China Sea observed by the Aquarius instrument. J. Geophys. Res. Oceans 2014, 119, 7016–7028. [Google Scholar] [CrossRef]
- Boëchat, I.G.; Giani, A. Seasonality affects diel cycles of seston biochemical composition in a tropical reservoir. J. Plankton Res. 2008, 30, 1417–1430. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, J.H.; Joo, H.; Song, H.J.; Yang, E.J.; Lee, S.H.; Lee, S.H. Macromolecular compositions of phytoplankton in the Amundsen Sea, Antarctica. Deep Res. Part II Top. Stud. Oceanogr. 2016, 123, 42–49. [Google Scholar] [CrossRef]
- Fabiano, M.; Povero, P.; Danovaro, R. Distribution and composition of particulate organic matter in the Ross Sea (Antarctica). Polar Biol. 1993, 3, 525–533. [Google Scholar] [CrossRef]
- Danovaro, R.; Dell’Anno, A.; Pusceddu, A.; Marrale, D.; Della Croce, N.; Fabiano, M.; Tselepides, A. Biochemical composition of pico-, nano-and micro-particulate organic matter and bacterioplankton biomass in the oligotrophic Cretan Sea (NE Mediterranean). Prog. Oceanogr. 2000, 46, 279–310. [Google Scholar] [CrossRef]
- Chen, C.-T.A. Distributions of nutrients in the East China Sea and the South China Sea connection. J. Oceanol. 2008, 64, 737–751. [Google Scholar] [CrossRef]
- Kim, D.-S.; Choi, S.-H.; Kim, K.-H.; Kim, C.-H. The distribution and interannual variation in suspended solid and particulate organic carbon in the northern East China Sea. Ocean Polar Res. 2009, 31, 219–229. [Google Scholar] [CrossRef]
- Kim, Y.; Youn, S.-H.; Oh, H.J.; Kang, J.J.; Lee, J.H.; Lee, D.; Kim, K.; Jang, H.K.; Lee, J.; Lee, S.H. Spatiotemporal variation in phytoplankton community driven by environmental factors in the northern East China Sea. Water 2020, 12, 2695. [Google Scholar] [CrossRef]
- DiTullio, G.R.; Laws, E.A. Estimates of phytoplankton N uptake based on 14CO2 incorporation into protein. Limnol. Oceanogr. 1983, 28, 177–185. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, H.; Dang, X.; Cheng, T.; Yang, S.; Wang, L.; Huang, L.; Cui, X. Spatial-temporal change of phytoplankton biomass in the East China Sea with MODIS data. J. Ocean Univ. China 2021, 20, 454–462. [Google Scholar] [CrossRef]
- Sverdrup, H. On conditions for the vernal blooming of phytoplankton. J. Cons. Int. Explor. Mer. 1953, 18, 287–295. [Google Scholar] [CrossRef]











| Variables | Abbreviation (Unit) | Spatial/Temporal Resolution | Dataset |
|---|---|---|---|
| Photosynthetic available radiation | PAR (Einstein m−2 d−1) | 4 km × 4 km, monthly | SNPP-VIIRS |
| Sea surface temperature | SST (°C) | 4 km × 4 km, monthly | SNPP-VIIRS |
| Suspended particulate matter | SPM (mg L−1) | 4 km × 4 km, monthly | Copernicus-Globcolour |
| Sea surface salinity | SSS (psu) | 0.083° × 0.083°, monthly | Copernicus-Global Ocean Physics Reanalysis |
| Mixed layer depth | MLD (m) | 0.083° × 0.083°, monthly | |
| Wind Speed | Wind speed (m s−1) | 0.25° × 0.25°, monthly | Cross-Calibrated Multi-Platform |
| Included Independent Variables | Regression Coefficient | p-Value | VIF | Adjusted R2 |
|---|---|---|---|---|
| Constant | 2.765 | |||
| Chla | 20.301 | 0.000 ** | 1.699 | 0.507 |
| PON | 0.822 | 0.000 ** | 1.699 | 0.613 |
| PRT | Chla | PON | SSS | SSN | SST | |
|---|---|---|---|---|---|---|
| PRT | 1 | |||||
| Chla | 0.76 ** | 1 | ||||
| PON | 0.73 ** | 0.67 ** | 1 | |||
| SSS | −0.15 | 0.12 | −0.16 | 1 | ||
| SSN | −0.07 | 0.01 | −0.004 | 0.22 * | 1 | |
| SST | −0.04 | −0.25 ** | −0.01 | −0.59 ** | −0.29 | 1 |
| Regions (Sampling Depth) | Study Period | Method | PRT Concentration (μg L−1) | References | ||
|---|---|---|---|---|---|---|
| Min. | Max. | Average ± S.D. | ||||
| Northern East Sea (euphotic depth) | October (2012) April–May (2015) | Field-measurement | 16 12 | 138 180 | 66 ± 27 75 ± 37 | Kang et al. [7] |
| Southwestern East Sea (euphotic depth) | April–November (2014) | Field-measurement | 27 | 174 | 85 ± 59 | Jo et al. [44] |
| Global coastal ocean (surface) | Monthly (1997–2013) | Satellite OC-CCI * data | 4 | 25 | 11 ± 6 | Roy [41] ** |
| Global open ocean (surface) | 2 | 9 | 5 ± 2 | |||
| Southwestern East Sea (surface) | Monthly (2003–2019) | Satellite Aqua-MODIS data | 27 | 138 | 54 ± 14 | Bae et al. [19] |
| Yellow Sea (euphotic depth) | February, April, August, and October (2018) | Field-measurement | 17 | 212 | 59 ± 43 | Jang et al. [40] |
| South Sea (euphotic depth) | 0 | 98 | 34 ± 29 | |||
| East Sea (euphotic depth) | 3 | 147 | 44 ± 35 | |||
| East China Sea (euphotic depth) | February, May, August, and November (2018) | 1 | 138 | 40 ± 29 | ||
| Western part of the NECS (surface) | 8 day (2012–2022) | Satellite SNPP-VIIRS data | 28 | 136 | 55 ± 15 | This study |
| Eastern part of the NECS (surface) | 17 | 63 | 37 ± 7 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Kim, S.; Lee, D.; Jang, H.-K.; Park, S.; Kim, Y.; Kim, J.; Youn, S.-H.; Joo, H.; Son, S.; et al. Spatiotemporal Protein Variations Based on VIIRS-Derived Regional Protein Algorithm in the Northern East China Sea. Remote Sens. 2024, 16, 829. https://doi.org/10.3390/rs16050829
Kim M, Kim S, Lee D, Jang H-K, Park S, Kim Y, Kim J, Youn S-H, Joo H, Son S, et al. Spatiotemporal Protein Variations Based on VIIRS-Derived Regional Protein Algorithm in the Northern East China Sea. Remote Sensing. 2024; 16(5):829. https://doi.org/10.3390/rs16050829
Chicago/Turabian StyleKim, Myeongseop, Sungjun Kim, Dabin Lee, Hyo-Keun Jang, Sanghoon Park, Yejin Kim, Jaesoon Kim, Seok-Hyun Youn, Huitae Joo, Seunghyun Son, and et al. 2024. "Spatiotemporal Protein Variations Based on VIIRS-Derived Regional Protein Algorithm in the Northern East China Sea" Remote Sensing 16, no. 5: 829. https://doi.org/10.3390/rs16050829
APA StyleKim, M., Kim, S., Lee, D., Jang, H. -K., Park, S., Kim, Y., Kim, J., Youn, S. -H., Joo, H., Son, S., & Lee, S. -H. (2024). Spatiotemporal Protein Variations Based on VIIRS-Derived Regional Protein Algorithm in the Northern East China Sea. Remote Sensing, 16(5), 829. https://doi.org/10.3390/rs16050829