Field Spectroscopy in the VNIR-SWIR Region to Discriminate between Mediterranean Native Plants and Exotic-Invasive Shrubs Based on Leaf Tannin Content
Abstract
:1. Introduction
2. Material and Methods
2.1. Site Description

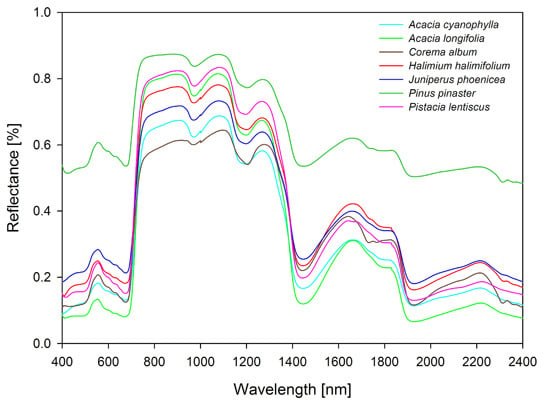
2.2. Spectral Data and Leaf Collection

| Species | Leaf Type | Leaf Size Length and Width | Leaf Sampling | Study Site | Number of Individuals | Number of Leaf Spectra | Used for Calibration | Used for Prediction | |
|---|---|---|---|---|---|---|---|---|---|
| A. longifolia | Phyllode | Large (>5 cm) | Broad (1–5 cm) | Several leaves | PDC, MEL, LDS | 15 | 225 | 155 | 70 |
| A. cyanophylla | Phyllode | Large | Broad | Several leaves | MEL | 5 | 75 | 50 | 25 |
| C. album | Needle | Small (<1 cm) | Thin (<1 cm) | Leaves stacked | PDC | 5 | 75 | 55 | 20 |
| H. halimifolium | Leaf | Medium (1–5 cm) | Broad | Several leaves | MEL, LDS | 10 | 150 | 100 | 50 |
| J. phoenicea | Needle | Small | Thin | Leaves stacked | MEL | 5 | 75 | 50 | 25 |
| P. pinea | Needle | Large | Thin | Leaves stacked | PDC | 5 | 75 | 50 | 25 |
| P. lentiscus | Leaf | Medium | Broad | Leaves stacked | PDC | 5 | 75 | 47 a | 25 |
| Total | 50 | 750 | 507 | 240 | |||||
2.3. Chemical Analysis of Tannin Concentration
2.4. Data Pre-Processing
2.5. Determination of Important Wavelength Regions
2.6. Discrimination of Species
3. Results
3.1. Tannin Concentration

| Species | A. cyanophylla | A. longifolia | C. album | H. halimifolium | J. phoenicea | P. pinea |
|---|---|---|---|---|---|---|
| A. longifolia | 0.476 | - | - | - | - | - |
| C. album | 0.476 | 0.021 | - | - | - | - |
| H. halimifolium | 0.013 | 0.001 | 0.013 | - | - | - |
| J. phoenicea | 0.383 | 0.021 | 1.000 | 0.037 | - | - |
| P. pinea | 1.000 | 0.383 | 0.103 | 0.013 | 0.107 | - |
| P. lentiscus | 0.103 | 0.021 | 0.103 | 0.810 | 0.169 | 0.103 |
3.2. Wavelength Selection for Classification

3.3. Discrimination of Species by PCA-LDA Classification
| Wavelengths | Overall Kappa | Overall Accuracy % | User’s Accuracy % | Producer’s Accuracy % | User’s Accuracy % | Producer’s Accuracy % |
|---|---|---|---|---|---|---|
| A. longifolia | A. longifolia | non-A. longifolia | non-A. longifolia | |||
| Euclidean | ||||||
| 400–2400 nm | 0.67 | 83.3 | 90.0 | 79.4 | 76.6 | 88.5 |
| 675–710 nm | 0.69 | 84.3 | 89.1 | 81.4 | 79.4 | 88.0 |
| 1060–1170 nm | 0.68 | 83.9 | 91.7 | 79.4 | 76.0 | 90.1 |
| 1360–1450 nm | 0.81 | 90.6 | 98.9 | 84.8 | 82.3 | 98.6 |
| 1630–1740 nm | 0.69 | 84.7 | 98.9 | 77.1 | 70.6 | 98.4 |
| all Tannin regions | 0.70 | 85.0 | 95.4 | 79.0 | 74.6 | 94.3 |
| sq. Euclidean | ||||||
| 400–2400 nm | 0.73 | 86.6 | 93.7 | 82.0 | 79.4 | 92.7 |
| 675–710 nm | 0.78 | 89.1 | 89.1 | 89.2 | 89.1 | 89.2 |
| 1060–1170 nm | 0.75 | 87.4 | 91.1 | 85.0 | 83.7 | 90.4 |
| 1360–1450 nm | 0.82 | 90.9 | 96.3 | 86.9 | 85.4 | 95.8 |
| 1630–1740 nm | 0.65 | 82.7 | 96.9 | 75.6 | 68.6 | 95.6 |
| all Tannin regions | 0.79 | 89.3 | 93.7 | 86.1 | 84.9 | 93.1 |
| Mahalanobis | ||||||
| 400–2,400 nm | 0.65 | 82.6 | 77.7 | 86.1 | 87.4 | 79.7 |
| 675–710 nm | 0.73 | 86.3 | 80.9 | 90.7 | 91.7 | 82.8 |
| 1060–1170 nm | 0.77 | 88.6 | 81.4 | 95.0 | 95.7 | 83.8 |
| 1360–1450 nm | 0.75 | 87.3 | 83.1 | 90.6 | 91.4 | 84.5 |
| 1630–1740nm | 0.53 | 76.3 | 55.7 | 94.6 | 96.9 | 68.6 |
| all Tannin regions | 0.73 | 86.3 | 80.9 | 90.7 | 91.7 | 82.8 |
4. Discussion
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hassanpour, S.; Maherisis, N.; Eshratkhah, B.; Mehmandar, F.B. Plants and secondary metabolites (Tannins): A review. IJFSE 2011, 1, 47–53. [Google Scholar]
- Ammar, H.; López, S.; González, J.S. Assessment of the digestibility of some Mediterranean shrubs by in vitro techniques. Anim. Feed Sci. Tech. 2005, 119, 323–331. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Ramos, G.; Giráldez, F.J.; Mantecón, A.R. Condensed tannin content of several shrub species from a mountain area in northern Spain, and its relationship to various indicators of nutritive value. Anim. Feed Sci. Tech. 2002, 95, 215–226. [Google Scholar] [CrossRef]
- Gallardo, A.; Merino, J. Nitrogen immobilization in leaf litter at two Mediterranean ecosystems of SW Spain. Biogeochemistry 1992, 15, 213–228. [Google Scholar] [CrossRef]
- Landau, S.; Perevolotsky, A.; Bonfil, D.; Barkai, D.; Silanikove, N. Utilization of low quality resources by small ruminants in Mediterranean agro-pastoral systems: The case of browse and aftermath cereal stubble. Livest. Prod. Sci. 2000, 64, 39–49. [Google Scholar] [CrossRef]
- Perevolotsky, A. Tannins in Mediterranean woodland species: Lack of response to browsing and thinning. Oikos 1994, 71, 333–340. [Google Scholar] [CrossRef]
- Rogosic, J.; Pfister, J.; Provenza, F.; Grbesa, D. Sheep and goat preference for and nutritional value of Mediterranean Maquis shrubs. Small Ruminant Res. 2006, 64, 169–179. [Google Scholar] [CrossRef]
- Ben Salem, H.; Nefzaoui, A.; Ben Salem, L.; Tisserand, J.L. Deactivation of condensed tannins in Acacia cyanophylla Lindl. foliage by polyethylene glycol in feed blocks: Effect on feed intake, diet digestibility, nitrogen balance, microbial synthesis and growth by sheep. Livest. Prod. Sci. 2000, 64, 51–60. [Google Scholar]
- Kumara Mahipala, M.B.P.; Krebs, G.L.; McCafferty, P.; Gunaratne, L.H.P. Chemical composition, biological effects of tannin and in vitro nutritive value of selected browse species grown in the West Australian Mediterranean environment. Anim. Feed Sci. Tech. 2009, 153, 203–215. [Google Scholar]
- Marchante, E.; Kjøller, A.; Struwe, S.; Freitas, H. Short- and long-term impacts of Acacia longifolia invasion on the belowground processes of a Mediterranean coastal dune ecosystem. Appl. Soil Ecol. 2008, 40, 210–217. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species—A global review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Gaertner, M.; Marchante, E.; Ens, E.-J.; Holmes, P.M.; Pauchard, A.; O’Farrell, P.J.; Rogers, A.M.; Blanchard, R.; Blignaut, J.; et al. Impacts of invasive Australian acacias: Implications for management and restoration. Divers. Distrib. 2011, 17, 1015–1029. [Google Scholar] [CrossRef]
- Rascher, K.G.; Große-Stoltenberg, A.; Máguas, C.; Meira-Neto, J.A.A.; Werner, C. Acacia longifolia invasion impacts vegetation structure and regeneration dynamics in open dunes and pine forests. Biol. Invasions 2011, 13, 1099–1113. [Google Scholar] [CrossRef]
- Werner, C.; Zumkier, U.; Beyschlag, W.; Máguas, C. High competitiveness of a resource demanding invasive Acacia under low resource supply. Plant Ecol. 2010, 206, 83–96. [Google Scholar] [CrossRef]
- Christine Hellmann, R.S. Influence of an exotic N2-fixing Acacia on community composition and N status of native Mediterranean species. Acta Oecologica 2011, 37, 43–50. [Google Scholar] [CrossRef]
- Rascher, K.G.; Große-Stoltenberg, A.; Máguas, C.; Werner, C. Understory invasion by Acacia longifolia alters the water balance and carbon gain of a Mediterranean pine forest. Ecosystems 2011, 14, 904–919. [Google Scholar] [CrossRef]
- Seigler, D.S.; Hernandez, J.F. Comparative tanning ability of extracts from four North American species of Acacia. J. Am. Leather Chem. Assoc. 1989, 84, 315–322. [Google Scholar]
- Furstenburg, D.; van Hoven, W. Condensed tannin as anti-defoliate agent against browsing by giraffe (Giraffa camelopardalis) in the Kruger National Park. Comp. Biochem. Physiol. A 1994, 107, 425–431. [Google Scholar] [CrossRef]
- Maiden, J.H. The Useful Native Plants of Australia, (Including Tasmania); Turner and Henderson: Sydney, Australia, 1889. [Google Scholar]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems: A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Barradas, M.C.D.; Zunzunegui, M.; Novo, F.G. Autecological traits of Halimium halimifolium in contrasting habitats under a Mediterranean type climate—A review. Folia Geobot. 1999, 34, 189–208. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Milton, E.J.; Schaepman, M.E.; Anderson, K.; Kneubühler, M.; Fox, N. Progress in field spectroscopy. Remote Sens. Environ. 2009, 113, S92–S109. [Google Scholar] [CrossRef]
- Asner, G.P.; Jones, M.O.; Martin, R.E.; Knapp, D.E.; Hughes, R.F. Remote sensing of native and invasive species in Hawaiian forests. Remote Sens. Environ. 2008, 112, 1912–1926. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.; Méndez-Rial, R.; Reguera-Salgado, J.; Martín-Herrero, J. Alien plant monitoring with ultralight airborne imaging spectroscopy. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Costa, J.C.; Espirito Santo, M.D.; Lousa, M. The vegetation of dunes of southwest Portugal. Silva Lusitana 1994, 2, 51–68. [Google Scholar]
- Carlos Silva Neto, J.C.C. Phytosociological associations and Natura 2000 habitats of Portuguese coastal dunes. Fitosociologia 2008, 44, 29–35. [Google Scholar]
- Roux, D.G. Photometric methods of tannin analysis for black wattle tannin. J. Soc. Leather Trades Chem. 1951, 35, 322–337. [Google Scholar]
- Rinnan, Å.; Berg, F. van den; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends in Analytical Chemistry 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Féret, J.-B.; François, C.; Gitelson, A.; Asner, G.P.; Barry, K.M.; Panigada, C.; Richardson, A.D.; Jacquemoud, S. Optimizing spectral indices and chemometric analysis of leaf chemical properties using radiative transfer modeling. Remote Sens. Environ. 2011, 115, 2742–2750. [Google Scholar] [CrossRef]
- Song, S.; Gong, W.; Zhu, B.; Huang, X. Wavelength selection and spectral discrimination for paddy rice, with laboratory measurements of hyperspectral leaf reflectance. ISPRS J. Photogramm. Remote Sens. 2011, 66, 672–682. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Suhaili, A.B. Sources of canopy chemical and spectral diversity in lowland bornean forest. Ecosystems 2012, 15, 504–517. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E. Spectral and chemical analysis of tropical forests: Scaling from leaf to canopy levels. Remote Sens. Environ. 2008, 112, 3958–3970. [Google Scholar] [CrossRef]
- Svante Wold, M.S. PLS-regression: A basic tool of chemometrics. Chemometr. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Abdi, H. Partial least squares regression and projection on latent structure regression (PLS Regression). Comp. Stat. 2010, 2, 97–106. [Google Scholar]
- Clark, M.L.; Roberts, D.A.; Clark, D.B. Hyperspectral discrimination of tropical rain forest tree species at leaf to crown scales. Remote Sens. Environ. 2005, 96, 375–398. [Google Scholar] [CrossRef]
- Naes, T.; Isaksson, T.; Fearn, T.; Davies, T. A user Friendly guide to Multivariate Calibration and Classification; NIR Publications: Chichester, UK, 2002. [Google Scholar]
- Pu, R.; Gong, P.; Tian, Y.; Miao, X.; Carruthers, R.I.; Anderson, G.L. Invasive species change detection using artificial neural networks and CASI hyperspectral imagery. Environ. Monit. Assess. 2008, 140, 15–32. [Google Scholar] [CrossRef] [PubMed]
- He, K.S.; Rocchini, D.; Neteler, M.; Nagendra, H. Benefits of hyperspectral remote sensing for tracking plant invasions. Divers. Distrib. 2011, 17, 381–392. [Google Scholar] [CrossRef]
- Devi, S.R.; Prasad, M.N.V. Tannins and related polyphenols from ten common Acacia species of India. Bioresour. Tech. 1991, 36, 189–192. [Google Scholar] [CrossRef]
- Humphries, S.G. The biosynthesis of tannins. In Biogenesis of Natural Compounds,, 2nd ed.; Pergamon Press: Oxford, UK, 1963; pp. 801–823. [Google Scholar]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (Tannins) as biological antioxidants. J. Agr. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.-Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Sardans, J.; Llusia, J.; Owen, S.M.; Silva, J.; Niinemets, U. Higher allocation to low cost chemical defenses in invasive species of Hawaii. J. Chem. Ecol. 2010, 36, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Siemann, E.; Wheeler, G.S.; Zhu, L.; Gu, X.; Ding, J. Genetic variation in anti-herbivore chemical defences in an invasive plant. J. Ecol. 2012, 100, 894–904. [Google Scholar] [CrossRef] [Green Version]
- Omelchuk, O. The Content of Phenolics and Tannins in Native and Invasive Solidago Species. Available online: http://phytomorphology.org/PDF/MP4/04091092.pdf (accessed on 13 October 2014).
- Blossey, B.; Notzold, R. Evolution of increased competitive ability in invasive nonindigenous plants: A hypothesis. J. Ecol. 1995, 83, 887–889. [Google Scholar] [CrossRef]
- Carvalho, S.; Schlerf, M.; van der Putten, W.H.; Skidmore, A.K. Hyperspectral reflectance of leaves and flowers of an outbreak species discriminates season and successional stage of vegetation. Int. J. Appl. Earth Obs. Geoinf. 2013, 24, 32–41. [Google Scholar] [CrossRef]
- Hagerman, A.E. Tannin Handbook; Miami University: Oxford, OH, USA, 2002. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Soukupova, J.; Rock, B.N.; Albrechtova, J. Spectral characteristics of lignin and soluble phenolics in the near infrared: A comparative study. Int. J. Remote Sens. 2002, 23, 3039–3055. [Google Scholar] [CrossRef]
- Ferwerda, J.G.; Skidmore, A.K.; Stein, A. A bootstrap procedure to select hyperspectral wavebands related to tannin content. Int. J. Remote Sens. 2006, 27, 1413–1424. [Google Scholar] [CrossRef]
- Porter, L.J. Structure and chemical properties of the condensed tannins. In Plant Polyphenols; Hemingway, R.W., Laks, P.E., Eds.; Springer: New Your, NY, USA, 1992; pp. 245–258. [Google Scholar]
- Daniel Cozzolino, W.U.C. Measurement of condensed tannins and dry matter in red grape homogenates using near infrared spectroscopy and partial least squares. J. Agr. Food Chem. 2008, 56, 7631–7636. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Clark, R.N. Spectroscopic determination of leaf biochemistry using band-depth analysis of absorption features and stepwise multiple linear regression. Remote Sens. Environ. 1999, 67, 267–287. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmann, J.R.K.; Große-Stoltenberg, A.; Römer, M.; Oldeland, J. Field Spectroscopy in the VNIR-SWIR Region to Discriminate between Mediterranean Native Plants and Exotic-Invasive Shrubs Based on Leaf Tannin Content. Remote Sens. 2015, 7, 1225-1241. https://doi.org/10.3390/rs70201225
Lehmann JRK, Große-Stoltenberg A, Römer M, Oldeland J. Field Spectroscopy in the VNIR-SWIR Region to Discriminate between Mediterranean Native Plants and Exotic-Invasive Shrubs Based on Leaf Tannin Content. Remote Sensing. 2015; 7(2):1225-1241. https://doi.org/10.3390/rs70201225
Chicago/Turabian StyleLehmann, Jan Rudolf Karl, André Große-Stoltenberg, Meike Römer, and Jens Oldeland. 2015. "Field Spectroscopy in the VNIR-SWIR Region to Discriminate between Mediterranean Native Plants and Exotic-Invasive Shrubs Based on Leaf Tannin Content" Remote Sensing 7, no. 2: 1225-1241. https://doi.org/10.3390/rs70201225
APA StyleLehmann, J. R. K., Große-Stoltenberg, A., Römer, M., & Oldeland, J. (2015). Field Spectroscopy in the VNIR-SWIR Region to Discriminate between Mediterranean Native Plants and Exotic-Invasive Shrubs Based on Leaf Tannin Content. Remote Sensing, 7(2), 1225-1241. https://doi.org/10.3390/rs70201225