Expansion of Industrial Plantations Continues to Threaten Malayan Tiger Habitat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Overview
2.3. Data
2.4. Natural Forest Mapping
2.5. Forest Loss Mapping
2.6. Sample-Based Adjustment of the Area Estimation
3. Results
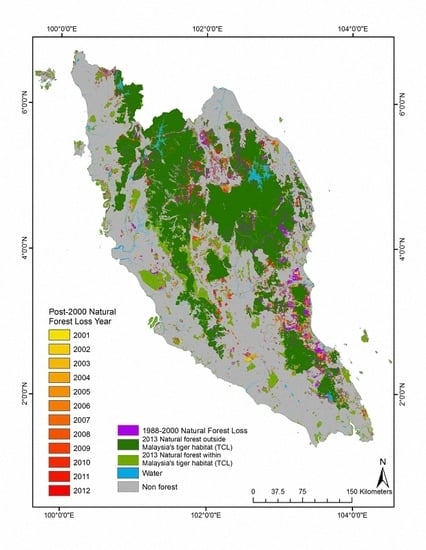
3.1. Natural Forest Extent and Change from 1988–2012
3.2. Tiger Habitat Loss and Change from 1988–2012
3.3. Accuracy Assessment of 1988–2000 and 2001–2012 Forest Loss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Convention on Biodiversity Diversity (CBD). Malaysia: Country Profile. Available online: https://www.cbd.int/countries/profile/default.shtml?country=my#facts (accessed on 10 December 2015).
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, K. Panthera tigris ssp. jacksoni. The IUCN Red List of Threatened Species 2015: E.T136893A50665029. 2015. Available online: http://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T136893A50665029.en (accessed on 10 October 2016).
- Seidensticker, J. Saving wild tigers: A case study in biodiversity loss and challenges to be met for recovery beyond 2010. Integr. Zool. 2010, 5, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, H.K.; Ruesch, A.S.; Achard, F.; Clayton, M.K.; Holmgren, P.; Ramankutty, N.; Foley, J.A. Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl. Acad. Sci. USA 2010, 107, 16732–16737. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, S.N.; Posa, R.C.M.; Lee, M.T.; Bickford, D.; Koh, P.L.; Brook, W.B. The state and conservation of Southeast Asian biodiversity. Biodivers. Conserv. 2010, 19, 317–328. [Google Scholar] [CrossRef]
- Kummer, D.M.; Turner, B.L. The human causes of deforestation in Southeast Asia. Bioscience 1994, 44, 323–328. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Nakagoshi, N. Forest fragmentation and its correlation to human land use change in the state of Selangor, peninsular Malaysia. For. Ecol. Manag. 2007, 241, 39–48. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Hezri, A.A. From forest landscape to agricultural landscape in the developing tropical country of Malaysia: Pattern, process, and their significance on policy. Environ. Manag. 2008, 42, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.P.; Wilcove, D.S. Is oil palm agriculture really destroying tropical biodiversity? Conserv. Lett. 2008, 1, 60–64. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Case Study of Tropical Forest Plantations in Malaysia by D.B.A Krishnapillay; Forest Plantations Working Paper 23; FAO: Rome, Italy, 2002; unpublished; Available online: http://www.fao.org/documents/card/en/c/8ac53512-c384-5b5a-baf4-f6d137e3a421/ (accessed on 11 July 2017).
- Byerlee, D. The Fall and Rise Again of Plantations in Tropical Asia: History Repeated? Land 2014, 3, 574–597. [Google Scholar] [CrossRef]
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Brookfield, H.; Byron, Y. Deforestation and timber extraction in Borneo and the Malay Peninsula: The record since 1965. Glob. Environ. Chang. 1990, 1, 42–56. [Google Scholar] [CrossRef]
- Clements, R.; Rayan, D.M.; Zafir, A.W.A.; Venkataraman, A.; Alfred, R.; Payne, J.; Ambu, L.; Sharma, D.S.K. Trio under threat: Can we secure the future of rhinos, elephants and tigers in Malaysia? Biodivers. Conserv. 2010, 19, 1115–1136. [Google Scholar] [CrossRef]
- Kawanishi, K.; Yatim, S.H.; Abu Hashim, A.K.; Topani, R. Distribution and potential population size of the tiger in Peninsular Malaysia. J. Wildl. Park. 2003, 21, 29–50. [Google Scholar]
- Joshi, A.R.; Dinerstein, E.; Wikramanayake, E.; Anderson, M.L.; Olson, D.; Jones, B.S.; Seidensticker, J.; Lumpkin, S.; Hansen, M.C.; Sizer, N.C.; et al. Tracking changes and preventing loss in critical tiger habitat. Sci. Adv. 2016, 2, e1501675. [Google Scholar] [CrossRef] [PubMed]
- World Bank (Washington, DC, USA). Global Tiger Recovery Program (2010–2022). Available online: http://documents.worldbank.org/curated/en/874191468331048098/Global-tiger-recovery-program-2010-2022 (accessed on 27 June 2017).
- Global Tiger Recovery Program (GTRP). Global Tiger Recovery Program Volume 2. Draft 31 July 2010. Washington, DC, USA, 2010. Available online: http://admin.indiaenvironmentportal.org.in/files/GTRP-Volume-2-July-31.pdf (accessed on 27 June 2017).
- Department of Wildlife and National Parks Peninsular Malaysia (DWNP). National Tiger Action Plan for Malaysia 2008–2020; DWNP: Kuala Lumpur, Malaysia, 2008.
- Hansen, M.C.; Stehman, S.V.; Potapov, P.V.; Loveland, T.R.; Townshend, J.R.; DeFries, R.S.; Pittman, K.W.; Arunarwati, B.; Stolle, F.; Steininger, M.K.; et al. Humid tropical forest clearing from 2000 to 2005 quantified by using multitemporal and multiresolution remotely sensed data. Proc. Natl. Acad. Sci. USA 2008, 105, 9439–9444. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.C.; Stehman, S.V.; Potapov, P.V. Quantification of global gross forest cover loss. Proc. Natl. Acad. Sci. USA 2010, 107, 8650–8655. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, J.; Shi, C.; Liew, S.C. Deforestation rates in insular Southeast Asia between 2000 and 2010. Glob. Chang. Biol. 2011, 17, 2261–2270. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Sexton, J.O.; Townshend, J.R. Accelerated deforestation in the humid tropics from the 1990s to the 2000s. Geophys. Res. Lett. 2015, 42, 3495–3501. [Google Scholar] [CrossRef] [PubMed]
- Cohen, W.B.; Goward, S.N. Landsat’s role in ecological applications of remote sensing. Bioscience 2004, 54, 535–545. [Google Scholar] [CrossRef]
- Margono, B.A.; Potapov, P.V.; Turubanova, S.; Stolle, F.; Hansen, M.C. Primary forest cover loss in Indonesia over 2000–2012. Nat. Clim. Chang. 2014, 4, 730–735. [Google Scholar] [CrossRef]
- Srinivas, A.; Koh, L.P. Oil palm expansion drives avifaunal decline in the Pucallpa region of Peruvian Amazonia. Glob. Ecol. Conserv. 2016, 7, 183–200. [Google Scholar] [CrossRef]
- Peh, K.S.H.; Sodhi, N.S.; De Jong, J.; Sekercioglu, C.H.; Yap, C.A.M.; Lim, S.L.H. Conservation value of degraded habitats for forest birds in southern Peninsular Malaysia. Divers. Distrib. 2006, 12, 572–581. [Google Scholar] [CrossRef]
- Maddox, T. The Conservation of Tigers and Other Wildlife in Oil Palm Plantations: Jambi Province, Sumatra, Indonesia; Zoological Society of London (ZSL): London, UK, 2007. [Google Scholar]
- Wicke, S.; Sikkema, R.; Dornburg, V.; Faaij, A. Exploring land use changes and the role of palm oil production in Indonesia and Malaysia. Land Use Policy 2011, 28, 193–206. [Google Scholar] [CrossRef]
- McMorrow, J.; Talip, M.A. Decline of forest area in Sabah, Malaysia: Relationship to state policies, land code and land capability. Glob. Environ. Chang. 2001, 11, 217–230. [Google Scholar] [CrossRef]
- Gunarso, P.; Hartoyo, M.; Agus, F.; Killeen, T. Oil Palm and Land Use Change in Indonesia, Malaysia and Papua New Guinea; Reports from the Technical Panels of the 2nd Greenhouse Gas Working Group of the Roundtable on Sustainable Palm Oil (RSPO); RSPO: Kuala Lumpur, Malaysia, 2013; pp. 29–64. Available online: http://www.rspo.org/key-documents/supplementary-materials (accessed on 20 June 2016).
- Food and Agriculture Organization of the United Nations (FAO). Countries: Geography Malaysia. 2012. Available online: http://www.fao.org/forestry/country/18310/en/mys/ (accessed on 22 June 2017).
- Forestry Department of Peninsular Malaysia (FDPM). Forest Types. 2016. Available online: https://www.forestry.gov.my/index.php/en/2016-06-07-02-31-39/2016-06-07-02-35-17/forest-type (accessed on 22 June 2017).
- Sanderson, E.; Forrest, J.; Loucks, C.; Ginsberg, J.; Dinerstein, E.; Seidensticker, J.; Leimgruber, P.; Songer, M.; Heydlauff, A.; O’Brien, T.; et al. Setting Priorities for the Conservation and Recovery of Wild Tigers: 2005–2015. The Technical Assessment; World Wildlife Fund: Washington, DC, USA; Wildlife Conservation Society: New York, NY, USA; Smithsonian, and National Fish and Wildlife Foundation: Washington, DC, USA, 2006. [Google Scholar]
- UNEP-WCMC and IUCN. Protected Planet: The World Database on Protected Areas (WDPA). UNEP-WCMC: Cambridge, UK, 2016. Available online: www.protectedplanet.net (accessed on 10 February 2016).
- Petersen, R.; Aksenov, D.; Goldman, E.; Sargent, S.; Harris, N.; Manisha, A.; Esipova, E.; Shevade, V.; Loboda, T. Mapping Tree Plantations with Multispectral Imagery: Preliminary Results for Seven Tropical Countries; Technical Note; World Resources Institute: Washington, DC, USA, 2016; Available online: http://www.wri.org/publication/mapping-tree-plantations (accessed on 23 September 2016).
- Potapov, P.V.; Turubanova, S.A.; Hansen, M.C.; Adusei, B.; Broich, M.; Altstatt, A.; Mane, L.; Justice, C.O. Quantifying forest cover loss in Democratic Republic of the Congo. 2000–2010, with Landsat ETM+ data. Remote Sens. Environ. 2012, 122, 106–116. [Google Scholar] [CrossRef]
- Potapov, P.V.; Turubanova, S.A.; Tyukavina, A.; Krylov, A.M.; McCarty, J.L.; Radeloff, V.C.; Hansen, M.C. Eastern Europe’s forest cover dynamics from 1985 to 2012 quantified from the full Landsat archive. Remote Sens. Environ. 2015, 159, 28–43. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Q.; Braswell, B.; Urbanski, S.; Boles, S.; Wofsy, S.C.; Moore, B.; Ojima, D. Modeling gross primary production of a deciduous broadleaf forest using satellite images and climate data. Remote Sens. Environ. 2004, 91, 256–270. [Google Scholar] [CrossRef]
- Breiman, L. Bagging predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef]
- Giree, N.; Stehman, S.V.; Potapov, P.; Hansen, M.C. A sample-based forest monitoring strategy using Landsat, AVHRR and MODIS data to estimate gross forest cover loss in Malaysia between 1990 and 2005. Remote Sens. 2013, 5, 1842–1855. [Google Scholar] [CrossRef]
- Olofsson, P.; Foody, G.M.; Herold, M.; Stehman, S.V.; Woodcock, C.E.; Wulder, M.A. Good practices for estimating area and assessing accuracy of land change. Remote Sens. Environ. 2014, 148, 42–57. [Google Scholar] [CrossRef]
- Olofsson, P.; Foody, G.M.; Stehman, S.V.; Woodcock, C.E. Making better use of accuracy data in land change studies: Estimating accuracy and area and quantifying uncertainty using stratified estimation. Remote Sens. Environ. 2013, 129, 122–131. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Institute for Global Environmental Strategies: Hayama, Japan, 2006. [Google Scholar]
- Tyukavina, A.; Stehman, S.V.; Potapov, P.V.; Turubanova, S.A.; Baccini, A.; Goetz, S.J.; Laporte, N.T.; Houghton, R.A.; Hansen, M.C. National-scale estimation of gross forest aboveground carbon loss: A case study of the Democratic Republic of the Congo. Environ. Res. Lett. 2013, 8, 044039. [Google Scholar] [CrossRef]
- Potapov, P.V.; Dempewolf, J.; Talero, Y.; Hansen, M.C.; Stehman, S.V.; Vargas, C.; Rojas, E.J.; Castillo, D.; Mendoza, E.; Calderón, A.; et al. National satellite-based humid tropical forest change assessment in Peru in support of REDD+ implementation. Environ. Res. Lett. 2014, 9, 124012. [Google Scholar] [CrossRef]
- Sheil, D.; Casson, A.; Meijaard, E.; van Nordwijk, M.; Gaskell, J.; Sunderland-Groves, J.; Wertz, K.; Kanninen, M. The Impacts and Opportunities of Oil Palm in Southeast Asia: What Do We Know and What Do We Need to Know? Occasional Paper No. 51; Center for International Forestry Research (CIFOR): Bogor, Indonesia, 2009. [Google Scholar]
- Gaveau, D.L.; Sheil, D.; Husnayaen, M.A.S.; Arjasakusuma, S.; Ancrenaz, M.; Pacheco, P.; Meijaard, E. Rapid conversions and avoided deforestation: Examining four decades of industrial plantation expansion in Borneo. Sci. Rep. 2016, 6, 32017. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.P.; Miettinen, J.; Liew, S.C.; Ghazoul, J. Remotely sensed evidence of tropical peatland conversion to oil palm. Proc. Natl. Acad. Sci. USA 2011, 108, 5127–5132. [Google Scholar] [CrossRef] [PubMed]
- Rayan, D.M.; Linkie, M. Conserving tigers in Malaysia: A science-driven approach for eliciting conservation policy change. Biol. Conserv. 2015, 184, 18–26. [Google Scholar] [CrossRef]
- Rayan, D.M.; Mohamad, S.W. The importance of selectively logged forests for tiger Panthera tigris conservation: A population density estimate in Peninsular Malaysia. Oryx 2009, 43, 48–51. [Google Scholar] [CrossRef]





| Types of Metrics | Multi-Temporal Metrics |
|---|---|
| Image Composites | 1. First and last cloud-free observations |
| 2. Mean and Median of first 3 observations | |
| 3. Mean and Median of last 3 observations | |
| Rank-based Metrics | 1. Percentiles representing minimum, 10%, 25%, 50%, 75%, 90% and maximum for band reflectance or index (NDVI, NDWI) values |
| 2. Symmetrical averages for various intervals: minimum–maximum, 10–90%, 25–75% | |
| 3. Asymmetrical averages for various intervals: minimum–10%, 10–25%, 25–50%, 50–75%, 75–90%, 90%-maximum | |
| Trend Analysis Metrics | 1. Slope of linear regression of band reflectance versus image date |
| 2. Standard deviation of band reflectance 1988–2000 | |
| 3. Maximum loss/gain of reflectance/index value between consecutive observations |
| Study Period | Total Loss | Total Loss within Plantations | Habitat Loss | Habitat Loss within Plantations |
|---|---|---|---|---|
| Pre-2000 | 0.59 ± 0.11 | 0.24 ± 0.05 | 0.42 ± 0.11 | 0.15 ± 0.04 |
| Post-2000 | 0.76 ± 0.13 | 0.41 ± 0.07 | 0.41 ± 0.10 | 0.23 ± 0.06 |
| Total | 1.35 ± 0.17 | 0.65 ± 0.08 | 0.83 ± 0.15 | 0.38 ± 0.07 |
| Reference | ||||||
|---|---|---|---|---|---|---|
| Loss | No Loss | Total | User’s | Producer’s | Overall | |
| Pre-2000 Forest | N = 475 | |||||
| Map | ||||||
| Loss | 0.06 | 0.02 | 0.08 | 0.75 | 0.75 | 0.96 |
| No Loss | 0.02 | 0.9 | 0.92 | 0.98 | 0.98 | |
| Total | 0.08 | 0.92 | 1 | |||
| Post-2000 Forest | N = 489 | |||||
| Map | ||||||
| Loss | 0.07 | 0.01 | 0.08 | 0.88 | 0.63 | 0.95 |
| No Loss | 0.04 | 0.88 | 0.92 | 0.96 | 0.99 | |
| Total | 0.12 | 0.88 | 1 | |||
| Pre-2000 Habitat | N = 314 | |||||
| Map | ||||||
| Loss | 0.05 | 0.01 | 0.06 | 0.90 | 0.55 | 0.95 |
| No Loss | 0.04 | 0.90 | 0.94 | 0.96 | 0.99 | |
| Total | 0.09 | 0.91 | 1.00 | |||
| Post-2000 Habitat | N = 358 | |||||
| Map | ||||||
| Loss | 0.06 | 0.005 | 0.06 | 0.92 | 0.57 | 0.95 |
| No Loss | 0.04 | 0.90 | 0.94 | 0.96 | 0.99 | |
| Total | 0.096 | 0.904 | 1.00 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevade, V.S.; Potapov, P.V.; Harris, N.L.; Loboda, T.V. Expansion of Industrial Plantations Continues to Threaten Malayan Tiger Habitat. Remote Sens. 2017, 9, 747. https://doi.org/10.3390/rs9070747
Shevade VS, Potapov PV, Harris NL, Loboda TV. Expansion of Industrial Plantations Continues to Threaten Malayan Tiger Habitat. Remote Sensing. 2017; 9(7):747. https://doi.org/10.3390/rs9070747
Chicago/Turabian StyleShevade, Varada S., Peter V. Potapov, Nancy L. Harris, and Tatiana V. Loboda. 2017. "Expansion of Industrial Plantations Continues to Threaten Malayan Tiger Habitat" Remote Sensing 9, no. 7: 747. https://doi.org/10.3390/rs9070747
APA StyleShevade, V. S., Potapov, P. V., Harris, N. L., & Loboda, T. V. (2017). Expansion of Industrial Plantations Continues to Threaten Malayan Tiger Habitat. Remote Sensing, 9(7), 747. https://doi.org/10.3390/rs9070747