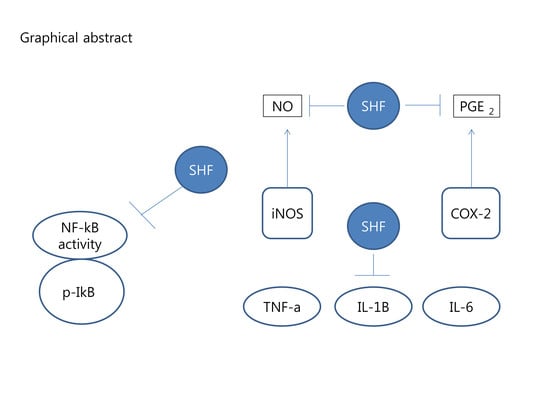
Anti-Inflammatory Effects of a Stauntonia hexaphylla Fruit Extract in Lipopolysaccharide-Activated RAW-264.7 Macrophages and Rats by Carrageenan-Induced Hind Paw Swelling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extraction
2.2. High-Performance Liquid Chromatography (HPLC) Analysis
2.3. Cell Culture
2.4. Cell Viability
2.5. Determination of NO and PGE2 Production
2.6. Measurement of COX Enzyme Activity
2.7. Measurement of Cytokine Production
2.8. Western Blot Analysis
2.9. Animals
2.10. Carrageenan-Induced Paw Edema in Rats
2.11. Statistical Analysis
3. Results
3.1. Analysis of the SHF Extract for Compounds with Anti-Inflammatory Activity
3.2. Effect of the SHF Extract on the Viability of RAW 264.7 Cells
3.3. Effect of the SHF Extract on LPS-Induced Production of NO and PGE2 in RAW 264.7 Cells
3.4. Effect of the SHF Extract on LPS-Induced COX Enzyme Activity and the Expression of iNOS Protein in RAW 264.7 Cells
3.5. Effect of the SHF Extract on LPS-Induced Production of Pro-Inflammatory Cytokines in RAW 264.7 Cells
3.6. Effect of the SHF Extract on LPS-Induced NF-κB Activity and IκB Phosphorylation in RAW 254.7 Cells
3.7. Effects of the SHF Extract on Carrageenan-Induced Rat Paw Edema
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Darshan, S.; Doreswamy, R. Patented antiinflammatory plant drug development from traditional medicine. Phytother. Res. 2004, 18, 343–357. [Google Scholar] [CrossRef] [PubMed]
- De las Heras, B.; Slowing, K. Antiinflammatory and antioxidant activity of plants used in traditional medicine in Ecuador. J. Ethnopharmacol. 1998, 61, 161–166. [Google Scholar] [CrossRef]
- Talhouk, R.S.; Karam, C. Anti-inflammatory bioactivities in plant extracts. J. Med. Food 2007, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, K. Traditional Indian spices and their health significance. Asia Pac. J. Clin. Nutr. 2008, 17, 265–268. [Google Scholar] [PubMed]
- Asongalem, E.A.; Foyet, H.S.; Ngogang, J.; Folefoc, G.N.; Dimo, T. Analgesic and antiinflammatory activities of Erigeron floribundus. J. Ethnopharmacol. 2004, 91, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; Kim, J.Y.; Kim, Y.S.; Chung, Y.C.; Hahm, K.S.; Jeong, H.G. Augmentation of macrophage functions by an aqueous extract isolated from Platycodon grandiflorum. Cancer Lett. 2001, 166, 17–25. [Google Scholar] [CrossRef]
- Talwar, S.; Nandakumar, K.; Nayak, P.G.; Bansal, P.; Mudgal, J.; Mor, V.; Rao, C.M.; Chamallamudi, M.R.; Lobo, R. Anti-inflammatory activity of Terminalia paniculata bark extract against acute and chronic inflammation in rats. J. Ethnopharmacol. 2011, 134, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Woldesellassie, M.; Eyasu, M.; Kelbessa, U. In vivo anti-inflammatory activities of leaf extracts of Ocimum lamiifolium in mice model. J. Ethnopharmacol. 2011, 134, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Huang, X.D.; Wang, H.; Leung, A.K.N.; Chan, C.L.; Fong, D.W.F.; Yu, Z.L. Anti-inflammatory and analgesic effects of the ethanol extract of Rosa multiflora Thunb. hips. J. Ethnopharmacol. 2008, 118, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Xian, Y.F.; Ip, S.P.; Su, Z.R.; Su, J.Y.; He, J.J.; Xie, Q.F.; Lai, X.P.; Lin, Z.X. Anti-inflammatory activity of patchouli alcohol isolated from Pogostemonis herba in animal models. Fitoterapia 2011, 82, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Park, Y.S.; Towantakavanit, K.; Park, J.O.; Kim, Y.M.; Jung, K.J.; Cho, J.Y.; Lee, K.D.; Heo, B.G. Chemical components and biological activity of Stauntonia hexaphylla. Korean J. Plant Resour. 2009, 22, 403–411. [Google Scholar]
- Wang, H.B.; Mayer, R.; Rocker, G.; Yang, J.J.; Matteson, D.S. A phenolic glycoside and triterpenoids from Stauntonia hexaphylla. Phytochemistry 1998, 47, 467–470. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kwon, S.H.; Kim, S.B.; Lim, S.S. Inhibitory activities of Stauntonia hexaphylla leaf constituents on rat lens aldose reductase and formation of advanced glycation end products and antioxidant. Biomed. Res. Int. 2017, 2017, 1–8. [Google Scholar]
- Ryu, H.W.; Lee, S.U.; Lee, S.; Song, H.H.; Son, T.H.; Kim, Y.U.; Yuk, H.J.; Ro, H.; Lee, C.K.; Hong, S.T.; et al. 3-methoxy-catalposide inhibited inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophages. Cytokine 2017, 91, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Ko, Y.J.; Kang, M.C.; Yang, H.M.; Roh, S.W.; Oda, T.; Jeon, Y.J.; Jung, W.K.; Heo, S.J.; Yoon, W.J.; et al. Anti-inflammatory effects of trans-1,3-diphenyl-2,3-epoxypropane-1-one mediated by suppression of inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2013, 53, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Flavell, R.A. The relationship of inflammation and initiation of autoimmune disease: Role of TNF super family members. Curr. Top. Microbiol. Immunol. 2002, 266, 1–9. [Google Scholar] [PubMed]
- Christodoulou, C.; Choy, E.H. Joint inflammation and cytokine inhibition in rheumatoid arthritis. Exp. Med. 2006, 6, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Jeffery, P.K.; Busse, W.W.; Johnson, M.; Vignola, A.M. Asthma: From bronchoconstriction to airways inflammation and remodeling. Am. J. Respir. Crit. Care Med. 2000, 161, 1720–1745. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Grisham, M.; Hodge, J.; Telliez, J.B. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: A hub for multiple inflammatory cytokines. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G155–G162. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Moreno, M.; Heinbockel, L.; Suwalsky, M.; Garidel, P.; Brandenburg, K. Biophysical study of the non-steroidal anti-inflammatory drugs (NSAID) ibuprofen, naproxen and diclofenac with phosphatidylserine bilayer membranes. Biochim. Biophys. Acta 2016, 1858, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.M.; Chung, S.W.; Kim, J.M.; Kim, D.H.; Kim, J.Y.; Lee, E.K.; Lee, J.; Kim, Y.J.; Yoo, M.A.; Jeong, K.S.; et al. Molecular activation of NF-κB, pro-inflammatory mediators, and signal pathways in γ-irradiated mice. Biotechnol. Lett. 2010, 32, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Song, S.M.; Ham, Y.M.; Ko, Y.J.; Ko, E.Y.; Oh, D.J.; Kim, C.S.; Kim, D.; Kim, K.M.; Yoon, W.J. Anti-inflammatory activities of the products of supercritical fluid extraction from Litsea japonica fruit in RAW 264.7 cells. J. Funct. Foods 2016, 22, 44–51. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Y.; Aisa, H.A. Anti-inflammatory effect of pomegranate flower in lipopolysaccharide (LPS)-stimulated RAW264. 7 macrophages. Pharm. Biol. 2017, 55, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Makarov, S.S. NF-kappaB as a therapeutic target in chronic inflammation: Recent advance. Mol. Med. Today 2000, 6, 441–448. [Google Scholar] [CrossRef]
- Hanada, T.; Yoshimura, A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002, 13, 413–421. [Google Scholar] [CrossRef]
- Liu, F.; Morris, S.; Epps, J.; Carroll, R. Demonstration of an activation regulated NF-kappaB/I-kappaBalpha complex in human platelets. Thromb. Res. 2002, 106, 199–203. [Google Scholar] [CrossRef]
- Chu, H.; Tang, Q.; Huang, H.; Hao, W.; Wei, X. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264.7 macrophages by suppressing MAPK and NF-κb signal pathways. Environ. Toxicol. Pharmacol. 2016, 41, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yang, S.Y.; Choi, C.Y. The Evaluation of the effect of herbal extract on osteoarthritis: In vitro and in vivo study. Prev. Nutr. Food Sci. 2016, 21, 310. [Google Scholar] [CrossRef] [PubMed]
- Bosca, L.; Zeini, M.; Traves, P.G.; Hortelano, S. Nitric oxide and cell viability in inflammatory cells: A role for NO in macrophage function and fate. Toxicology 2005, 208, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Beharka, A.A.; Wu, D.; Serafini, M.; Meydani, N. Mechanism of vitamin E inhibition of cyclooxygenase activity in macrophages from old mice: Role of peroxynitrite. Free Racdic. Biol. Med. 2002, 32, 503–511. [Google Scholar] [CrossRef]
- Jiang, Q.; Elson-Schwab, I.; Courtemanche, C.; Ames, B.N. γ-tocopherol and its major metabolite, in contrast to α-tocopherol, inhibited cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11494–11499. [Google Scholar] [CrossRef] [PubMed]
- Masresha, B.; Makonnen, E.; Debella, A. In vivo anti-inflammatory activities of Ocimum suave in mice. J. Ethnopharmacol. 2012, 142, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Li, Y.; Li, W.; Hu, U.; Yao, H.; Li, H.; Mu, Q. The anti-inflammatory effects of Caragana tangutica ethyl acetate extract. J. Ethnopharmacol. 2014, 152, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Moon, S.M.; Choi, Y.H.; Han, S.H.; Park, B.R.; Choi, M.S.; Kim, J.S.; Kim, Y.H.; Kim, D.K.; Kim, C.S. Aqueous extract of Codium fragile suppressed inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells and carrageenan-induced rats. Biomed. Pharmacother. 2017, 93, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.B.; Zhang, Y.H.; Zhao, B.J.; Li, C.; Tian, G.; Niu, B.; Qi, H.; Feng, L.; Shao, J.G. Screening for anti-inflammatory components from Corydalis bungeana Turcz. based on macrophage binding combined with HPLC. BMC Complement. Altern. Med. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.; Pan, M.H.; Ho, C.T.; Simon, J.E.; Wu, Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2016, 190, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.S.; Lim, H.S.; Jeong, S.J.; Ha, H.; Shin, H.K. HPLC-PDA analysis and anti-inflammatory effects of Mori Cortex Radicis. Nat. Prod. Commun. 2013, 8, 1443–1446. [Google Scholar] [PubMed]
- Francisco, V.; Costa, G.; Figueirinha, A.; Marques, C.; Paulo, P.; Neves, B.M.; Lopes, M.C.; García-Rodríguez, C.; Cruz, M.T.; Batista, M.T. Anti-inflammatory activity of Cymbopogon citratus leaves infusion via proteasome and nuclear factor-κB pathway inhibition: Contribution of chlorogenic acid. J. Ethnopharmacol. 2013, 14, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, H.; Choi, H.; Jo, A.; Kang, H.; Yun, H.; Choi, C.; Im, S. Anti-Inflammatory Effects of a Stauntonia hexaphylla Fruit Extract in Lipopolysaccharide-Activated RAW-264.7 Macrophages and Rats by Carrageenan-Induced Hind Paw Swelling. Nutrients 2018, 10, 110. https://doi.org/10.3390/nu10010110
Kim J, Kim H, Choi H, Jo A, Kang H, Yun H, Choi C, Im S. Anti-Inflammatory Effects of a Stauntonia hexaphylla Fruit Extract in Lipopolysaccharide-Activated RAW-264.7 Macrophages and Rats by Carrageenan-Induced Hind Paw Swelling. Nutrients. 2018; 10(1):110. https://doi.org/10.3390/nu10010110
Chicago/Turabian StyleKim, Jaeyong, Heesook Kim, Hakjoon Choi, Ara Jo, Huwon Kang, Hyojeong Yun, Chulyung Choi, and Sojeong Im. 2018. "Anti-Inflammatory Effects of a Stauntonia hexaphylla Fruit Extract in Lipopolysaccharide-Activated RAW-264.7 Macrophages and Rats by Carrageenan-Induced Hind Paw Swelling" Nutrients 10, no. 1: 110. https://doi.org/10.3390/nu10010110
APA StyleKim, J., Kim, H., Choi, H., Jo, A., Kang, H., Yun, H., Choi, C., & Im, S. (2018). Anti-Inflammatory Effects of a Stauntonia hexaphylla Fruit Extract in Lipopolysaccharide-Activated RAW-264.7 Macrophages and Rats by Carrageenan-Induced Hind Paw Swelling. Nutrients, 10(1), 110. https://doi.org/10.3390/nu10010110