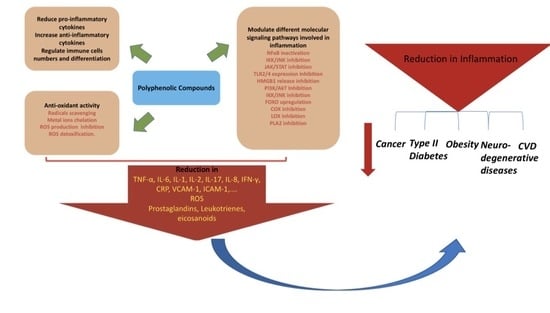
The Immunomodulatory and Anti-Inflammatory Role of Polyphenols
Abstract
:1. Introduction
2. Polyphenols and Inflammation
3. Polyphenol and Cytokine Modulation
4. Polyphenols, Inflammation, and Modulation of Different Signaling Pathways
4.1. NFκ B Signaling Pathway
4.2. MAPK Signaling Pathway
4.3. Arachidonic Acid Signaling Pathway
5. Polyphenols, Oxidative Stress, and Inflammation
6. Polyphenols, Chronic Diseases and Cancer
6.1. Polyphenols and Insulin Resistance
6.2. Polyphenols and Inflammatory Cardiovascular Diseases (CVD)
6.3. Polyphenols and Inflammatory Neurological Diseases
6.4. Polyphenols and Inflammatory Obesity
6.5. Polyphenols and Cancer
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| COX | Cyclooxygenase |
| NOX | NADPH oxidase |
| SOD | Superoxide dismutase |
| GSH | Glutathione |
| Px | Peroxidase |
| PLA2 | Phospholipase A2 |
| PGs | Prostaglandins |
| LTs | Leukotrienes |
| MAPK | Mitogen-activated protein Kinase |
| IKK | Inhibitor of kappa kinase |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Th1 | T helper 1 |
| NK | Natural killer |
| DCs | Dendritic cells |
| ECGC | Epigallocatechin gallate |
| Treg | Regulatory T cells |
| AhR | Aryl hydrocarbon receptor |
| iNOS | Inducible nitric oxide synthase |
| LPS | Lipopolysaccharide |
| MCP-1 | Monocyte chemoattractant protein-1 |
| VEGF | Vascular endothelial growth Factor |
| IL | Interleukin |
| JNK | c-Jun amino-terminal kinases |
| CREB | cAMP response element-binding protein |
| AA | Arachidonic acid |
| AMPK | AMP-activated protein kinase |
| ERK | Extra-cellular signal regulated kinases |
| ATP | Adenosine triphosphate |
| CVD | Cardiovascular disease |
| PI3K | Phosphatidylinositide 3-kinases |
| Akt | Protein kinase B |
| LDL | Low density lipoprotein |
| HDL | High density lipoprotein |
| RNS | Reactive nitrogen species |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| CYPs | Cytochrome P450 enzymes |
| MMP-9 | Matrix metallopeptidase-9 |
| NPC | Nasopharyngeal carcinoma |
| CSCs | Cancer stem cells |
| EMT | Epithelial-to-mesenchymal transition |
| FDA | Food and drug administration |
| ICAM | Intercellular adhesion molecule |
| VCAM | Vascular cell adhesion molecule |
| HIV | Human immunodeficiency diarrhea |
| HMG-CoA | 3-Hydroxy-3-methyl-glutaryl-coenzyme A |
| VEGF | Vascular endothelial growth factor |
| TLR | Toll-like receptor |
| NO | Nitric oxide |
| eNOS | Endothelial nitric oxide synthase |
| PDE | Phosphodiesterase |
| ACE | Angiotensin converting enzyme |
References
- Recio, M.; Andujar, I.; Rios, J. Anti-Inflammatory Agents from Plants: Progress and Potential. Curr. Med. Chem. 2012, 19, 2088–2103. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant Activity of Fresh Apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Russo, M.; Bilotto, S.; Tedesco, I.; Laratta, B.; Russo, G.L. Dietary Polyphenols in Cancer Prevention: The Example of the Flavonoid Quercetin in Leukemia. Ann. N. Y. Acad. Sci. 2012, 1259, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Étenne-Selloum, N.; Li, H.; Martínez, M.C.; Schini-Kerth, V.B.; Laher, I. Molecular Mechanisms of the Cardiovascular Protective Effects of Polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Kosinska-Cagnazzo, A.; Kerr, W.L.; Amarowicz, R.; Swanson, R.B.; Pegg, R.B. Separation and Characterization of Soluble Esterified and Glycoside-Bound Phenolic Compounds in Dry-Blanched Peanut Skins by Liquid Chromatography-Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2014, 62, 11488–11504. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheynier, V. Polyphenols in Food Are More Complex Then Often Thought. Am. J. Clin. Nutr. 2005, 81, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macia, A.; Romero, M.-P.; Motilua, M.-J.; Rubio, L. Application of in Vitro Gastrointestinal Digestion and Colonic\nfermentation Models to Pomegranate Products (Juice, Pulp and Peel\nextract) to Study the Stability and Catabolism of Phenolic Compounds. J. Funct. Food 2015, 14, 529–540. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and Biological Activity of Wild Blueberry (Vaccinium Angustifolium) Polyphenols during Simulated in Vitro Gastrointestinal Digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of Phenolic Compounds for Food Preservation: Food Additive and Active Packaging. In Phenolic Compounds-Biological Activity; InTech: London, UK, 2017. [Google Scholar] [Green Version]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging Role of Phenolic Compounds as Natural Food Additives in Fish and Fish Products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Maestre, R.; Micol, V.; Funes, L.; Medina, I. Incorporation and Interaction of Grape Seed Extract in Membranes and Relation with Efficacy in Muscle Foods. J. Agric. Food Chem. 2010, 58, 8365–8374. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.T. Evidence for Nutritional Benefits in Prolonging Wellness. Am. J. Clin. Nutr. 2006, 8, 16470004. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Acute and “Chronic” Phase Reaction-a Mother of Disease. Clin. Nutr. 2004, 23, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C. The Effect of Minor Constituents of Olive Oil on Cardiovascular Disease: New Findings. Nutr. Rev. 1998, 56, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C. The Role of Antioxidants in the Mediterranean Diet. Lipids 2001, 36, S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease, and Cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Urquiaga, J.; Leighton, F. Plant Polyphenol Antioxidants and Oxidative Stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Baek, S.J. Molecular Targets of Dietary Polyphenols with Anti-Inflammatory Properties. Yonsei Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Malireddy, S.; Kotha, S.R.; Secor, J.D.; Gurney, T.O.; Abbott, J.L.; Maulik, G.; Maddipati, K.R.; Parinandi, N.L. Phytochemical Antioxidants Modulate Mammalian Cellular Epigenome: Implications in Health and Disease. Antioxid. Redox Signal. 2012, 17, 327–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Di Benedetto, R.; Filesi, C.; Masella, R. Polyphenols, Intracellular Signalling and Inflammation. Ann. Ist. Super. Sanita 2007, 43, 394–405. [Google Scholar] [PubMed]
- Karasawa, K.; Uzuhashi, Y.; Hirota, M.; Otani, H. A Matured Fruit Extract of Date Palm Tree (Phoenix dactylifera L.) Stimulates the Cellular Immune System in Mice. J. Agric. Food Chem. 2011, 59, 11287–11293. [Google Scholar] [CrossRef] [PubMed]
- John, C.M.; Sandrasaigaran, P.; Tong, C.K.; Adam, A.; Ramasamy, R. Immunomodulatory Activity of Polyphenols Derived from Cassia Auriculata Flowers in Aged Rats. Cell. Immunol. 2011, 271, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Mohar, D.; Malik, S. The Sirtuin System: The Holy Grail of Resveratrol? J. Clin. Exp. Cardiol. 2012, 3, 216. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Chirafisi, J.; Saija, A.; Cimino, F. Nutritional Antioxidants and Adaptive Cell Responses: An Update. Curr. Mol. Med. 2011, 11, 770–789. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Mattarei, A.; Zoratti, M. Resveratrol and Health: The Starting Point. ChemBioChem 2012, 13, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Capiralla, H.; Vingtdeux, V.; Venkatesh, J.; Dreses-werringloer, U.; Zhao, H.; Davies, P.; Marambaud, P. Identification of Potent Small? Molecule Inhibitors of STAT3 with Anti? Inflammatory Properties in RAW 264.7 Macrophages. FEBS J. 2012, 279, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Leiherer, A.; Mündlein, A.; Drexel, H. Phytochemicals and Their Impact on Adipose Tissue Inflammation and Diabetes. Vasc. Pharmacol. 2013, 58, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.M.; Cui, X.; Wu, R.; Dong, W.; Zhou, M.; Hu, M.; Simms, H.H.; Wang, P. The Anti-Inflammatory Effect of Curcumin in an Experimental Model of Sepsis Is Mediated by up-Regulation of Peroxisome Proliferator-Activated Receptor-γ. Crit. Care Med. 2006, 34, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and Curcumin-like Molecules: From Spice to Drugs. Curr. Med. Chem. 2014, 21, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Ashkani-Esfahani, S. A Review of Therapeutic Effects of Curcumin. Curr. Pharm. Des. 2013, 19, 2032–2046. [Google Scholar] [PubMed]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by Curcumin as Revealed by Molecular Interaction Studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Bae, J. Role of High Mobility Group Box 1 in Inflammatory Disease: Focus on Sepsis. Arch. Pharm. Res. 2012, 35, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Egawa, T.; Ma, X.; Oshima, R.; Kurogi, E.; Hayashi, T. Coffee Polyphenol Caffeic Acid but Not Chlorogenic Acid Increases 5’AMP-Activated Protein Kinase and Insulin-Independent Glucose Transport in Rat Skeletal Muscle. J. Nutr. Biochem. 2012, 23, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Akyol, S.; Ozturk, G.; Ginis, Z.; Amutcu, F.; Yigitoglu, M.; Akyol, O. In Vivo and in Vitro Antıneoplastic Actions of Caffeic Acid Phenethyl Ester (CAPE): Therapeutic Perspectives. Nutr. Cancer 2013, 65, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J. Recent Advances on Tea Polyphenols. Front. Biosci. 2012, E4, 111–131. [Google Scholar] [CrossRef]
- Domitrovic, R. The Molecular Basis for the Pharmacological Activity of Anthocyans. Curr. Med. Chem. 2011, 18, 4454–4469. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Shankar, S.; Sriivastava, R. Green Tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Landis-Piwowar, K.; Chen, D.; Foldes, R.; Chan, T.-H.; Dou, Q.P. Novel Epigallocatechin Gallate Analogs as Potential Anticancer Agents: A Patent Review (2009–Present). Expert Opin. Ther. Pat. 2013, 23, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3 + Regulatory T Cells in the Human Immune System. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Boissier, M.C.; Assier, E.; Biton, J.; Denys, A.; Falgarone, G.; Bessis, N. Regulatory T Cells (Treg) in Rheumatoid Arthritis. J. Bone Spine 2009, 76, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.S.; Larché, M.; Durham, S.R. Tregs and Allergic Disease. J. Clin. Investig. 2004, 114, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.; Nguyen, L.P.; Noh, S.K.; Bray, T.M.; Bruno, R.S.; Ho, E. Induction of Regulatory T Cells by Green Tea Polyphenol EGCG. Immunol. Lett. 2011, 139, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, X.; Li, M. Baicalin, a Natural Compound, Promotes Regulatory T Cell Differentiation. IBMC Complement. Altern. Med. 2012, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.K.; Yeh, C.H.; Iwamoto, T.; Satsu, H.; Shimizu, M.; Totsuka, M. Dietary Flavonoid Naringenin Induces Regulatory T Cells via an Aryl Hydrocarbon Receptor Mediated Pathway. J. Agric. Food Chem. 2012, 60, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pae, M.; Meydani, S.N.; Wu, D. Green Tea Epigallocatechin-3-Gallate Modulates Differentiation of Naïve CD4+T Cells into Specific Lineage Effector Cells. J. Mol. Med. 2013, 91, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, X.; Chu, Y.; Li, M. Identification of Baicalin as an Immunoregulatory Compound by Controlling TH17 Cell Differentiation. PLoS ONE 2011, 6, e21359178. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and Pathogenic Functions of Macrophage Subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of Flavonoids and Other Polyphenols on Inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ping, S.; Huang, S.; Hu, L.; Xuan, H.; Zhang, C.; Hu, F. Molecular Mechanisms Underlying the In Vitro Anti-Inflammatory Effects of a Fla Vonoid -Rich Ethanol Extract from Chinese Propolis (Poplar Type). Cell 2013, 2013, 127672. [Google Scholar]
- Park, K.I.; Kang, S.R.; Park, H.S.; Lee, D.H.; Nagappan, A.; Kim, J.A.; Shin, S.C.; Kim, E.H.; Lee, W.S.; Chung, H.J.; et al. Regulation of Proinflammatory Mediators via NF-ΚB and P38 MAPK-Dependent Mechanisms in RAW 264.7 Macrophages by Polyphenol Components Isolated from Korea Lonicera Japonica THUNB. Evid.-Based Complement. Altern. Med. 2012, 2012, 22611435. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.-R.; Ho, Y.-L.; Huang, S.-C.; Huang, T.-H.; Lai, S.-C.; Tsai, J.-C.; Wang, C.-Y.; Huang, G.-J.; Chang, Y.-S. Antioxidant, Anti-Inflammatory and Antiproliferative Activities of Kalanchoe gracilis (L.) DC Stem. Am. J. Chin. Med. 2011, 39, 1275–1290. [Google Scholar] [CrossRef] [PubMed]
- Bohstam, M.; Asgary, S.; Kouhpayeh, S.; Shariati, L.; Khanhamad, H. Aptamers Against Pro- and Anti-Inflammatory Cytokines: A Review. Inflamm. Febr. 2017, 40, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Mykkänen, O.; Kirjavainen, P.V.; Leppänen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimiä, R.; Pulkkinen, L.; et al. Bilberries Reduce Low-Grade Inflammation in Individuals with Features of Metabolic Syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Cladellas, M.; de la Torre, R.; Martí, J.; Muñoz, D.; Schröder, H.; Alcántara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Ló-Sabater, M.C.; et al. Anti-Inflammatory Effect of Virgin Olive Oil in Stable Coronary Disease Patients: A Randomized, Crossover, Controlled Trial. Eur. J. Clin. Nutr. 2008, 62, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Bitler, C.M.; Viale, T.M.; Damaj, B.; Crea, R. Hydrolyzed Olive Vegetation Water in Mice Has Anti-Inflammatory Activity. J. Nutr. 2005, 135, 1475–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comalada, M.; Ballester, I.; Bailon, E.; Sierra, S.; Xaus, J.; de Medina, F.; Zarzuelo, A. Inhibition of pro-Inflammatory Markers in Primary Bone Marrow-Derived Mouse Macrophages by Naturally Occurring Flavonoids: Analysis of the Structure-Activity Relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Blonska, M.; Czuba, Z.P.; Krol, W. Effect of Flavone Derivatives on Interleukin-1beta (IL-1beta) MRNA Expression and IL-1beta Protein Synthesis in Stimulated RAW 264.7 Macrophages. Scand. J. Immunol. 2003, 57, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Mishra, M.; Ghosh, S.; Tewari, R.; Basu, A.; Seth, P.; Sen, E. Modulation of Interleukin-1beta Mediated Inflammatory Response in Human Astrocytes by Flavonoids: Implications in Neuroprotection. Brain Res. Bull. 2007, 73, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Miyazaki, T.; Kambe, F.; Maeda, K.; Seo, H. Quercetin, a Bioflavonoid, Inhibits the Induction of Interleukin 8 and Monocyte Chemoattractant Protein-1 Expression by Tumor Necrosis Factor-Alpha in Cultured Human Synovial Cells. J. Rheumatol. 1997, 24, 1680–1684. [Google Scholar] [PubMed]
- Min, Y.; Choi, C.; Bark, H.; Son, H.; Park, H.; Lee, S.; Park, J.; Park, E.; Shin, H.; Kim, S. Quercetin Inhibits Expression of Inflammatory Cytokines through Attenuation of NFkappaB and P38 MAPK in HMC-1 Human Mast Cell Line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.Y.; Park, W.B. Production of Cytokine and NO by RAW 264.7 Macrophages and PBMC in Vitro Incubation with Flavonoids. Arch. Pharm. Res. 2005, 28, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I.; et al. Inhibition of the NF-ΚB Signaling Pathway by the Curcumin Analog, 3,5-Bis(2-Pyridinylmethylidene)-4-Piperidone (EF31): Anti-Inflammatory and Anti-Cancer Properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.M.; Harbourne, N.; Marete, E.; Martyn, D.; Jacquier, J.C.; O’Riordan, D.; Gibney, E.R. Inhibition of Proinflammatory Biomarkers in THP1 Macrophages by Polyphenols Derived from Chamomile, Meadowsweet and Willow Bark. Phyther. Res. 2013, 27, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Schindler, R.; Mancilla, J.; Endres, S.; Ghorbani, R.; Clark, S.C.; Dinarello, C.A. Correlations and Interactions in the Production of Interleukin-6 (IL-6), IL-1, and Tumor Necrosis Factor (TNF) in Human Blood Mononuclear Cells: IL-6 Suppresses IL-1 and TNF. Blood 1990, 75, 40–47. [Google Scholar] [PubMed]
- Essafi-Benkhadir, K.; Refai, A.; Riahi, I.; Fattouch, S.; Karoui, H.; Essafi, M. Quince (Cydonia oblonga Miller) Peel Polyphenols Modulate LPS-Induced Inflammation in Human THP-1-Derived Macrophages through NF-ΚB, P38MAPK and Akt Inhibition. Biochem. Biophys. Res. Commun. 2012, 418, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Iwaki, K.; Koya-Miyata, S.; Tanimoto, T.; Kohno, K.; Ikeda, M.; Kurimoto, M. The Flavonoid Kaempferol Suppresses the Graft-versus-Host Reaction by Inhibiting Type 1 Cytokine Production and CD8+T Cell Engraftment. Clin. Immunol. 2002, 103, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Crouvezier, S.; Powell, B.; Keir, D.; Yaqoob, P. The Effects of Phenolic Components of Tea on the Production of Pro- and Anti-Inflammatory Cytokines by Human Leukocytes in Vitro. Cytokine 2001, 13, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Nam, N. Naturally Occurring NF-kappa B Inhibitors. Mini Rev. Med. Chem. 2006, 6, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-KappaB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J. Redox Regulation of pro-Inflammatory Cytokines and IkappaB-Alpha/NF-KappaB Nuclear Translocation And. Biochem. Biophys. Res. Commun. 2002, 296, 847–856. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation Meets Ubiquitination: The Control of NF-[Kappa]B Activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. Integrating Cell-Signalling Pathways with NF-ΚB and IKK Function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Yamamoto, Y.; Wang, Q.M. The IKK NF-ΚB System: A Treasure Trove for Drug Development. Nat. Rev. Drug Discov. 2004, 3, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Biswas, S.; Kirkham, P. Regulation of Inflammation and Redox Signaling by Dietary Polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Marwick, J.; Kirkham, P. Redox Modulation of Chromatin Remodeling: Impact on Histone Acetylation and Deacetylation, NF-KappaB and pro-Inflammatory Gene Expression. Biochem. Pharmacol. 2004, 68, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, D.; Maiuri, M.C.; Simeon, V.; Grassia, G.; Soscia, A.; Cinelli, M.P.; Carnuccio, R. Lycopene, Quercetin and Tyrosol Prevent Macrophage Activation Induced by Gliadin and IFN-γ. Eur. J. Pharmacol. 2007, 566, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gálvez, J.; Zarzuelo, A. In Vivo Quercitrin Anti-Inflammatory Effect Involves Release of Quercetin, Which Inhibits Inflammation through down-Regulation of the NF-ΚB Pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.A.; Braune, A.; HÖlzlwimmer, G.; Quintanilla-Fend, L.; Haller, D. Quercetin Inhibits TNF-Induced NF-ΚB Transcription Factor Recruitment to Proinflammatory Gene Promoters in Murine Intestinal Epithelial Cells. J. Nutr. 2007, 137, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Ho, F.M.; Chao, P.D.L.; Chen, C.P.; Jeng, K.C.G.; Hsu, H.B.; Lee, S.T.; Wen, T.W.; Lin, W.W. Inhibition of INOS Gene Expression by Quercetin Is Mediated by the Inhibition of IκB Kinase, Nuclear Factor-Kappa B and STAT1, and Depends on Heme Oxygenase-1 Induction in Mouse BV-2 Microglia. Eur. J. Pharmacol. 2005, 521, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Lafuente, A.; Guillamon, E.; Villares, A.; Rostagno, M.; Martinez, J. Flavonoids as Anti-Inflammatory Agents: Implications in Cancer and Cardiovascular Disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, Y.; Yamamoto, K.; Yoshida, M.; Azuma, T.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. Intestinal Anti-Inflammatory Activity of Luteolin: Role of the Aglycone in NF-ΚB Inactivation in Macrophages Co-Cultured with Intestinal Epithelial Cells. Biofactors 2013, 39, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhang, Y.; Yang, Q.; Cheng, S.; Hao, J.; Zhao, X.; Jiang, Z. Genistein Suppresses LPS-Induced Inflammatory Response through Inhibiting NF-ΚB Following AMP Kinase Activation in RAW 264.7 Macrophages. PLoS ONE 2012, 7, e23300870. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Bae, Y.; Kim, S.H. Galangin Attenuates Mast Cell-Mediated Allergic Inflammation. Food Chem. Toxicol. 2013, 57, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.S.; Catravas, J.D.; Odoms, K.; Denenberg, A.; Malhotra, V.; Wong, H.R. Epigallocatechin-3-Gallate, a Green Tea-Derived Polyphenol, Inhibits IL-1 Beta-Dependent Proinflammatory Signal Transduction in Cultured Respiratory Epithelial Cells. J. Nutr. 2004, 134, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Aneja, R.; Hake, P.W.; Burroughs, T.J.; Denenberg, A.G.; Wong, H.R.; Zingarelli, B. Epigallocatechin, a Green Tea Polyphenol, Attenuates Myocardial Ischemia Reperfusion Injury in Rats. Mol. Med. 2004, 10, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, D.; Matsui, A.; Imai, M.; Sonoda, Y.; Kasahara, T. Effect of Various Catechins on the IL-12 p40 Production by Murine Peritoneal Macrophages and A. Biol. Pharm. Bull. 2004, 27, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, J. Epigallocatechin-3-Gallate Blocks the Induction of Nitric Oxide Synthase by Down-Regulating Lipopolysaccharide-Induced Activity of Transcription Factor Nuclear Factor-κB. Mol. Pharmacol. 1997, 472, 465–472. [Google Scholar] [CrossRef]
- Mackenzie, G.; Carrasquedo, F.; Delfino, J.; Keen, C.; Fraga, C.; Oteiza, P. Epicatechin, Catechin, and Dimeric Procyanidins Inhibit PMA? Induced NF? KappaB Activation at Multiple Steps in Jurkat T Cells. FASEB J. 2004, 18, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive Oil and Red Wine Antioxidant Polyphenols Inhibit Endothelial Activation: Antiatherogenic Properties of Mediterranean Diet Phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP Kinase Signalling Cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting Multiple Signaling Pathways by Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate. 1 Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Author Information Full. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Kolch, W. Coordinating ERK/MAPK Signalling through Scaffolds and Inhibitors. Nat. Rev. Mol. Cell Biol. 2005, 6, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, S. ERK1/2 MAP Kinases in Cell Survival and Apoptosis. IUBMB Life 2006, 58, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Mayor, F.; Jurado-Pueyo, M.; Campos, P.M.; Murga, C. Interfering with MAP Kinase Docking Interactions: Implications and Perspective for the P38 Route. Cell Cycle 2007, 6, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. MAPK Signalling Pathways as Molecular Targets for Anti-Inflammatory Therapy—From Molecular Mechanisms to Therapeutic Benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Inflammation-Activated Protein Kinases as Targets for Drug Development. Proc. Am. Thorac. Soc. 2005, 2, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, A.; Roussos, C.; Papapetropoulos, A. Inhibition of LPS-Stimulated Pathways in Macrophages by the Flavonoid Luteolin. Br. J. Pharmacol. 2002, 136, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chow, M.; Huang, W.; Lin, Y.; Chang, Y. Flavonoids Inhibit Tumor Necrosis Factor-Alpha-Induced up-Regulation of Intercellular Adhesion Molecule-1 (ICAM-1) in Respiratory Epithelial Cells through Activator Protein-1 and Nuclear Factor-KappaB: Structure-Activity Relationships. Mol. Pharmacol. 2004, 66, 683–693. [Google Scholar] [PubMed]
- Wadsworth, T.L.; McDonald, T.L.; Koop, D.R. Effects of Ginkgo Biloba Extract (EGb 761) and Quercetin on Lipopolysaccharide-Induced Signaling Pathways Involved in the Release of Tumor Necrosis Factor-Alpha. Biochem. Pharmacol. 2001, 62, 963–974. [Google Scholar] [CrossRef]
- Cho, S.; Park, S.; Kwon, M.; Jeong, T.; Bok, S.; Choi, W.; Jeong, W.; Ryu, S.; Do, S.; Song, C.; et al. Quercetin Suppresses Proinflammatory Cytokines Production through MAP Kinases AndNF-Kappa B Pathway in Lipopolysaccharide-Stimulated Macrophage. Mol. Cell. Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Surh, Y.J. Epigallocatechin Gallate Inhibits Phorbol Ester-Induced Activation of NF-ΚB and CREB in Mouse Skin Role of P38 MAPK. Ann. N. Y. Acad. Sci. 2007, 1095, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Pasten, C.; Olave, N.; Zhou, L.; Tabengwa, E.; Wolkowicz, P.; Grenett, H. Polyphenols Downregulate PAI-1 Gene Expression in Cultured Human Coronary Artery Endothelial Cells: Molecular Contributor to Cardiovascular Protection. Thromb. Res. 2007, 121, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, N.V.; Dai, H.; Roos, K.L.T.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a Cyclooxygenase-1 Variant Inhibited by Acetaminophen and Other Analgesic/Antipyretic Drugs: Cloning, Structure, and Expression. Proc. Natl. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar] [CrossRef] [PubMed]
- Needleman, P.; Isakson, P. The Discovery and Function of COX-2. J. Rheumatol. Suppl. 2018, 49, 6–8. [Google Scholar]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-Inflammatory Plant Flavonoids and Cellular Action Mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Welton, A.F.; Tobias, L.D.; Fiedler-Nagy, C.; Anderson, W.; Hope, W.; Meyers, K.; Coffey, J.W. Effect of Flavonoids on Arachidonic Acid Metabolism. Prog. Clin. Biol. Res. 1986, 213, 231–242. [Google Scholar] [PubMed]
- Laughton, M.; Evans, P.; Moroney, M.; Hoult, J.; Halliwell, B. Inhibition of Mammalian 5-Lipoxygenase and Cyclo-Oxygenase by Flavonoids and Phenolic Dietary Additives. Relationship to Antioxidant Activity and to Iron Ion-Reducing Ability. Biochem. Pharmacol. 1991, 42, 1673–1681. [Google Scholar] [CrossRef]
- Aviram, M.; Fuhrman, B. Polyphenolic Flavonoids Inhibit Macrophage-Mediated Oxidation of LDL and Attenuate Atherogenesis. Atherosclerosis 1998, 137, 9694541. [Google Scholar] [CrossRef]
- Ferrandiz, M.L.; Alcaraz, M.J. Ferrandiz 1991-Anti-Inflammatory Activity and Inhibition of Arachidonic Acid Metabolism by Flavonoids. Agent Action 1991, 32, 283–288. [Google Scholar] [CrossRef]
- Kim, H.; Mani, I.; Iversen, L.; Ziboh, V. Effects of Naturally-Occurring Flavonoids and Biflavonoids on Epidermal Cyclooxygenase and Lipoxygenase from Guinea-Pigs. Prostaglandin Leukot. Essent. Fat. Acid. 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Luceri, C.; Caderni, G.; Sanna, A.; Dolara, P. Red Wine and Black Tea Polyphenols Modulate the Expression of Cycloxygenase-2, Inducible Nitric Oxide Synthase and Glutathione-Related Enzymes in Azoxymethane-Induced F344 Rat Colon Tumors. J. Nutr. 2002, 132, 1376–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, D.X.; Luo, D.; Tanigawa, S.; Hashimoto, F.; Uto, T.; Masuzaki, S.; Fujii, M.; Sakata, Y. Prodelphinidin B-4 3′-O-Gallate, a Tea Polyphenol, Is Involved in the Inhibition of COX-2 and INOS via the Downregulation of TAK1-NF-ΚB Pathway. Biochem. Pharmacol. 2007, 74, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Masuzaki, S.; Hashimoto, F.; Uto, T.; Tanigawa, S.; Fujii, M.; Sakata, Y. Green Tea Proanthocyanidins Inhibit Cyclooxygenase-2 Expression in LPS-Activated Mouse Macrophages: Molecular Mechanisms and Structure? Activity Relationship. Arch. Biochem. Biophys. 2007, 460, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Zoubouli, P.; Calder, P.C. Differential Anti-Inflammatory Effects of Phenolic Compounds from Extra Virgin Olive Oil Identified in Human Whole Blood Cultures. Nutrition 2005, 21, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Tuck, K.L.; Hayball, P.J. Major Phenolic Compounds in Olive Oil: Metabolism and Health Effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- De la Puerta, R.; Gutierrez, V.R.; Hoult, J. Inhibition of Leukocyte 5 Lipoxygenase by Phenolics from Virgin Olive Oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-like Activity in Extra-Virgin Olive Oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Berlett, B.S.; Stadtman, E.R.; Berlett, B.S.; Stadtman, E.R. Protein Oxidation in Aging, Disease, and Oxidative Stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzano, S.; Checconi, P.; Hanschmann, E.-M.; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, D.; Mengozzi, M.; Coppo, L.; Sacre, S.; et al. Linkage of Inflammation and Oxidative Stress via Release of Glutathionylated Peroxiredoxin-2, Which Acts as a Danger Signal. Proc. Natl. Acad. Sci. USA 2014, 111, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and Prevention of Chronic Disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wohlert, S.; Hunt, J.A. Reactive Oxygen Species (ROS)-A Family of Fate Deciding Molecules Pivotal in Constructive Inflammation and Wound Healing. Eur. Cells Mater. 2012, 24, 249–265. [Google Scholar] [CrossRef]
- Naik, E.; Dixit, V.M. Mitochondrial Reactive Oxygen Species Drive Proinflammatory Cytokine Production: Figure 1. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Valente, A.J. Nuclear Factor Kappa B Activation by NADPH Oxidases. Mech. Ageing Dev. 2004, 125, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M.; Leto, T.L. The Nox Family of NAD(P)H Oxidases: Host Defense and Beyond. J. Biol. Chem. 2004, 279, 51715–51718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia Variegata Leaf Extracts Exhibit Considerable Antibacterial, Antioxidant, and Anticancer Activities. Biomed. Res. Int. 2013, 2013, 915436. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kumar, S.; Pandey, A.K. Scientific Validation of the Medicinal Efficacy of Tinospora Cordifolia. Sci. World J. 2013, 2013, 292934. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J.; Riggins, J.N.; West, J.D. Endogenous Generation of Reactive Oxidants and Electrophiles and Their Reactions with DNA and Protein. J. Clin. Investig. 2003, 111, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Prousek, J. Fenton Chemistry in Biology and Medicine. Pure Appl. Chem. 2007, 79, 2007–2010. [Google Scholar] [CrossRef]
- Deby-Dupont, G.; Mouithys-Mickalad, A.; Serteyn, D.; Lamy, M.; Deby, C. Resveratrol and Curcumin Reduce the Respiratory Burst of Chlamydia-Primed THP-1 Cells. Biochem. Biophys. Res. Commun. 2005, 333, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.E.; Hshu, Y.C.; Wang, J.S.; Chen, J.K. Resveratrol Attenuates OxLDL-Stimulated NADPH Oxidase Activity and Protects Endothelial Cells from Oxidative Functional Damages. J. Appl. Physiol. 2007, 102, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Petrônio, M.S.; Zeraik, M.L.; Da Fonseca, L.M.; Ximenes, V.F. Apocynin: Chemical and Biophysical Properties of a NADPH Oxidase Inhibitor. Molecules 2013, 18, 2821–2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Ji, H.F. Insights into the Inhibition of Xanthine Oxidase by Curcumin. Bioorg. Med. Chem. Lett. 2009, 19, 5990–5993. [Google Scholar] [CrossRef] [PubMed]
- Aucamp, J. Inhibition of Xanthine Oxidase by Tea Catechins (Camellia Sinensis). Method Mol. Biol. 1997, 702, 47–60. [Google Scholar]
- Schmidt, A.; Böhmer, A.E.; Antunes, C.; Schallenberger, C.; Porciuncula, L.; Elisabetsky, E.; Lara, D.; Souza, D. Anti-Nociceptive Properties of the Xanthine Oxidase Inhibitor Allopurinol in Mice: Role of A1 Adenosine Receptors. Br. J. Pharmacol. 2009, 156, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.T.; Nguyen, N.T. Xanthine Oxidase Inhibitors from Vietnamese Blume balsamifer L. Phyther. Res. 2012, 26, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, Anthocyanins and Procyanidins from Aronia Melanocarpa as Radical Scavengers and Enzyme Inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.F.; Li, H.Q.; Shi, L.; Xue, J.Y.; Ruan, B.F.; Zhu, H.L. Synthesis of Resveratrol Analogues, and Evaluation of Their Cytotoxic and Xanthine Oxidase Inhibitory Activities. Chem. Biodivers. 2008, 5, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Cheon, B.S.; Kim, Y.H.; Son, K.S.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of Prenylated Flavonoids and Biflavonoids on Lipopolysaccharide-Induced Nitric Oxide Production from the Mouse Macrophage Cell Line RAW 264.7. Planta Med. 2000, 66, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Bhaduri, A. Black Tea Is a Powerful Chemopreventor of Reactive Oxygen and Nitrogen Species: Comparison with Its Individual Catechin Constituents and Green Tea. Biochem. Biophys. Res. Commun. 2001, 284, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Liby, K.T. NRF2 and Cancer: The Good, the Bad and the Importance of Context. Nat. Rev. Cancer 2012, 12, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Chu, A. Antagonism by Bioactive Polyphenols Against Inflammation: A Systematic View. Inflamm. Allergy Drug Targets 2014, 13, 34–64. [Google Scholar] [CrossRef] [PubMed]
- Meydani, M.; Hasan, S.T. Dietary Polyphenols and Obesity. Nutrients 2010, 2, 737–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahfoufi, N.; Mallet, J.F.; Graham, E.; Matar, C. Role of Probiotics and Prebiotics in Immunomodulation. Curr. Opin. Food Sci. 2018, 20, 82–91. [Google Scholar] [CrossRef]
- Roy, D.; Perreault, M.; Marette, A. Insulin Stimulation of Glucose Uptake in Skeletal Muscles and Adipose Tissues in Vivo Is NO Dependent. Am. J. Physiol. Endocrinol. Metab. 1998, 274, E692–E699. [Google Scholar] [CrossRef]
- Fryer, L.G.; Hajduch, E.; Rencurel, F.; Salt, I.P.; Hundal, H.S.; Hardie, D.G.; Carling, D. Activation of Glucose Transport by AMP-Activated Protein Kinase via Stimulation of Nitric Oxide Synthase. Diabetes 2000, 49, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Barnard, R.J.; Scheck, S.H.; Balon, T.W. Exercise-Stimulated Glucose Transport in Skeletal Muscle Is Nitric Oxide Dependent. Am. J. Physiol. 1997, 273, E220–E225. [Google Scholar] [PubMed]
- Peters, U.; Poole, C.; Arab, L. Does Tea Affect Cardiovascular Disease? A Meta-Analysis. Am. J. Epidemiol. 2001, 154, 495–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hébert, R.; Helliwell, B.; Hill, G.; McDowell, I. Risk Factors for Alzheimer’s Disease: A Prospective Analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Truelsen, T.; Thudium, D.; Grønbaek, M.; Copenhagen City Heart Study. Amount and Type of Alcohol and Risk of Dementia: The Copenhagen City Heart Study. Neurology 2002, 59, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.M.; Asad, S.F.; Singh, S.; Ahmad, A. Putative Mechanism for Anticancer and Apoptosis-Inducing Properties of Plant-Derived Polyphenolic Compounds. IUBMB Life 2000, 50, 167–171. [Google Scholar] [PubMed] [Green Version]
- Park, S.; Ahmad, F.; Philip, A.; Baar, K.; William, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussing, R.; Brown, A.; et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting CAMP Phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Forstermann, U. Resveratrol, a Polyphenolic Phytoalexin Present in Red Wine, Enhances Expression and Activity of Endothelial Nitric Oxide Synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-Glucosidase Inhibitors from Plants: A Natural Approach to Treat Diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Di Castelnuovo, A.; Rotondo, S.; Iacoviello, L.; Donati, M.B.; De Gaetano, G. Meta-Analysis of Wine and Beer Consumption in Relation to Vascular Risk. Circulation 2002, 105, 2836–2844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Cornu, K.A.; Le Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, Flavonoid-Rich Foods, and Cardiovascular Risk: A Meta-Analysis of Randomized Controlled Trials 1, 2. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhao, L.; Wu, R.X.; Yue, S.Q.; Pei, J.M. The Vasorelaxing Effect of Resveratrol on Abdominal Aorta from Rats and Its Underlying Mechanisms. Vasc. Pharmacol. 2013, 58, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Raptis, S.A. Advanced Glycation End Products and Cardiovascular Disease. Curr. Diabete Rev. 2008, 4, 92–100. [Google Scholar] [CrossRef]
- Huang, S.M.; Wu, C.H.; Yen, G.C. Effects of Flavonoids on the Expression of the Pro-Inflammatory Response in Human Monocytes Induced by Ligation of the Receptor for AGEs. Mol. Nutr. Food Res. 2006, 50, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, E.K.; Kim, D.H.; Yu, B.P.; Chung, H.Y. Kaempferol Modulates Pro-Inflammatory NF-ΚB Activation by Suppressing Advanced Glycation Endproducts-Induced NADPH Oxidase. Age 2010, 32, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-Berka, J.L.; Rana, I.; Armani, R.; Agrotis, A. Reactive Oxygen Species, Nox and Angiotensin II in Angiogenesis: Implications for Retinopathy. Clin. Sci. 2013, 124, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Thomasset, S.; Teller, N.; Cai, H.; Marko, D.; Berry, D.; Steward, W.; Gescher, A. Do Anthocyanins and Anthocyanidins, Cancer Chemopreventive Pigments in the Diet, Merit Development as Potential Drugs? Cancer Chemother. Pharmacol. 2009, 64, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Fuhrman, B. Wine Flavonoids Protect against LDL Oxidation and Atherosclerosis. Ann. N. Y. Acad. Sci. 2002, 957, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.F. Intake of Flavonoids and Risk of Dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Borenstein, A.R.; Wu, Y.; Jackson, J.C.; Larson, E.B. Fruit and Vegetable Juices and Alzheimer’s Disease: The Kame Project. Am. J. Med. 2006, 119, 751–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Associations of Vegetable and Fruit Consumption with Age-Related Cognitive Change. Neurology 2006, 67, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Checkoway, H.; Powers, K.; Smith-Weller, T.; Franklin, G.M.; Longstreth, W.T.; Swanson, P.D. Parkinson’s Disease Risks Associated with Cigarette Smoking, Alcohol Consumption, and Caffeine Intake. Am. J. Epidemiol. 2002, 155, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Lee, Y.S. Molecular Mechanisms of Curcumin Action: Signal Transduction. Biofactors 2013, 39, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Nguyen, T.T.J. Natural Mood Foods: The Actions of Polyphenols against Psychiatric and Cognitive Disorders. Nutr. Neurosci. 2012, 15, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Vafeiadou, K.; Rice-Evans, C.; Williams, R.J.; Spencer, J.P.E. Activation of Pro-Survival Akt and ERK1/2 Signalling Pathways Underlie the Anti-Apoptotic Effects of Flavanones in Cortical Neurons. J. Neurochem. 2007, 103, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadou, K.; Vauzour, D.; Lee, H.Y.; Rodriguez-Mateos, A.; Williams, R.J.; Spencer, J.P.E. The Citrus Flavanone Naringenin Inhibits Inflammatory Signalling in Glial Cells and Protects against Neuroinflammatory Injury. Arch. Biochem. Biophys. 2009, 484, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Ma, G.; Ye, M.; Lu, G. Genistein Protects Dopaminergic Neurons by Inhibiting Microglial Activation. Neuroreport 2005, 16, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.R.; Feinstein, D.L.; Shen, Q.; Bhat, A.N. P38 MAPK-Mediated Transcriptional Activation of Inducible Nitric-Oxide Synthase in Glial Cells: Roles of Nuclear Factors, Nuclear Factor ΚB, CAMP Response Element-Binding Protein, CCAAT/Enhancer-Binding Protein-β, and Activating Transcription Factor-2. J. Biol. Chem. 2002, 277, 29584–29592. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.; Derbyshire, E.; Tiwari, B.K. Capsaicinoids and Capsinoids. A Potential Role for Weight Management? A Systematic Review of the Evidence. Appetite 2012, 59, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yoneshiro, T. Capsinoids and Related Food Ingredients Activating Brown Fat Thermogenesis and Reducing Body Fat in Humans. Curr. Opin. Lipidol. 2013, 24, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Dusting, G.J.; Peshavariya, H.; Jiang, F.; Hsiao, S.T.-F.; Chan, E.C.; Liu, G.-S. Differentiation of Human Adipose-Derived Stem Cells into Fat Involves Reactive Oxygen Species and Forkhead Box O1 Mediated Upregulation of Antioxidant Enzymes. Stem Cell Dev. 2013, 22, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, M.; Irii, H.; Tahara, Y.; Ishii, H.; Hirao, A.; Udagawa, H.; Hiramoto, M.; Yasuda, K.; Takanishi, A.; Shibata, S.; et al. Synthesis of a New [6]-Gingerol Analogue and Its Protective Effect with Respect to the Development of Metabolic Syndrome in Mice Fed a High-Fat Diet. J. Med. Chem. 2011, 54, 6295–6304. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Soflaei, S.S.; Majeed, M.; Sahebkar, A. Effects of Supplementation with Curcumin on Serum Adipokine Concentrations: A Randomized Controlled Trial. Nutrition 2016, 32, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of Carcinogenesis by Dietary Polyphenolic Compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.; Kuntz, S.; Brendel, M.D.; Daniel, H. Dietary Flavone Is a Potent Apoptosis Inducer in Human Colon Carcinoma Cells. Cancer Res. 2000, 60, 3823–3831. [Google Scholar] [PubMed]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxid. Med. Cell. Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef] [PubMed]
- Wessner, B.; Strasser, E.-M.; Koitz, N.; Schmuckenschlager, C.; Unger-Manhart, N.; Roth, E. Green Tea Polyphenol Administration Partly Ameliorates Chemotherapy-Induced Side Effects in the Small Intestine of Mice. J. Nutr. 2007, 137, 634–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, C.E.; Patel, B.B.; Wang, J.; Eltoum, I.A.; Lamartiniere, C.A. Epigallocatechin-3-Gallate Suppresses Early Stage, but Not Late Stage Prostate Cancer in TRAMP Mice: Mechanisms of Action. Prostate 2007, 67, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.E.; Cheng, A.L.; Lin, J.K.; Kuo, M.L. Inhibition by Curcumin of Diethylnitrosamine-Induced Hepatic Hyperplasia, Inflammation, Cellular Gene Products and Cell-Cycle-Related Proteins in Rats. Food Chem. Toxicol. 2000, 38, 991–995. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Goel, A. Cancer Chemoprevention by Dietary Polyphenols: Promising Role for Epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.E.; Gescher, A.J. Cancer Chemoprevention: Lessons Learned and Future Directions. Br. J. Cancer 2005, 93, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-J.; Yen, G.-C. Chemopreventive Effects of Dietary Phytochemicals against Cancer Invasion and Metastasis: Phenolic Acids, Monophenol, Polyphenol, and Their Derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.-Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Schuller Levis, G.B.; Lee, E.B.; Levis, W.R.; Lee, D.W.; Kim, B.S.; Park, S.Y.; Park, E. Platycodin D and D3 Isolated from the Root of Platycodon Grandiflorum Modulate the Production of Nitric Oxide and Secretion of TNF-Alpha in Activated RAW 264.7 Cells. Int. Immunopharmacol. 2004, 4, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Amararathna, M.; Johnston, M.R.; Rupasinghe, H.P.V. Plant Polyphenols as Chemopreventive Agents for Lung Cancer. Int. J. Mol. Sci. 2016, 17, 1352. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, P.A.; Walle, T. Inhibition of Benzo[a]Pyrene-Activating Enzymes and DNA Binding in Human Bronchial Epithelial BEAS-2B Cells by Methoxylated Flavonoids. Carcinogenesis 2006, 27, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lin, M.; Zhang, F.; Hu, Y.; Xu, X.; Li, Y.; Liu, K.; Ma, X.; Tian, X.; Yao, J. Dietary Flavonoid Genistein Induces Nrf2 and Phase II Detoxification Gene Expression via ERKs and PKC Pathways and Protects against Oxidative Stress in Caco-2 Cells. Mol. Nutr. Food Res. 2013, 57, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The Antioxidant and Pro-Oxidant Activities of Green Tea Polyphenols: A Role in Cancer Prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, T.; Ito, K.; Ikeda, Y.; Kizaki, M. Green Tea Component, Catechin, Induces Apoptosis of Human Malignant B Cells via Production of Reactive Oxygen Species. Clin. Cancer Res. 2005, 11, 6040–6049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howells, L.M.; Mitra, A.; Manson, M.M. Comparison of Oxaliplatin- and Curcumin-Mediated Antiproliferative Effects in Colorectal Cell Lines. Int. J. Cancer 2007, 121, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Efimova, T.; Eckert, R.L. Green Tea Polyphenol Stimulates a Ras, MEKK1, MEK3, and P38 Cascade to Increase Activator Protein 1 Factor-Dependent Involucrin Gene Expression in Normal Human Keratinocytes. J. Biol. Chem. 2002, 277, 1828–1836. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-L.; Kuo, Y.-M.; Lee, Y.-R.; Yang, S.-F.; Chen, W.-R.; Lee, H.-J. Apple Polyphenol Induces Cell Apoptosis, Cell Cycle Arrest at G2/M Phase, and Mitotic Catastrophe in Human Bladder Transitional Carcinoma Cells. J. Funct. Food 2015, 14, 384–394. [Google Scholar] [CrossRef]
- Singh, M.; Singh, R.; Bhui, K.; Tyagi, S.; Mahmood, Z.; Shukla, Y. Tea Polyphenols Induce Apoptosis through Mitochondrial Pathway and by Inhibiting Nuclear Factor-KappaB and Akt Activation in Human Cervical Cancer Cells. Oncol. Res. 2011, 19, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, A.; Urdaci, M.C.; Pinchuk, I.V.; López-Moratalla, N.; Martínez-Irujo, J.J. Flavonoids Induce Apoptosis in Human Leukemia U937 Cells through Caspase- and Caspase-Calpain-Dependent Pathways. Nutr. Cancer 2004, 50, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of Cancer Cell Apoptosis by Flavonoids Is Associated with Their Ability to Inhibit Fatty Acid Synthase Activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yumnam, S.; Hong, G.E.; Raha, S.; Saralamma, V.V.G.; Lee, H.J.; Heo, J.D.; Lee, S.J.; Lee, W.-S.; Kim, E.-H.; et al. Flavonoids of Korean Citrus Aurantium L. Induce Apoptosis via Intrinsic Pathway in Human Hepatoblastoma HepG2 Cells. Phyther. Res. PTR 2015, 29, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Pichardo, L.; Dharmawardhane, S.F. Grape Polyphenols Inhibit Akt/Mammalian Target of Rapamycin Signaling and Potentiate the Effects of Gefitinib in Breast Cancer. Nutr. Cancer 2012, 64, 1058–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepporta, M.V.; Fuccelli, R.; Rosignoli, P.; Ricci, G.; Servili, M.; Morozzi, G.; Fabiani, R. Oleuropein Inhibits Tumour Growth and Metastases Dissemination in Ovariectomised Nude Mice with MCF-7 Human Breast Tumour Xenografts. J. Funct. Food 2014, 8, 269–273. [Google Scholar] [CrossRef]
- Rivera, A.R.; Castillo-Pichardo, L.; Gerena, Y.; Dharmawardhane, S. Anti-Breast Cancer Potential of Quercetin via the Akt/AMPK/Mammalian Target of Rapamycin (MTOR) Signaling Cascade. PLoS ONE 2016, 11, e0157251. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Verma, I.M. NF-ΚB, an Active Player in Human Cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Noh, E.-M.; Kwon, K.-B.; Kim, J.-S.; You, Y.-O.; Hwang, J.-K.; Hwang, B.-M.; Kim, B.-S.; Lee, S.-H.; Lee, S.J.; et al. Curcumin Suppresses the TPA-Induced Invasion through Inhibition of PKCα-Dependent MMP-Expression in MCF-7 Human Breast Cancer Cells. Phytomed. Int. J. Phyther. Phytopharm. 2012, 19, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. The Role of Nutraceuticals in the Regulation of Wnt and Hedgehog Signaling in Cancer. Cancer Metastasis Rev. 2010, 29, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Nuclear Factor-KappaB: The Enemy Within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.; Nerlich, A.G.; Iancu, C.M.; Cilli, M.; Schleicher, E.; Vené, R.; Dell’Eva, R.; Jochum, M.; Albini, A.; Pfeffer, U. The Chemopreventive Polyphenol Curcumin Prevents Hematogenous Breast Cancer Metastases in Immunodeficient Mice. Cell. Physiol. Biochem. 2007, 19, 137–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhangi, B.; Alizadeh, A.M.; Khodayari, H.; Khodayari, S.; Dehghan, M.J.; Khori, V.; Heidarzadeh, A.; Khaniki, M.; Sadeghiezadeh, M.; Najafi, F. Protective Effects of Dendrosomal Curcumin on an Animal Metastatic Breast Tumor. Eur. J. Pharmacol. 2015, 758, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Yang, J. Epithelial–Mesenchymal Plasticity in Carcinoma Metastasis. Gene Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, E.; Kim, W.; Seong, K.M.; Youn, H.; Kim, J.W.; Kim, J.; Youn, B. Rhamnetin and Cirsiliol Induce Radiosensitization and Inhibition of Epithelial-Mesenchymal Transition (EMT) by MiR-34a-Mediated Suppression of Notch-1 Expression in Non-Small Cell Lung Cancer Cell Lines. J. Biol. Chem. 2013, 288, 27343–27357. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Shen, Y.-A.; Hung, P.-H.; Yu, Y.-B.; Chen, Y.-J. Epigallocathechin Gallate, Polyphenol Present in Green Tea, Inhibits Stem-like Characteristics and Epithelial-Mesenchymal Transition in Nasopharyngeal Cancer Cell Lines. BMC Complement. Altern. Med. 2012, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Tsai, P.-H.; Kandaswami, C.C.; Cheng, C.-H.; Ke, F.-C.; Lee, P.-P.; Hwang, J.-J.; Lee, M.-T. Effects of Dietary Flavonoids, Luteolin, and Quercetin on the Reversal of Epithelial-Mesenchymal Transition in A431 Epidermal Cancer Cells. Cancer Sci. 2011, 102, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y. Tea Catechins and Their Applications as Supplements and Pharmaceutics. Pharmacol. Res. 2011, 64, 100–104. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. https://doi.org/10.3390/nu10111618
Yahfoufi N, Alsadi N, Jambi M, Matar C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients. 2018; 10(11):1618. https://doi.org/10.3390/nu10111618
Chicago/Turabian StyleYahfoufi, Nour, Nawal Alsadi, Majed Jambi, and Chantal Matar. 2018. "The Immunomodulatory and Anti-Inflammatory Role of Polyphenols" Nutrients 10, no. 11: 1618. https://doi.org/10.3390/nu10111618
APA StyleYahfoufi, N., Alsadi, N., Jambi, M., & Matar, C. (2018). The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients, 10(11), 1618. https://doi.org/10.3390/nu10111618