Alcohol Pattern Consumption Differently Affects the Efficiency of Macrophage Reverse Cholesterol Transport in Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Biometric and Clinical Analysis
2.3. Macrophage RCT in Vivo
2.4. Hepatic Lipid Content
2.5. Evaluation of Cholesterol Efflux Capacity (CEC)
2.6. Cholesterol Efflux Assay
2.7. RNA-Preparation, Reverse Transcription, and Quantitative Real-Time PCR (qPCR)
2.8. Western Blot
2.9. Immunofluorescence Analysis of Liver Cryosections
2.10. Statistical Analysis
3. Results
3.1. Effect of Moderate and Binge Alcohol Consumption on the Plasma Lipoprotein Profile
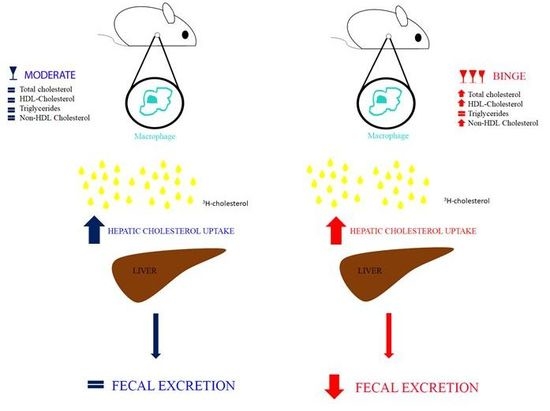
3.2. Effecst of Moderate and Binge Alcohol Consumption on the Reverse Cholesterol Transport in Vivo
3.3. Hepatic Cholesterol Content of Mice undergoing Moderate and Binge Alcohol Consumption
3.4. Effect of Moderate and Binge Alcohol Consumption on the First Step of RCT
3.5. Effect of Moderate and Binge Alcohol Consumption on Hepatic Expression of SR-BI
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parry, C.D.; Patra, J.; Rehm, J. Alcohol consumption and non-communicable diseases: Epidemiology and policy implications. Addiction 2011, 106, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Baliunas, D.; Borges, G.L.G.; Graham, K.; Irving, H.; Kehoe, T.; Parry, C.D.; Patra, J.; Popova, S.; Poznyak, V.; et al. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction 2010, 105, 817–843. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Biochemical and molecular basis of alcohol-in. N. Engl. J. Med. 1988, 319, 1639. [Google Scholar] [PubMed]
- Rocco, A. Alcoholic disease: Liver and beyond. World J Gastroenterol. 2014, 20, 14652–14659. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sola, J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat. Rev. Cardiol. 2015, 12, 576–587. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Alcohol Collaborators Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [CrossRef]
- Di Castelnuovo, A.; Costanzo, S.; Bagnardi, V.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Alcohol Dosing and Total Mortality in Men and Women. Arch. Intern. Med. 2014, 166, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Roerecke, M.; Rehm, J. Alcohol consumption, drinking patterns, and ischemic heart disease: A narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Foerster, M.; Marques-Vidal, P.; Gmel, G.; Daeppen, J.-B.; Cornuz, J.; Hayoz, D.; Pécoud, A.; Mooser, V.; Waeber, G.; Vollenweider, P.; et al. Alcohol drinking and cardiovascular risk in a population with high mean alcohol consumption. Am. J. Cardiol. 2009, 103, 361–368. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.; Britton, A.; Hannah, M.K.; Goldberg, M.; Kuh, D.; Khaw, K.T.; Bell, S. Association of longitudinal alcohol consumption trajectories with coronary heart disease: A meta-analysis of six cohort studies using individual participant data. BMC Med. 2018, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342, d636. [Google Scholar] [CrossRef] [PubMed]
- Brinton, E.A. Effects of ethanol intake on lipoproteins. Curr. Atheroscler. Rep. 2012, 14, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Redmond, E.M.; Morrow, D.; Cullen, J.P. Differential effects of daily-moderate versus weekend-binge alcohol consumption on atherosclerotic plaque development in mice. Atherosclerosis 2011, 219, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rader, D.J.; Alexander, E.T.; Weibel, G.L.; Billheimer, J.; Rothblat, G.H. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 2009, 50, S189–S194. [Google Scholar] [CrossRef]
- Rigotti, A.; Miettinen, H.E.; Krieger, M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr. Rev. 2003, 24, 357–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Da Silva, J.R.; Reiliy, M.; Billheimer, J.T.; Rothblat, G.H.; Rader, D.J. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2005, 115, 2870–2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanotti, I.; Maugeais, C.; Pedrelli, M.; Gomaraschi, M.; Salgam, P.; Calabresi, L.; Bernini, F.; Kempen, H. The thienotriazolodiazepine Ro 11-1464 increases plasma apoA-I and promotes reverse cholesterol transport in human apoA-I transgenic mice. Br. J. Pharmacol. 2011, 164, 1642–1651. [Google Scholar] [CrossRef]
- Zanotti, I.; Greco, D.; Lusardi, G.; Zimetti, F.; Potì, F.; Arnaboldi, L.; Corsini, A.; Bernini, F. Cyclosporine A Impairs the Macrophage Reverse Cholesterol Transport in Mice by Reducing Sterol Fecal Excretion. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, I.; Pedrelli, M.; Poti, F.; Stomeo, G.; Gomaraschi, M.; Calabresi, L.; Bernini, F. Macrophage, but not systemic, apolipoprotein E is necessary for macrophage reverse cholesterol transport in vivo. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Iverson, S.J.; Lang, S.L.; Cooper, M.H. Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 2001, 36, 1283–1287. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Zanotti, I.; Favari, E.; Bernini, F. Cellular cholesterol efflux pathways: Impact on intracellular lipid trafficking and methodological considerations. Curr. Pharm. Biotechnol. 2012, 13, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Eileen, M. Redmond, David Morrow, Sreenath Kundimi, Carol, L. Miller-Graziano, and J.P.C. Acetaldehyde stimulates monocyte adhesion in a P-selectin- and TNFα-dependent manner. Atherosclerosis 2009, 204, 372–380. [Google Scholar]
- Bébarová, M.; Matejovic, P.; Simurdová, M. Acetaldehyde at Clinically Relevant Concentrations Inhibits Inward Rectifier Potassium Current I^ sub K1^ in Rat Ventricular Myocytes. Physiol. Res. 2015, 8408, 939–943. [Google Scholar]
- Ruidavets, J.-B.; Ducimetiere, P.; Evans, A.; Montaye, M.; Haas, B.; Bingham, A.; Yarnell, J.; Amouyel, P.; Arveiler, D.; Kee, F.; et al. Patterns of alcohol consumption and ischaemic heart disease in culturally divergent countries: The Prospective Epidemiological Study of Myocardial Infarction (PRIME). BMJ 2010, 341, c6077. [Google Scholar] [CrossRef] [PubMed]
- Avdulov, N.A.; Chochina, S.V.; Igbavboa, U.; Wood, W.G. Cholesterol efflux to high-density lipoproteins and apolipoprotein A-I phosphatidylcholine complexes is inhibited by ethanol: Role of apolipoprotein structure and cooperative interaction of phosphatidylcholine and cholesterol. Biochemistry 2000, 39, 10599–10606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, J.; Zhang, X.; Costa, L.G.; Guizzetti, M. Prenatal Ethanol Exposure Up-Regulates the Cholesterol Transporters ATP-Binding Cassette A1 and G1 and Reduces Cholesterol Levels in the Developing Rat Brain. Alcohol Alcohol. 2014, 49, 626–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guizzetti, M.; Chen, J.; Oram, J.F.; Tsuji, R.; Dao, K.; Möller, T.; Costa, L.G. Ethanol induces cholesterol efflux and up-regulates ATP-binding cassette cholesterol transporters in fetal astrocytes. J. Biol. Chem. 2007, 282, 18740–18749. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Zimetti, F.; Billheimer, J.T.; Wang, N.; Rader, D.J.; Phillips, M.C.; Rothblat, G.H. The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 2007, 48, 2453–2462. [Google Scholar] [CrossRef] [Green Version]
- Zani, I.A.; Stephen, S.L.; Mughal, N.A.; Russell, D.; Homer-Vanniasinkam, S.; Wheatcroft, S.B.; Ponnambalam, S. Scavenger receptor structure and function in health and disease. Cells 2015, 4, 178–201. [Google Scholar] [CrossRef]
- Li, M.; Diao, Y.; Liu, Y.; Huang, H.; Li, Y.; Tan, P. Chronic Moderate Alcohol Intakes Accelerate SR-B1 Mediated Reverse Cholesterol Transport. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]






| Placebo | Moderate | Binge | |
|---|---|---|---|
| Body weight at baseline (g) | 24.9 ± 1.5 | 24.3 ± 2.0 | 24.4 ± 2.3 |
| Body weight on day 28 (g) | 25.4 ± 1.8 | 25.6 ±1.3 | 24.5 ± 2.0 |
| Liver weight (g) | 1.10 ± 0.11 | 1.14 ± 0.22 | 1.25 ± 0.21 |
| Hepatic triglycerides (mg/g liver) | 9.5 ± 3.8 | 18.4 ± 4.3 | 27.5 ± 3.3 §§ |
| ALT (U/L) | 2.83 ± 1.25 | 2.59 ± 1.22 | 3.18 ± 0.93 |
| Total cholesterol (mg/dL) | 328 ± 24 | 304 ± 39 | 478 ± 50 §§§§, **** |
| HDL-c (mg/dL) | 11 ± 1 | 9 ± 1 | 22 ± 7 §§, **** |
| Triglycerides (mg/dL) | 107 ± 25 | 151 ± 42 | 147 ± 26 |
| Non-HDL-c (mg/dL) | 316 ± 24 | 295 ± 39 | 455 ± 47 §§§§, **** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, D.; Battista, S.; Mele, L.; Piemontese, A.; Papotti, B.; Cavazzini, S.; Potì, F.; Di Rocco, G.; Poli, A.; Bernini, F.; et al. Alcohol Pattern Consumption Differently Affects the Efficiency of Macrophage Reverse Cholesterol Transport in Vivo. Nutrients 2018, 10, 1885. https://doi.org/10.3390/nu10121885
Greco D, Battista S, Mele L, Piemontese A, Papotti B, Cavazzini S, Potì F, Di Rocco G, Poli A, Bernini F, et al. Alcohol Pattern Consumption Differently Affects the Efficiency of Macrophage Reverse Cholesterol Transport in Vivo. Nutrients. 2018; 10(12):1885. https://doi.org/10.3390/nu10121885
Chicago/Turabian StyleGreco, Daniela, Simone Battista, Laura Mele, Antonio Piemontese, Bianca Papotti, Stefania Cavazzini, Francesco Potì, Giulia Di Rocco, Andrea Poli, Franco Bernini, and et al. 2018. "Alcohol Pattern Consumption Differently Affects the Efficiency of Macrophage Reverse Cholesterol Transport in Vivo" Nutrients 10, no. 12: 1885. https://doi.org/10.3390/nu10121885
APA StyleGreco, D., Battista, S., Mele, L., Piemontese, A., Papotti, B., Cavazzini, S., Potì, F., Di Rocco, G., Poli, A., Bernini, F., & Zanotti, I. (2018). Alcohol Pattern Consumption Differently Affects the Efficiency of Macrophage Reverse Cholesterol Transport in Vivo. Nutrients, 10(12), 1885. https://doi.org/10.3390/nu10121885