Inulin Improves Postprandial Hypertriglyceridemia by Modulating Gene Expression in the Small Intestine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Ethics Statement
2.3. Lipid Tolerance Test
2.4. Real-Time Quantitative PCR
2.5. Biochemical Analysis
2.6. Histological Analysis
2.7. Statistical Analysis
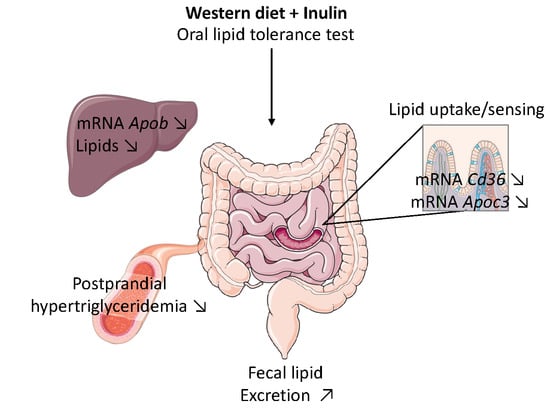
3. Results
3.1. Inulin Does Not Influence Body Weight, Adipose Tissue and Liver Weight
3.2. Inulin Supplementation Improves Postprandial Hypertriglyceridemia Induced by Western Diet
3.3. Inulin Modifies Intestinal Lipid Metabolism by Changing Gene Expression in the Jejunum
3.3.1. Inulin Decreases Cd36 Expression, a Receptor Involved in Fatty Acid Absorption and Lipid Sensing
3.3.2. Inulin Supplementation Increases Fecal Lipid Excretion When Fed with Western Diet
3.3.3. Western Diet and Inulin Supplementation Does Not Impact Lipid Accumulation in the Enterocytes
3.4. Effect of Dietary Intervention on Lipoprotein Lipase Activity and on the Expression of Genes Involved in Its Regulation
3.5. Inulin Decreases Hepatic Lipid Content and Changes the Hepatic Expression of Genes Involved in VLDL Secretion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Hassing, H.C.; Surendran, R.P.; Mooij, H.L.; Stroes, E.S.; Nieuwdorp, M.; Dallinga-Thie, G.M. Pathophysiology of hypertriglyceridemia. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Matikainen, N.; Adiels, M.; Taskinen, M.R. Postprandial hypertriglyceridemia as a coronary risk factor. Clin. Chim. Acta 2014, 431, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Miyoshi, T.; Yunoki, K.; Ito, H. Postprandial hyperlipidemia as a potential residual risk factor. J. Cardiol. 2016, 67, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Miranda, J.; Williams, C.; Lairon, D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br. J. Nutr. 2007, 98, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. Postprandial lipemia as a cardiometabolic risk factor. Curr. Med. Res. Opin. 2014, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kalala, G.; Kambashi, B.; Everaert, N.; Beckers, Y.; Richel, A.; Pachikian, B.; Neyrinck, A.M.; Delzenne, N.M.; Bindelle, J. Characterization of fructans and dietary fibre profiles in raw and steamed vegetables. Int. J. Food Sci. Nutr. 2017, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [PubMed]
- Delzenne, N.M.; Williams, C.M. Prebiotics and lipid metabolism. Curr. Opin. Lipidol. 2002, 13, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Daubioul, C.; Neyrinck, A.; Lasa, M.; Taper, H.S. Inulin and oligofructose modulate lipid metabolism in animals: Review of biochemical events and future prospects. Br. J. Nutr. 2002, 87 (Suppl. 2), S255–S259. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Cani, P.D. Gut microbiota and metabolic disorders: How prebiotic can work? Br. J. Nutr. 2013, 109 (Suppl. 2), S81–S85. [Google Scholar] [CrossRef] [PubMed]
- Kok, N.; Roberfroid, M.; Delzenne, N. Dietary oligofructose modifies the impact of fructose on hepatic triacylglycerol metabolism. Metabolism 1996, 45, 1547–1550. [Google Scholar] [CrossRef]
- Kok, N.; Roberfroid, M.; Robert, A.; Delzenne, N. Involvement of lipogenesis in the lower VLDL secretion induced by oligofructose in rats. Br. J. Nutr. 1996, 76, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Kok, N.; Taper, H.S.; Delzenne, N.M. Oligofructose modulates lipid metabolism alterations induced by a fat-rich diet in rats. J. Appl. Toxicol. 1998, 18, 47–53. [Google Scholar] [CrossRef]
- Daubioul, C.A.; Taper, H.S.; De Wispelaere, L.D.; Delzenne, N.M. Dietary oligofructose lessens hepatic steatosis, but does not prevent hypertriglyceridemia in obese zucker rats. J. Nutr. 2000, 130, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Catry, E.; Bindels, L.B.; Tailleux, A.; Lestavel, S.; Neyrinck, A.M.; Goossens, J.F.; Lobysheva, I.; Plovier, H.; Essaghir, A.; Demoulin, J.B.; et al. Targeting the gut microbiota with inulin-type fructans: Preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut 2018, 67, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, F. Dietary fructans and serum triacylglycerols: A meta-analysis of randomized controlled trials. J. Nutr. 2007, 137, 2552S–2556S. [Google Scholar] [CrossRef] [PubMed]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Systematic Review Metabolic benefits of dietary prebiotics in human subjects : A systematic review of randomised controlled trials. Br. J. Nutr. 2013, 111, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Prabhakar, M.; Ju, J.; Long, H.; Zhou, H.W. Effect of inulin-type fructans on blood lipid profile and glucose level: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2017, 71, 9–20. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.W.; Gibb, R.D.; McKeown, N.M. Inulin-type fructans have no significant beneficial effects on lipid or glucose metabolism. Eur. J. Clin. Nutr. 2017, 71, 667. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Liu, M.; Portincasa, P.; Wang, D.Q. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur. J. Clin. Investig. 2013, 43, 1203–1223. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Pachikian, B.; Taminiau, B.; Daube, G.; Frédérick, R.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Intestinal sucrase as a novel target contributing to the regulation of glycemia by prebiotics. PLoS ONE 2016, 11, e0160488. [Google Scholar] [CrossRef] [PubMed]
- Capitan-Canadas, F.; Ortega-Gonzalez, M.; Guadix, E.; Zarzuelo, A.; Suárez, M.D.; de Medina, F.S.; Martínez-Augustin, O. Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4. Mol. Nutr. Food Res. 2014, 58, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G. A Simple method for the isolation and purification of total lipidsfrom animal tissues. J. Biol. Chem. 1987, 55, 999–1033. [Google Scholar]
- Suriano, F.; Bindels, L.B.; Verspreet, J.; Courtin, C.M.; Verbeke, K.; Cani, P.D.; Neyrinck, A.M.; Delzenne, N.M. Fat binding capacity and modulation of the gut microbiota both determine the effect of wheat bran fractions on adiposity. Sci. Rep. 2017, 7, 5621. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Muccioli, G.G.; Deldicque, L.; Bindels, L.B.; Pachikian, B.D.; Sohet, F.M.; et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J. Nutr. Biochem. 2011, 22, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.D.; Widmaier, E.P. Involvement of CD36 and intestinal alkaline phosphatases in fatty acid transport in enterocytes, and the response to a high-fat diet. Life Sci. 2011, 88, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.T.; Poirier, H.; Clément, L.; Nassir, F.; Pelsers, M.M.; Petit, V.; Degrace, P.; Monnot, M.C.; Glatz, J.F.; Abumrad, N.A.; et al. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J. Biol. Chem. 2011, 286, 25201–25210. [Google Scholar] [CrossRef] [PubMed]
- Buttet, M.; Poirier, H.; Traynard, V.; Gaire, K.; Tran, T.T.; Sundaresan, S.; Besnard, P.; Abumrad, N.A.; Niot, I. Deregulated lipid sensing by intestinal CD36 in diet-induced hyperinsulinemic obese mouse model. PLoS ONE 2016, 11, e0145626. [Google Scholar] [CrossRef] [PubMed]
- Geloen, A.; Helin, L.; Geeraert, B.; Malaud, E.; Holvoet, P.; Marguerie, G. CD36 inhibitors reduce postprandial hypertriglyceridemia and protect against diabetic dyslipidemia and atherosclerosis. PLoS ONE 2012, 7, e37633. [Google Scholar] [CrossRef] [PubMed]
- Lairon, D.; Play, B.; Jourdheuil-Rahmani, D. Digestible and indigestible carbohydrates: Interactions with postprandial lipid metabolism. J. Nutr. Biochem. 2007, 18, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Druart, C.; Neyrinck, A.M.; Dewulf, E.M.; De Backer, F.C.; Possemiers, S.; Van de Wiele, T.; Moens, F.; De Vuyst, L.; Cani, P.D.; Larondelle, Y.; et al. Implication of fermentable carbohydrates targeting the gut microbiota on conjugated linoleic acid production in high-fat-fed mice. Br. J. Nutr. 2013, 110, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Nauli, A.M.; Nassir, F.; Zheng, S.; Yang, Q.; Lo, C.M.; Vonlehmden, S.B.; Lee, D.; Jandacek, R.J.; Abumrad, N.A.; Tso, P. CD36 Is Important for Chylomicron Formation and Secretion and May Mediate Cholesterol Uptake in the Proximal Intestine. Gastroenterology 2006, 131, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Goudriaan, J.R.; Dahlmans, V.E.H.; Febbraio, M.; Teusink, B.; Romijn, J.A.; Havekes, L.M.; Voshol, P.J. Intestinal lipid absorption is not affected in CD36 deficient mice. Mol. Cell. Biochem. 2002, 239, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.D.; Malik, N.; Chon, S.H.; Wells, K.; Zhou, Y.X.; Choi, A.S.; Joseph, L.B.; Storch, J. Intestinal mucosal triacylglycerol accumulation secondary to decreased lipid secretion in obese and high fat fed mice. Front. Physiol. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Borén, J. Why Is Apolipoprotein CIII Emerging as a Novel Therapeutic Target to Reduce the Burden of Cardiovascular Disease? Curr. Atheroscler. Rep. 2016, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.J.; Lee, R.G.; Bell, T.A.; Fu, W.; Mullick, A.E.; Alexander, V.J.; Singleton, W.; Viney, N.; Geary, R.; Su, J.; et al. Antisense oligonucleotide inhibition of apolipoprotein c-iii reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ. Res. 2013, 112, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Dallinga-Thie, G.M.; Kroon, J.; Borén, J.; Chapman, M.J. Triglyceride-Rich Lipoproteins and Remnants: Targets for Therapy? Curr. Cardiol. Rep. 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Dallinga-Thie, G.M.; Franssen, R.; Mooij, H.L.; Visser, M.E.; Hassing, H.C.; Peelman, F.; Kastelein, J.J.P.; Péterfy, M.; Nieuwdorp, M.; Metrics, P. The metabolism of triglyceride rich lipoproteins revisited; new player new insight. Atherosclerosis 2014, 211, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fiordaliso, M.; Kok, N.; Desager, J.P.; Goethals, F.; Deboyser, D.; Roberfroid, M.; Delzenne, N. Dietary oligofructose lowers triglycerides, phospholipids and cholesterol in serum and very low density lipoproteins of rats. Lipids 1995, 30, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Daubioul, C.; Rousseau, N.; Demeure, R.; Gallez, B.; Taper, H.; Declerck, B.; Delzenne, N. Dietary fructans, but not cellulose, decrease triglyceride accumulation in the liver of obese Zucker fa/fa rats. J. Nutr. 2002, 132, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Aidy, S.E.; van den Bogert, B.; Kleerebezem, M. The small intestine microbiota, nutritional modulation and relevance for health. Curr. Opin. Biotechnol. 2015, 32, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, E.A.; Rieckhoff, D.; Erbersdobler, H.F. Dietary inulin lowers plasma cholesterol and triacylglycerol and alters biliary bile acid profile in hamsters. J. Nutr. 1998, 128, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.M.; Merhige, P.M.; Hagey, L.R. The Effect of Dietary Prebiotics and Probiotics on Body Weight, Large Intestine Indices, and Fecal Bile Acid Profile in Wild Type and IL10−/− Mice. PLoS ONE 2013, 8, e60270. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Wahlström, A.; Marschall, H.-U. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. 2018, 29, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Porez, G.; Prawitt, J.; Gross, B.; Staels, B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J. Lipid Res. 2012, 53, 1723–1737. [Google Scholar] [CrossRef] [PubMed]






| Tissues | CT | CT + I | WD | WD + I |
|---|---|---|---|---|
| Liver (g) | 0.87 ± 0.04 | 0.89 ± 0.02 | 1.00 ± 0.05 | 0.91 ± 0.05 |
| Subcutaneous adipose tissue (g) | 0.41 ± 0.05 | 0.29 ± 0.01 | 0.85 ± 0.13 *,§ | 0.75 ± 0.12 § |
| Epididymal adipose tissue (g) | 0.40 ± 0.04 | 0.28 ± 0.01 | 1.00 ± 0.17 *,§ | 0.88 ± 0.17 *,§ |
| Visceral adipose tissue (g) | 0.18 ± 0.02 | 0.12 ± 0.01 | 0.37 ± 0.06 *,§ | 0.29 ± 0.05 § |
| Cecal tissue (g) | 0.039 ± 0.002 | 0.054 ± 0.002 * | 0.033 ± 0.002 § | 0.046 ± 0.003 † |
| Cecal content (g) | 0.15 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.23 ± 0.02 *,† |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiel, S.; Neyrinck, A.M.; Rodriguez, J.; Pachikian, B.D.; Bouzin, C.; Thissen, J.-P.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Inulin Improves Postprandial Hypertriglyceridemia by Modulating Gene Expression in the Small Intestine. Nutrients 2018, 10, 532. https://doi.org/10.3390/nu10050532
Hiel S, Neyrinck AM, Rodriguez J, Pachikian BD, Bouzin C, Thissen J-P, Cani PD, Bindels LB, Delzenne NM. Inulin Improves Postprandial Hypertriglyceridemia by Modulating Gene Expression in the Small Intestine. Nutrients. 2018; 10(5):532. https://doi.org/10.3390/nu10050532
Chicago/Turabian StyleHiel, Sophie, Audrey M. Neyrinck, Julie Rodriguez, Barbara D. Pachikian, Caroline Bouzin, Jean-Paul Thissen, Patrice D. Cani, Laure B. Bindels, and Nathalie M. Delzenne. 2018. "Inulin Improves Postprandial Hypertriglyceridemia by Modulating Gene Expression in the Small Intestine" Nutrients 10, no. 5: 532. https://doi.org/10.3390/nu10050532
APA StyleHiel, S., Neyrinck, A. M., Rodriguez, J., Pachikian, B. D., Bouzin, C., Thissen, J. -P., Cani, P. D., Bindels, L. B., & Delzenne, N. M. (2018). Inulin Improves Postprandial Hypertriglyceridemia by Modulating Gene Expression in the Small Intestine. Nutrients, 10(5), 532. https://doi.org/10.3390/nu10050532