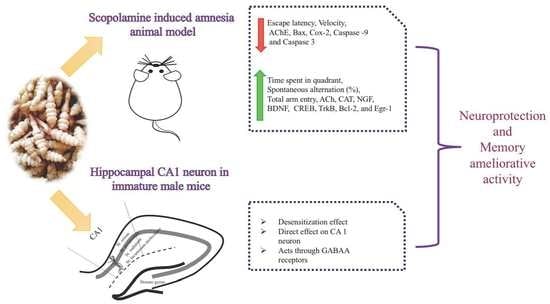
Stachys sieboldii Extract Supplementation Attenuates Memory Deficits by Modulating BDNF-CREB and Its Downstream Molecules, in Animal Models of Memory Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Stachys Sieboldii Extract
2.2. UHPLC-MS/MS
2.3. Animals and Experimental Groups
2.4. Morris Water Maze Test
2.5. Y-maze Test
2.6. Sample Collection
2.7. Determination of Acetylcholine, Choline Acetyl Transferase, and Acetyl Choline Esterase Levels in Animal Model of Impaired Memory
2.8. Quantitative Reverse Transcription PCR
2.9. Brain Slice Preparation and Electrophysiology
2.10. Statistical Analysis
3. Results
3.1. Effects of SS on Memory Function in Animal Model of Impaired Memory
3.2. Effects of SS on Hippocampal and Frontal Cortical, ACh, CAT, and AChE Levels in Animal Model of Impaired Memory
3.3. Effect of SS on mRNA Expression in the Hippocampal and Frontal Cortical Area on Memory Impaired Animals
3.4. Electrophysiology Results
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Canevelli, M.; Blasimme, A.; Cesari, M. Societal and global implications of the “dementia epidemic”: The example of the London Heathrow airport. Eur. J. Epidemiol. 2017, 32, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Tarawneh, R.; Holtzman, D.M. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb. Perspect. Med. 2012, 2, a006148. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Maloney, A.J.F. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 2, 1403. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Riba-Llena, I.; Serra-Basante, C.; Alom, J.; Boopathy, R.; Sáez-Valero, J. Altered Levels of Acetylcholinesterase in Alzheimer Plasma. PLoS ONE 2010, 5, e8701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 703–1718. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.M.; Franks, K.M.; Titterness, A.K.; Russell, K.; Merrill, D.A.; Christie, B.R.; Sejnowski, T.J.; Tuszynski, M.H. NGF is essential for hippocampal plasticity and learning. J. Neurosci. 2009, 29, 10883–10889. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Bekinschtein, P.; Cammarota, M.; Vianna, M.R.; Izquierdo, I.; Medina, J.H. Endogenous BDNF is required for long-term memory formation in the rat parietal cortex. Learn. Mem. 2005, 12, 504–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhao, H.F.; Zhang, Z.F.; Liu, Z.G.; Pei, X.R.; Wang, J.B.; Li, Y. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience 2009, 159, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Maddox, S.A.; Monsey, M.S.; Schafe, G.E. Early growth response gene 1 (Egr-1) is required for new and reactivated fear memories in the lateral amygdala. Learn. Mem. 2011, 18, 24–38. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, M.; Cavallucci, V.; Cecconi, F. Neuronal caspase-3 signaling: Not only cell death. Cell Death Differ. 2010, 17, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.N.; Commins, S.; O’mara, S.M. Deficits in spatial learning and synaptic plasticity induced by the rapid and competitive broad-spectrum cyclooxygenase inhibitor ibuprofen are reversed by increasing endogenous brain-derived neurotrophic factor. Eur. J. Neurosci. 2003, 17, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sheng, M. Caspases in synaptic plasticity. Mol. Brain 2012, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, M.L.; Pilli, J.; Lorenz-Guertin, J.M.; Das, S.; Moon, C.E.; Graff, N.; Jacob, T.C. Depolarizing, inhibitory GABA type A receptor activity regulates GABAergic synapse plasticity via ERK and BDNF signaling. Neuropharmacology 2018, 128, 324–339. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.I. Brain-derived neurotrophic factor promotes gephyrin protein expression and GABAA receptor clustering in immature cultured hippocampal cells. Neurochem. Int. 2014, 72, 14–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, G.A.; Hanrahan, J.R.; Chebib, M.; Duke, R.K.; Mewett, K.N. Modulation of ionotropic GABA receptors by natural products of plant origin. Adv. Pharmacol. 2006, 54, 285–316. [Google Scholar] [PubMed]
- Bhattarai, J.P.; Park, S.J.; Han, S.K. Potentiation of NMDA receptors by Withania somnifera on hippocampal CA1 pyramidal neurons. Am. J. Chin. Med. 2013, 41, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Abinaya, R.; Pichiah, P.B.T.; Sara Thomas, S.; Kim, S.G.; Han, D.W.; Song, Y.S.; Oh, S.H.; Cha, Y.S. γ-amino butyric acid-enriched barley bran lowers adrenocorticotropic hormone and corticosterone levels in immobilized stressed rats. J. Food Biochem. 2017, 41, e12324. [Google Scholar] [CrossRef]
- Yamahara, J.; Kitani, T.; Kobayashi, H.; Kawahara, Y. Studies on Stachys sieboldii MIQ. II. Anti-anoxia action and the active constituents. Yakugaku zasshi: J. Pharm. Soc. Jpn. 1990, 110, 932–935. [Google Scholar] [CrossRef]
- Hayashi, K.; Nagamatsu, T.; Ito, M.; Hattori, T.; Suzuki, Y. Acteoside, a component of Stachys sieboldii MIQ, may be a promising antinephritic agent: Effect of acteoside on crescentic-type anti-GBM nephritis in rats. Jpn. J. Pharmacol. 1994, 65, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yang, G.; Wang, S.; Chen, Y. Purification and determination of stachyose in Chinese artichoke (Stachys Sieboldii Miq.) by high-performance liquid chromatography with evaporative light scattering detection. Talanta 2006, 70, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Abinaya, R.; Kim, M.; Lee, S.J.; Cha, Y.S. Protective effects of Stachys sieboldii MIQ extract in SK-N-SH cells and its memory ameliorative effect in mice. J. Food Biochem. 2017, 41, e12411. [Google Scholar] [CrossRef]
- Nishimura, H.; Sasaki, H.; Inagaki, N.; Masao, C.; Chen, Z.; Mitsuhashi, H. Nine phenethyl alcohol glycosides from Stachys sieboldii. Phytochemistry 1991, 30, 965–969. [Google Scholar] [CrossRef]
- Agrawal, R.; Tyagi, E.; Shukla, R.; Nath, C. Effect of insulin and melatonin on acetylcholinesterase activity in the brain of amnesic mice. Behav. Brain Res. 2008, 189, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Freireich, E.J.; Gehan, E.A.; Rall, D.P.; Schmidt, L.H.; Skipper, H.E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey and man. Cancer Chemother Rep. 1966, 50, 219–244. [Google Scholar] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Ma, M.; Chen, Y.; He, J.; Zeng, T.; Wang, J. Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience 2007, 147, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, J.P.; Han, S.K. Phasic and tonic type A γ-Aminobutryic acid receptor mediated effect of Withania somnifera on mice hippocampal CA1 pyramidal Neurons. J. Ayurveda Integr. Med. 2014, 5, 216–222. [Google Scholar] [PubMed]
- Sattayasai, J.; Chaonapan, P.; Arkaravichie, T.; Soi-Ampornkul, R.; Junnu, S.; Charoensilp, P.; Samer, J.; Jantaravinid, J.; Masaratana, P.; Suktitipat, B.; et al. Protective effects of mangosteen extract on H2O2-induced cytotoxicity in SK-N-SH cells and scopolamine-induced memory impairment in mice. PLoS ONE 2013, 8, e85053. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Lim, D.W.; Son, H.J.; Cho, S.; Lee, C. Phlorotannin-rich fraction from Ishige foliacea brown seaweed prevents the scopolamine-induced memory impairment via regulation of ERK-CREB-BDNF pathway. J. Funct. Foods 2018, 40, 110–116. [Google Scholar] [CrossRef]
- Ballinger, E.; Ananth, M.; Talmage, D.A.; Role, L. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesulam, M.M.; Mufson, E.J.; Levey, A.I.; Wainer, B.H. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol. 1983, 214, 170–197. [Google Scholar] [CrossRef] [PubMed]
- Batool, Z.; Sadir, S.; Liaquat, L.; Tabassum, S.; Madiha, S.; Rafiq, S.; Haider, S. Repeated administration of almonds increases brain acetylcholine levels and enhances memory function in healthy rats while attenuates memory deficits in animal model of amnesia. Brain Res. Bull. 2016, 120, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borba, E.M.; Duarte, J.A.; Bristot, G.; Scotton, E.; Camozzato, A.L.; Chaves, M.L.F. Brain-derived neurotrophic factor serum levels and hippocampal volume in mild cognitive impairment and dementia due to alzheimer disease. Dement. Geriatr. Cogn. Disord. Extra 2016, 6, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, R.M. Apoptosis and caspases in neurodegenerative diseases. N. Engl. J. Med. 2003, 348, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, J.P.; Ah Park, S.; Han, S.K. The methanolic extract of Withania somnifera ACTS on GABAA receptors in gonadotropin releasing hormone (GnRH) neurons in mice. Phytother. Res. 2010, 24, 1147–1150. [Google Scholar] [PubMed]
- Mehta, A.; Binkley, P.; Gandhi, S.; Ticku, M. Pharmacological effects of Withania somnifera root extract on GABAA receptor complex. Indian J. Med. Res. 1991, 94, 312–315. [Google Scholar] [PubMed]
- Porcher, C.; Hatchett, C.; Longbottom, R.E.; McAinch, K.; Sihra, T.S.; Moss, S.J.; Thomson, A.M.; Jovanovic, J.N. Positive feedback regulation between γ-aminobutyric acid type A (GABAA) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons. J. Biol. Chem. 2011, 286, 21667–21677. [Google Scholar] [CrossRef] [PubMed]









| Gene Name | Primers | Sequence (5′–3′) |
|---|---|---|
| NGF | Forward | ACCTCTTCGGACACTCTGG |
| Reverse | CGTGGCTGTGGTCTTATCTC | |
| BDNF | Forward | CGAGACCAAGTGTAATCCCA |
| Reverse | TCTATCCTTATGAACCGCCA | |
| Trkb | Forward | TCTCATTTTAGGCCGCTTTG |
| Reverse | GGGTTTGAGGTGGGTGAAG | |
| CREB | Forward | TACCCAGGGAGGAGCAATAC |
| Reverse | GAGGCAGCTTGAACAACAAC | |
| Egr-1 | Forward | CCAGTGCCCACCTCTTACTC |
| Reverse | TGCAGACTGGAAGGTGCTG | |
| Bcl-2 | Forward | TTGACGCTCTCCACACACATG |
| Reverse | GGTGGAGGAACTCTTCAGGGA | |
| Bax | Forward | CTGGAAGAAGATGGGCTGAGG |
| Reverse | ACCTGAGGTTTATTGGCACCT | |
| Cox-2 | Forward | GGCACAAATATGATGTTCGC |
| Reverse | CCTCGCTTCTGATCTGTCTTGA | |
| Caspase-3 | Forward | AATTCAAGGGACGGGTCATG |
| Reverse | GCTTGTGCGCGTACAGTTTC | |
| Caspase-9 | Forward | CTGTCCCGTGAAGCAAGGAT |
| Reverse | TGGTACATCGGCAGAGAAGC | |
| GAPDH | Forward | TGCACCACCAACTGCTTAGC |
| Reverse | GGCATGGACTGTGGTCATGAG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravichandran, V.A.; Kim, M.; Han, S.K.; Cha, Y.S. Stachys sieboldii Extract Supplementation Attenuates Memory Deficits by Modulating BDNF-CREB and Its Downstream Molecules, in Animal Models of Memory Impairment. Nutrients 2018, 10, 917. https://doi.org/10.3390/nu10070917
Ravichandran VA, Kim M, Han SK, Cha YS. Stachys sieboldii Extract Supplementation Attenuates Memory Deficits by Modulating BDNF-CREB and Its Downstream Molecules, in Animal Models of Memory Impairment. Nutrients. 2018; 10(7):917. https://doi.org/10.3390/nu10070917
Chicago/Turabian StyleRavichandran, Vijaya Abinaya, Mina Kim, Seong Kyu Han, and Youn Soo Cha. 2018. "Stachys sieboldii Extract Supplementation Attenuates Memory Deficits by Modulating BDNF-CREB and Its Downstream Molecules, in Animal Models of Memory Impairment" Nutrients 10, no. 7: 917. https://doi.org/10.3390/nu10070917
APA StyleRavichandran, V. A., Kim, M., Han, S. K., & Cha, Y. S. (2018). Stachys sieboldii Extract Supplementation Attenuates Memory Deficits by Modulating BDNF-CREB and Its Downstream Molecules, in Animal Models of Memory Impairment. Nutrients, 10(7), 917. https://doi.org/10.3390/nu10070917