Probiotic Enrichment and Reduction of Aflatoxins in a Traditional African Maize-Based Fermented Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients for Probiotic Kwete
2.2. Preparation of Probiotic Kwete
2.3. Inoculation Approaches
2.4. Enumeration of Colony Forming Units
2.5. Determination of Titratable Acidity and pH
2.6. Consumer Acceptability of Probiotic Kwete
2.7. Aflatoxin Analysis
2.7.1. Total Aflatoxin Concentration in Kwete
2.7.2. Aflatoxin B1 Binding to Lactobacillus rhamnosus
3. Results
3.1. Fermentation of Kwete Using the Yoba Starter Culture
3.2. Acceptability of Probiotic Kwete
3.3. Shelf Stability of Probiotic Kwete
3.4. Effect of Inoculation Method
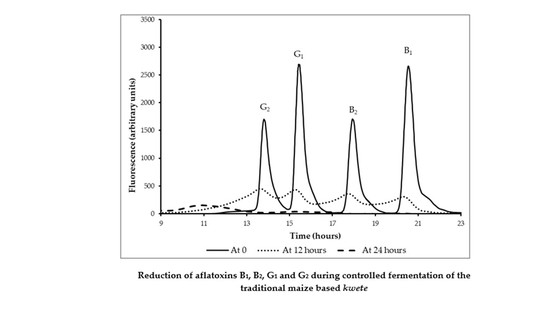
3.5. Reduction Aflatoxins B1, B2, G1, and G2 by Fermentation
3.6. Binding of Aflatoxin B1 by Lactobacillus rhamnosus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mpofu, A.; Linnemann, A.R.; Sybesma, W.; Kort, R.; Nout, M.; Smid, E.J. Development of a locally sustainable functional food based on mutandabota, a traditional food in southern Africa. J. Dairy Sci. 2014, 97, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, J. World Agriculture: Towards 2015/2030: An FAO Perspective; Routledge: London, UK, 2003. [Google Scholar]
- Awika, J.M.; Piironen, V.; Bean, S. Advances in Cereal Science: Implications to Food Processing and Health Promotion; ACS Publications: Washington, DC, USA, 2011. [Google Scholar]
- Kikafunda, J.; Tumwine, J. Diet and socio-economic factors and their association with the nutritional status of pre-school children in a low income suburb of Kampala city, Uganda. East Afr. Med. J. 2006, 83, 565–574. [Google Scholar] [CrossRef]
- Acham, H.; Tumuhimbise, G.A.; Kikafunda, J.K. Simple food group diversity as a proxy indicator for iron and vitamin a status of rural primary school children in Uganda. Food Nutr. Sci. 2013, 4, 1271. [Google Scholar] [CrossRef]
- Mukisa, I. Sensory characteristics, microbial diversity and starter culture development for obushera, a traditional cereal fermented beverage from Uganda. Ph.D. Thesis, Norwegian University of Life Sciences, Aas, Norway, 2012. [Google Scholar]
- Muyanja, C.; Kikafunda, J.; Narvhus, J.; Helgetun, K.; Langsrud, T. Production methods and composition of bushera: A Ugandan traditional fermented cereal beverage. Afr. J. Food Agric. Nutr. Dev. 2003, 3, 10–19. [Google Scholar] [CrossRef]
- Muyanja, C.; Namugumya, B. Traditional processing, microbiological, physiochemical and sensory characteristics of kwete, a ugandan fermented maize based beverage. Afr. J. Food Agric. Nutr. Dev. 2009, 9, 1046–1059. [Google Scholar] [CrossRef]
- Mukisa, I.; Muyanja, C.M.; Byaruhanga, Y.; Langsrud, T.; Narvhus, J. Changes in physico-chemical properties and flavour compounds during fermentation of different obushera (sorghum and millet) beverages. Afr. J. Food Agric. Nutr. Dev. 2012, 12, 6665–6685. [Google Scholar]
- Pratiwi, C.; Rahayu, W.P.; Lioe, H.N.; Herawati, D.; Broto, W.; Ambarwati, S. The effect of temperature and relative humidity for Aspergillus flavus BIO 2237 growth and aflatoxin production on soybeans. Int. Food Res. J. 2015, 22, 82–87. [Google Scholar]
- Makun, H.; Dutton, M.; Njobeh, P.; Gbodi, T.; Ogbadu, G. Aflatoxin contamination in foods and feeds: A special focus on Africa. In Trends in Vital Food and Control Engineering; InTech Open Access Publisher: Rijeka, Croatia, 2012; pp. 187–234. [Google Scholar]
- Najjumba, I.; Bunjo, C.; Kyaddondo, D.; Misinde, C. Improving learning in Aganda volume i: Community-led school feeding practices and issues for consideration; World Bank Publications: Washington, DC, USA, 2013; Volume 1, pp. 1–85. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Outbreak of aflatoxin poisoning--eastern and central provinces, Kenya, January–July 2004. MMWR. Morb. Mortal. Wkly. Rep. 2004, 53, 790–793. [Google Scholar]
- Asiki, G.; Seeley, J.; Srey, C.; Baisley, K.; Lightfoot, T.; Archileo, K.; Agol, D.; Abaasa, A.; Wakeham, K.; Routledge, M.N. A pilot study to evaluate aflatoxin exposure in a rural Ugandan population. Trop. Med. Int. Health 2014, 19, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Kew, M.C. Epidemiology of hepatocellular carcinoma in sub-Saharan Africa. Ann. Hepatol. 2015, 12, 173–182. [Google Scholar]
- Jiang, Y.; Jolly, P.E.; Ellis, W.O.; Wang, J.-S.; Phillips, T.D.; Williams, J.H. Aflatoxin B1 albumin adduct levels and cellular immune status in Ghanaians. Int. Immunol. 2005, 17, 807–814. [Google Scholar] [CrossRef] [PubMed]
- The Foodborne Disease Burden Epidemiology Reference Group (FERG) WHO Initiative to Estimate the Global Burden of Foodborne Diseases. 2008. Available online: https://www.who.int/foodsafety/foodborne_disease/FERG2_report.pdf (accessed on 24 January 2019).
- Gong, Y.Y.; Turner, P.C.; Hall, A.J.; Wild, C.P. Aflatoxin exposure and impaired child growth in West Africa: An unexplored international public health burden. Mycotoxins Detect. Methods, Manage. Publ. Health Agri. Trade 2008, 53–66. [Google Scholar]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for detection of aflatoxins in agricultural food crops. J. Appl. Chem. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Wacoo, P.A. Development of a cysteine based electroless silver biosensor platform for electrochemical detection of aflatoxin B1. Master’s Thesis, Makerere University, Kampala, Uganda, 2016. [Google Scholar]
- Wacoo, P.A.; Ocheng, M.; Wendiro, D.; Vuzi, P.C.; Hawumba, F.J. Development and characterization of an electroless plated silver/cysteine sensor platform for the electrochemical determination of aflatoxin B1. J. Sens. 2016, 2015, 1–8. [Google Scholar] [CrossRef]
- Wacoo, A.P.; Wendiro, D.; Nanyonga, S.; Hawumba, J.F.; Sybesma, W.; Kort, R. Feasibility of a novel on-site detection method for aflatoxin in maize flour from markets and selected households in Kampala, Uganda. Toxins 2018, 10, 327. [Google Scholar]
- East African Community. East African Standard. Milled maize (corn) products—specification. Arusha, Tanzania, 2011; pp. 1–11. Available online: https://law.resource.org/pub/eac/ibr/eas.44.2011.html (accessed on 29 December 2018).
- Assohoun, M.C.; Djeni, T.N.; Koussémon-Camara, M.; Brou, K. Effect of fermentation process on nutritional composition and aflatoxins concentration of doklu, a fermented maize based food. Food Nutr. Sci. 2013, 4, 1120–1127. [Google Scholar]
- Ahlberg, S.H.; Joutsjoki, V.; Korhonen, H.J. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food Microbial. 2015, 207, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.; Chelule, P.; Gqaleni, N. The toxicity and decreased concentration of aflatoxin B1 in natural lactic acid fermented maize meal. J. Appl. Microbiol. 2006, 100, 773–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verheecke, C.; Liboz, T.; Mathieu, F. Microbial degradation of aflatoxin B1: Current status and future advances. Int. J. Food Microbiol. 2016, 237, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gnonlonfin, G.J.B.; Hell, K.; Adjovi, Y.; Fandohan, P.; Koudande, D.; Mensah, G.; Sanni, A.; Brimer, L. A review on aflatoxin contamination and its implications in the developing world: A sub-Saharan African perspective. Crit. Rev. Food Sci. Nutr. 2013, 53, 349–365. [Google Scholar] [CrossRef]
- Allen, S.J.; Martinez, E.G.; Gregorio, G.V.; Dans, L.F. Probiotics for treating acute infectious diarrhoea. Cochrane Database Systematic Rev. 2010. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003048.pub3/full (accessed on 24 January 2019).[Green Version]
- Segers, M.E.; Lebeer, S. In Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb. Cell Factories 2014, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kort, R.; Westerik, N.; Serrano, L.M.; Douillard, F.P.; Gottstein, W.; Mukisa, I.M.; Tuijn, C.J.; Basten, L.; Hafkamp, B.; Meijer, W.C. A novel consortium of Lactobacillus rhamnosus and Streptococcus thermophilus for increased access to functional fermented foods. Microb. Cell Factories 2015, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Kort, R.; Sybesma, W. Probiotics for every body. Trends Biotechnol. 2012, 30, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Zobrist, S.; Donahue, C.; Edick, C.; Mansen, K.; Hassan Zade Nadjari, M.; Heerikhuisen, M.; Sybesma, W.; Molenaar, D.; Diallo, A. Naturally fermented milk from northern senegal: Bacterial community composition and probiotic enrichment with Lactobacillus rhamnosus. Front. Microbiol. 2018, 9, 2218. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, E.; White, J.; Seney, S.; Hekmat, S.; McDowell, T.; Sumarah, M.; Reid, G. A novel millet-based probiotic fermented food for the developing world. Nutrients 2017, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.; Malcata, F.X. Bifidobacterium spp. and Lactobacillus acidophilus: Biological, biochemical, technological and therapeutical properties relevant for use as probiotics. Trends Food Sci. Technol. 1999, 10, 139–157. [Google Scholar] [CrossRef]
- Nyanzi, R.; Jooste, P.J.; Abu, J.O.; Beukes, E.M. Consumer acceptability of a synbiotic version of the maize beverage mageu. Dev. South. Afr. 2010, 27, 447–463. [Google Scholar] [CrossRef]
- Rathore, S.; Salmerón, I.; Pandiella, S.S. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with lactic acid bacteria cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef]
- Westerik, N.; Wacoo, A.P.; Sybesma, W.; Kort, R. Novel production protocol for small-scale manufacture of probiotic fermented foods. JoVE (J. Visual. Exp.) 2016, 115, e54365. [Google Scholar] [CrossRef]
- Garner, D.; Crisosto, C.; Wiley, P.; Crisosto, G. Measurement of pH and titratable acidity. In Quality Evaluation Methodology; Kearney Agricultural Center: Parlier, CA, USA, 2005. [Google Scholar]
- Wichchukit, S.; O’Mahony, M. The 9-point hedonic scale and hedonic ranking in food science: Some reappraisals and alternatives. J. Sci. Food Agric. 2015, 95, 2167–2178. [Google Scholar] [CrossRef]
- Franz, C.M.; Huch, M.; Mathara, J.M.; Abriouel, H.; Benomar, N.; Reid, G.; Galvez, A.; Holzapfel, W.H. African fermented foods and probiotics. Int. J. Food Microbiol. 2014, 190, 84–96. [Google Scholar] [CrossRef] [PubMed]
- De Roos, N.M.; Katan, M.B. Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: A review of papers published between 1988 and 1998. Am. J. Clin. Nutr. 2000, 71, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Drisko, J.A.; Giles, C.K.; Bischoff, B.J. Probiotics in health maintenance and disease prevention. Altern. Med. Rev. 2003, 8, 143–155. [Google Scholar] [PubMed]
- Guandalini, S. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2011, 45, S149–S153. [Google Scholar] [CrossRef]
- Horvath, A.; Dziechciarz, P.; Szajewska, H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment. Pharmacol. Ther. 2011, 33, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Kunene, N.; Hastings, J.; Von Holy, A. Bacterial populations associated with a sorghum-based fermented weaning cereal. Int. J. Food Microbiol. 1999, 49, 75–83. [Google Scholar] [CrossRef]
- Nout, M. Accelerated natural lactic fermentation of cereal-based formulas at reduced water activity. Int. J. Food Microbiol. 1992, 16, 313–322. [Google Scholar] [CrossRef]
- Nout, M.R.; Rombouts, F.; Havelaar, A. Effect of accelerated natural lactic fermentation of infant good ingredients on some pathogenic microorganisms. Int. J. Food Microbiol. 1989, 8, 351–361. [Google Scholar] [CrossRef]
- Steinkraus, K. Handbook of Indigenous Fermented Foods, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Helland, M.H.; Wicklund, T.; Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria, in maize porridge with added malted barley. Int. J. Food Microbiol. 2004, 91, 305–313. [Google Scholar] [CrossRef]
- Byakika, S. Evaluation of sorghum malt as a growth and carrier medium for Lactobacillus plantarum MNC 21 biomass. Master’s Thesis, Makerere University, Kampala, Uganda, 2015. [Google Scholar]
- Sekwati-Monang, B. Microbiological and chemical characterisation of ting, a sorghum-based gluten-free fermented cereal product from Botswana. Ph.D. Thesis, University of Alberta, Edmonton, Canada, 2011. [Google Scholar]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of potentially probiotic lactic acid bacteria on the physicochemical composition and acceptance of fermented cereal beverages. J. Funct. Foods 2015, 15, 106–115. [Google Scholar] [CrossRef]
- Tripathi, M.; Giri, S. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Knorr, D. Technology aspects related to microorganisms in functional foods. Trends Food Sci. Technol. 1998, 9, 295–306. [Google Scholar] [CrossRef]
- Aka, S.; Konan, G.; Fokou, G.; Dje, K.M.; Bonfoh, B. Review on African traditional cereal beverages. Am. J. Res. Commun. 2014, 2, 103–153. [Google Scholar]
- Oluwafemi, F.; Kumar, M.; Bandyopadhyay, R.; Ogunbanwo, T.; Ayanwande, K.B. Bio-detoxification of aflatoxin B1 in artificially contaminated maize grains using lactic acid bacteria. Toxin Rev. 2010, 29, 115–122. [Google Scholar] [CrossRef]
- Pizzolitto, R.P.; Bueno, D.J.; Armando, M.R.; Cavaglieri, L.; Dalcero, A.M.; Salvano, M.A. Binding of aflatoxin B1 to lactic acid bacteria and Saccharomyces cerevisiae in vitro: A useful model to determine the most efficient microorganism. In Aflatoxins-Biochemistry and Molecular Biology; InTech Open Access Publisher: Rijeka, Croatia, 2011; Volume 16, pp. 323–346. [Google Scholar]
- Haskard, C.A.; El-Nezami, H.S.; Kankaanpää, P.E.; Salminen, S.; Ahokas, J.T. Surface binding of aflatoxin B1 by lactic acid bacteria. Appl. Environ. Microbiol. 2001, 67, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- El-Nezami, H.; Kankaanpaa, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef]
- Peltonen, K.; El-Nezami, H.; Haskard, C.; Ahokas, J.; Salminen, S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy Sci. 2001, 84, 2152–2156. [Google Scholar] [CrossRef]
- El-Nezami, H.; Mykkänen, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Ability of Lactobacillus and Propionibacterium strains to remove aflatoxin B1 from the chicken duodenum. J. Food Prot. 2000, 63, 549–552. [Google Scholar] [CrossRef]
- Gratz, S.; Täubel, M.; Juvonen, R.; Viluksela, M.; Turner, P.; Mykkänen, H.; El-Nezami, H. Lactobacillus rhamnosus strain GG modulates intestinal absorption, fecal excretion, and toxicity of aflatoxin B1 in rats. Appl. Environ. Microbiol. 2006, 72, 7398–7400. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Haskard, C.A.; Ouwehand, A.C.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 to cell wall components of Lactobacillus rhamnosus strain GG. Food Addit. Contam. 2004, 21, 158–164. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, A.; Guzman-de-Peña, D.; Garcia, H. Key role of teichoic acids on aflatoxin B1 binding by probiotic bacteria. J. Appl. Microbiol. 2009, 107, 395–403. [Google Scholar] [CrossRef] [PubMed]




| Sample | Acceptability Scores | |||||
|---|---|---|---|---|---|---|
| Appearance | Color | Aroma | Taste | Overall | ||
| kwete | Probiotic | 6.2 ± 1.1 | 6.6 ± 1.9 | 6.7 ± 0.8 | 6.8 ± 1.4 | 6.4 ± 1.7 |
| Commercial brand | 6.8 ± 2.2 | 6.1 ± 1.2 | 6.3 ± 2.1 | 6.5 ± 1.6 | 6.5 ± 2.0 | |
| p-value | >0.05 | >0.05 | >0.05 | <0.05 | >0.05 | |
| Parameter | Time (hours) | Inoculation Methods for kwete | ||
|---|---|---|---|---|
| Prefermented Milk | Dried Starter | Prefermented Maize | ||
| L. rhamnosus (log cfu g−1) | 0 | 6.5 | 6.3 | 5.7 |
| 24 | 8.4 | 8.7 | 8.9 | |
| S. thermophilus (log cfu g−1) | 0 | 6.8 | 7.1 | 7.3 |
| 24 | 9.1 | 7.8 | 7.9 | |
| pH | 0 | 5.6 | 6.3 | 6.2 |
| 24 | 3.9 | 4.2 | 3.9 | |
| Acidity (% acid) | 0 | 0.2 | 0.2 | 0.2 |
| 24 | 0.3 | 0.4 | 0.5 | |
| Experimental Runs | Initial pH | Final pH | Initial Counts * (log cfu g−1) | Final Counts * (log cfu g−1) | Aflatoxin Concentration (ng mL−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | G1 | G2 | Total | ||||||
| Controls | no spike, no starter | 6.3 ± 0.1 | 6.3 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| no spike, starter | 6.3 ± 0.3 | 4.2 ± 0.1 | 6.5 ± 0.2 | 9.4 ± 0.3 | 0 | 0 | 0 | 0 | 0 | |
| spike (t = 0 h), no starter | 6.3 ± 0.0 | 6.1± 0.3 | 0 | 0 | 2.4 ± 0.1 | 1.1 ± 0.0 | 2.4 ± 0.1 | 1.1 ± 0.0 | 7.0 | |
| spike (t = 0 h), lactic acid, no starter | 6.3 ± 0.1 | 4.4 ± 0.2 | 0 | 0 | 2.4 ± 0.1 | 1.1 ± 0.0 | 2.4 ± 0.1 | 1.0 ± 0.5 | 6.9 | |
| spike (t = 24 h), starter | 6.3 ± 0.5 | 3.9 ± 0.2 | 6.2 ± 0.4 | 9.0 ± 0.2 | 2.4 ± 0.3 | 0.9 ± 0.1 | 2.4 ± 0.1 | 1.1 ± 0.0 | 6.8 | |
| Experiment (spike, starter) | t = 0 h | 6.3 ± 0.5 | 6.1 ± 0.4 | 6.1 ± 0.5 | 6.5 ± 0.2 | 2.4 ± 0.2 | 1.2 ± 0.1 | 2.4 ± 0.3 | 0.9 ± 0.1 | 6.9 |
| t = 12 h | 6.3 ± 0.1 | 4.7 ± 0.2 | 6.1 ± 0.5 | 7.5 ± 0.2 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.6 | |
| t = 24 h | 6.3 ± 0.1 | 4.1± 0.1 | 6.3 ± 0.6 | 8.9 ± 0.1 | 0 | 0 | 0 | 0 | 0 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wacoo, A.P.; Mukisa, I.M.; Meeme, R.; Byakika, S.; Wendiro, D.; Sybesma, W.; Kort, R. Probiotic Enrichment and Reduction of Aflatoxins in a Traditional African Maize-Based Fermented Food. Nutrients 2019, 11, 265. https://doi.org/10.3390/nu11020265
Wacoo AP, Mukisa IM, Meeme R, Byakika S, Wendiro D, Sybesma W, Kort R. Probiotic Enrichment and Reduction of Aflatoxins in a Traditional African Maize-Based Fermented Food. Nutrients. 2019; 11(2):265. https://doi.org/10.3390/nu11020265
Chicago/Turabian StyleWacoo, Alex Paul, Ivan Muzira Mukisa, Rehema Meeme, Stellah Byakika, Deborah Wendiro, Wilbert Sybesma, and Remco Kort. 2019. "Probiotic Enrichment and Reduction of Aflatoxins in a Traditional African Maize-Based Fermented Food" Nutrients 11, no. 2: 265. https://doi.org/10.3390/nu11020265
APA StyleWacoo, A. P., Mukisa, I. M., Meeme, R., Byakika, S., Wendiro, D., Sybesma, W., & Kort, R. (2019). Probiotic Enrichment and Reduction of Aflatoxins in a Traditional African Maize-Based Fermented Food. Nutrients, 11(2), 265. https://doi.org/10.3390/nu11020265