Enhancement of Exercise Performance by 48 Hours, and 15-Day Supplementation with Mangiferin and Luteolin in Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. General Procedures
2.3. Exercise Protocol
2.4. Power Output and VO2max
2.5. Maximal Fat Oxidation
2.6. Exercise Efficiency, Supramaximal Exercise O2 Demand, and Oxygen Deficit
2.7. Cerebral and Musculus Vastus Lateralis Oxygenation
2.8. Diet Analysis
2.9. Statistics
3. Results
3.1. Incremental Exercise Test
3.2. Sprint Exercise after Ischemia-Reperfusion
3.3. Wingate Tests
3.4. Final Time Trial
3.5. Quadriceps Muscle O2 Extraction during Ischemia
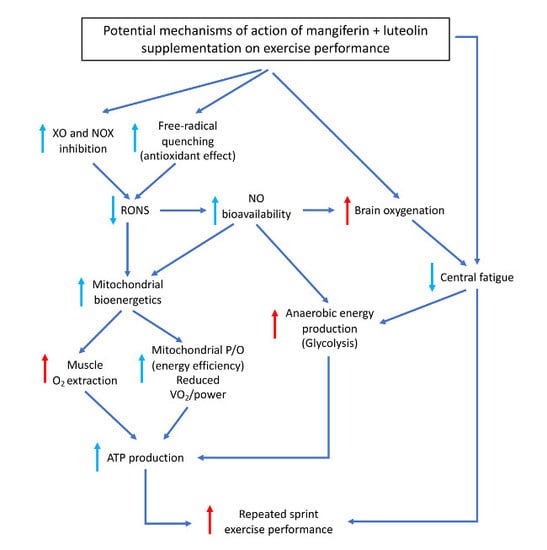
4. Discussion
4.1. A Combination of Mangiferin and Luteolin Botanical Extracts Improves Muscle O2 Extraction
4.2. A Combination of Mangiferin and Luteolin Botanical Extracts Enhances Sprint Performance after Ischemia-Reperfusion
4.3. A Combination of Mangiferin and Luteolin Botanical Extracts Increases Frontal Lobe Oxygenation during Repeated Sprint Exercise
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larsen, F.J.; Schiffer, T.A.; Ortenblad, N.; Zinner, C.; Morales-Alamo, D.; Willis, S.J.; Calbet, J.A.; Holmberg, H.C.; Boushel, R. High-intensity sprint training inhibits mitochondrial respiration through aconitase inactivation. FASEB J. 2016, 30, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Place, N.; Ivarsson, N.; Venckunas, T.; Neyroud, D.; Brazaitis, M.; Cheng, A.J.; Ochala, J.; Kamandulis, S.; Girard, S.; Volungevicius, G.; et al. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc. Natl. Acad. Sci. USA 2015, 112, 15492–15497. [Google Scholar] [CrossRef]
- Romagnoli, M.; Gomez-Cabrera, M.C.; Perrelli, M.G.; Biasi, F.; Pallardo, F.V.; Sastre, J.; Poli, G.; Vina, J. Xanthine oxidase-induced oxidative stress causes activation of nf-kappab and inflammation in the liver of type i diabetic rats. Free Radic. Biol. Med. 2010, 49, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Westerblad, H.; Allen, D.G. Emerging roles of ros/rns in muscle function and fatigue. Antioxid. Redox Signal. 2011, 15, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Debold, E.P. Potential molecular mechanisms underlying muscle fatigue mediated by reactive oxygen and nitrogen species. Front. Physiol. 2015, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B.; Haack, K.E.; Franchek, K.M.; Valberg, P.A.; Kobzik, L.; West, M.S. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J. Appl. Physiol. 1992, 73, 1797–1804. [Google Scholar] [CrossRef]
- Vitiello, D.; Boissiere, J.; Doucende, G.; Gayrard, S.; Polge, A.; Faure, P.; Goux, A.; Tanguy, S.; Obert, P.; Reboul, C.; et al. Beta-adrenergic receptors desensitization is not involved in exercise-induced cardiac fatigue: Nadph oxidase-induced oxidative stress as a new trigger. J. Appl. Physiol. 2011, 111, 1242–1248. [Google Scholar] [CrossRef]
- Aguiar, A.S., Jr.; Boemer, G.; Rial, D.; Cordova, F.M.; Mancini, G.; Walz, R.; de Bem, A.F.; Latini, A.; Leal, R.B.; Pinho, R.A.; et al. High-intensity physical exercise disrupts implicit memory in mice: Involvement of the striatal glutathione antioxidant system and intracellular signaling. Neuroscience 2010, 171, 1216–1227. [Google Scholar] [CrossRef]
- Reid, M.B. Redox interventions to increase exercise performance. J. Physiol. 2016, 594, 5125–5133. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Calbet, J.A. Free radicals and sprint exercise in humans. Free Radic. Res. 2014, 48, 30–42. [Google Scholar] [CrossRef]
- Mason, S.A.; Morrison, D.; McConell, G.K.; Wadley, G.D. Muscle redox signalling pathways in exercise. Role of antioxidants. Free Radic. Biol. Med. 2016, 98, 29–45. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Martin-Rincon, M.; Santana, A.; Perez-Suarez, I.; Dorado, C.; Calbet, J.A.L.; Morales-Alamo, D. Antioxidants facilitate high-intensity exercise il-15 expression in skeletal muscle. Int. J. Sports Med. 2019, 40, 16–22. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Zwergel, C.; Valente, S.; Mai, A. DNA methyltransferases inhibitors from natural sources. Curr. Top. Med. Chem. 2016, 16, 680–696. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Medina-Remon, A.; Tresserra-Rimbau, A.; Pons, A.; Tur, J.A.; Martorell, M.; Ros, E.; Buil-Cosiales, P.; Sacanella, E.; Covas, M.I.; Corella, D.; et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The predimed randomized trial. Nutr. Metab. Cardiovasc. Dis. 2014, 25, 60–67. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef]
- Luczkiewicz, P.; Kokotkiewicz, A.; Dampc, A.; Luczkiewicz, M. Mangiferin: A promising therapeutic agent for rheumatoid arthritis treatment. Med. Hypotheses 2014, 83, 570–574. [Google Scholar] [CrossRef]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef]
- Menendez, J.A.; Joven, J.; Aragones, G.; Barrajon-Catalan, E.; Beltran-Debon, R.; Borras-Linares, I.; Camps, J.; Corominas-Faja, B.; Cufi, S.; Fernandez-Arroyo, S.; et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: A new family of gerosuppressant agents. Cell Cycle 2013, 12, 555–578. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, Z.Y. Tea and cancer. J. Natl. Cancer Inst. 1993, 85, 1038–1049. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef]
- Pinto, M.M.; Sousa, M.E.; Nascimento, M.S. Xanthone derivatives: New insights in biological activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Chang, C.J.; Lin, C.S.; Lu, C.C.; Ko, Y.F.; Tseng, S.F.; Lai, H.C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160. [Google Scholar] [CrossRef]
- Trebaticka, J.; Durackova, Z. Psychiatric disorders and polyphenols: Can they be helpful in therapy? Oxid. Med. Cell. Longev. 2015, 2015, 248529. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Nguyen, T.T. Natural mood foods: The actions of polyphenols against psychiatric and cognitive disorders. Nutr. Neurosci. 2012, 15, 127–133. [Google Scholar] [CrossRef]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Gericke, N.; Perez-Valera, M.; Curtelin, D.; Galvan-Alvarez, V.; Lopez-Rios, L.; Morales-Alamo, D.; Calbet, J.A.L. Mangifera indica l. Leaf extract in combination with luteolin or quercetin enhances vo2peak and peak power output, and preserves skeletal muscle function during ischemia-reperfusion in humans. Front. Physiol. 2018, 9, 740. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Hopkins, W.G. Impact of dietary antioxidants on sport performance: A review. Sports Med. 2015, 45, 939–955. [Google Scholar] [CrossRef]
- Myburgh, K.H. Polyphenol supplementation: Benefits for exercise performance or oxidative stress? Sports Med. 2014, 44 (Suppl. 1), S57–S70. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar]
- Morales-Alamo, D.; Calbet, J.A. Ampk signaling in skeletal muscle during exercise: Role of reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2016, 98, 68–77. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Ponce-Gonzalez, J.G.; Guadalupe-Grau, A.; Rodriguez-Garcia, L.; Santana, A.; Cusso, R.; Guerrero, M.; Dorado, C.; Guerra, B.; Calbet, J.A. Critical role for free radicals on sprint exercise-induced camkii and ampkalpha phosphorylation in human skeletal muscle. J. Appl. Physiol. 2013, 114, 566–577. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Hopkins, W.G.; Lowe, T.E. Effects of dietary antioxidants on training and performance in female runners. Eur. J. Sport Sci. 2014, 14, 160–168. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Kerksick, C.M.; Lamprecht, M.; McAnulty, S.R. Does vitamin c and e supplementation impair the favorable adaptations of regular exercise? Oxid. Med. Cell. Longev. 2012, 2012, 707941. [Google Scholar] [CrossRef]
- Ryan, M.J.; Jackson, J.R.; Hao, Y.; Leonard, S.S.; Alway, S.E. Inhibition of xanthine oxidase reduces oxidative stress and improves skeletal muscle function in response to electrically stimulated isometric contractions in aged mice. Free Radic. Biol. Med. 2011, 51, 38–52. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Pareja-Galeano, H.; Gomez-Cabrera, M.C.; Candel, J.; Lippi, G.; Salvagno, G.L.; Mann, G.E.; Vina, J. Allopurinol prevents cardiac and skeletal muscle damage in professional soccer players. Scand. J. Med. Sci. Sports 2015, 25, e110–e115. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Pallardo, F.V.; Sastre, J.; Vina, J.; Garcia-del-Moral, L. Allopurinol and markers of muscle damage among participants in the tour de france. JAMA 2003, 289, 2503–2504. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, J.; Liu, H.Y.; Gao, L.H.; Feng, G.H.; Liu, X.; Li, L. Hypouricaemic action of mangiferin results from metabolite norathyriol via inhibiting xanthine oxidase activity. Pharm. Biol. 2016, 54, 1680–1686. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Jeffery, S.; Saw, C.L.; Shu, L.; Su, Z.Y.; Kong, A.N. Induction of nrf2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug Dispos. 2015, 36, 440–451. [Google Scholar] [CrossRef]
- Makino, J.; Nakanishi, R.; Kamiya, T.; Hara, H.; Ninomiya, M.; Koketsu, M.; Adachi, T. Luteolin suppresses the differentiation of thp-1 cells through the inhibition of nox2 mrna expression and the membrane translocation of p47phox. J. Nat. Prod. 2013, 76, 1285–1290. [Google Scholar] [CrossRef]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin protects huvecs from tnf-alpha-induced oxidative stress and inflammation via its effects on the nox4/ros-nf-kappab and mapk pathways. J. Atheroscler. Thromb. 2014, 21, 768–783. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Roy, A.; Sil, P.C. Mangiferin exerts hepatoprotective activity against d-galactosamine induced acute toxicity and oxidative/nitrosative stress via nrf2-nfkappab pathways. Toxicol. Appl. Pharmacol. 2012, 260, 35–47. [Google Scholar] [CrossRef]
- Andreu, G.P.; Delgado, R.; Velho, J.A.; Curti, C.; Vercesi, A.E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur. J. Pharmacol. 2005, 513, 47–55. [Google Scholar] [CrossRef]
- Cooper-Mullin, C.; McWilliams, S.R. The role of the antioxidant system during intense endurance exercise: Lessons from migrating birds. J. Exp. Biol. 2016, 219, 3684–3695. [Google Scholar] [CrossRef]
- Hou, S.; Wang, F.; Li, Y.; Li, Y.; Wang, M.; Sun, D.; Sun, C. Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chem. 2012, 132, 289–294. [Google Scholar] [CrossRef]
- Wittemer, S.M.; Ploch, M.; Windeck, T.; Muller, S.C.; Drewelow, B.; Derendorf, H.; Veit, M. Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of artichoke leaf extracts in humans. Phytomedicine 2005, 12, 28–38. [Google Scholar] [CrossRef]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of tnf and IL-6. Transl. Psychiatry 2015, 5, e647. [Google Scholar] [CrossRef]
- Curtelin, D.; Morales-Alamo, D.; Torres-Peralta, R.; Rasmussen, P.; Martin-Rincon, M.; Perez-Valera, M.; Siebenmann, C.; Perez-Suarez, I.; Cherouveim, E.; Sheel, A.W.; et al. Cerebral blood flow, frontal lobe oxygenation and intra-arterial blood pressure during sprint exercise in normoxia and severe acute hypoxia in humans. J. Cereb. Blood Flow Metab. 2018, 38, 136–150. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Losa-Reyna, J.; Torres-Peralta, R.; Martin-Rincon, M.; Perez-Valera, M.; Curtelin, D.; Ponce-Gonzalez, J.G.; Santana, A.; Calbet, J.A. What limits performance during whole-body incremental exercise to exhaustion in humans? J. Physiol. 2015, 593, 4631–4648. [Google Scholar] [CrossRef]
- Achten, J.; Gleeson, M.; Jeukendrup, A.E. Determination of the exercise intensity that elicits maximal fat oxidation. Med. Sci. Sports Exerc. 2002, 34, 92–97. [Google Scholar] [CrossRef]
- Ponce-Gonzalez, J.G.; Rodriguez-Garcia, L.; Losa-Reyna, J.; Guadalupe-Grau, A.; Rodriguez-Gonzalez, F.G.; Diaz-Chico, B.N.; Dorado, C.; Serrano-Sanchez, J.A.; Calbet, J.A. Androgen receptor gene polymorphisms influence fat accumulation: A longitudinal study from adolescence to adult age. Scand. J. Med. Sci. Sports 2016, 26, 1313–1320. [Google Scholar] [CrossRef]
- Dorado, C.; Sanchis-Moysi, J.; Calbet, J.A. Effects of recovery mode on performance, O2 uptake, and O2 deficit during high-intensity intermittent exercise. Can. J. Appl. Physiol. 2004, 29, 227–244. [Google Scholar] [CrossRef]
- Calbet, J.A.; Chavarren, J.; Dorado, C. Fractional use of anaerobic capacity during a 30- and a 45-s wingate test. Eur. J. Appl. Physiol. 1997, 76, 308–313. [Google Scholar] [CrossRef]
- Chavarren, J.; Calbet, J.A. Cycling efficiency and pedalling frequency in road cyclists. Eur. J. Appl. Physiol. 1999, 80, 555–563. [Google Scholar] [CrossRef]
- Van der Zee, P.; Cope, M.; Arridge, S.R.; Essenpreis, M.; Potter, L.A.; Edwards, A.D.; Wyatt, J.S.; McCormick, D.C.; Roth, S.C.; Reynolds, E.O.; et al. Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv. Exp. Med. Biol. 1992, 316, 143–153. [Google Scholar]
- Gregory, A.J.; Hatem, M.A.; Yee, K.; Grocott, H.P. Optimal placement of cerebral oximeter monitors to avoid the frontal sinus as determined by computed tomography. J. Cardiothorac. Vasc. Anesth. 2016, 30, 127–133. [Google Scholar] [CrossRef]
- Rasmussen, P.; Nielsen, J.; Overgaard, M.; Krogh-Madsen, R.; Gjedde, A.; Secher, N.H.; Petersen, N.C. Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J. Physiol. 2010, 588, 1985–1995. [Google Scholar] [CrossRef]
- Santos-Concejero, J.; Billaut, F.; Grobler, L.; Olivan, J.; Noakes, T.D.; Tucker, R. Brain oxygenation declines in elite kenyan runners during a maximal interval training session. Eur. J. Appl. Physiol. 2017, 117, 1017–1024. [Google Scholar] [CrossRef]
- Ortega, R.M.; Andres, P.; Lopez-Sobaler, A.M.; Rodriguez-Rodriguez, E.; Aparicio, A.; Bermejo, L.M.; Garcia-Gonzalez, L.; Basabe, B. Changes in thiamin intake and blood levels in young, overweight/obese women following hypocaloric diets based on the increased relative consumption of cereals or vegetables. Eur. J. Clin. Nutr. 2007, 61, 77–82. [Google Scholar] [CrossRef]
- Amann, M.; Calbet, J.A. Convective oxygen transport and fatigue. J. Appl. Physiol. 2008, 104, 861–870. [Google Scholar] [CrossRef]
- Lundby, C.; Robach, P.; Boushel, R.; Thomsen, J.J.; Rasmussen, P.; Koskolou, M.; Calbet, J.A. Does recombinant human epo increase exercise capacity by means other than augmenting oxygen transport? J. Appl. Physiol. 2008, 105, 581–587. [Google Scholar] [CrossRef]
- Thomsen, J.J.; Rentsch, R.L.; Robach, P.; Calbet, J.A.L.; Boushel, R.; Rasmussen, P.; Juel, C.; Lundby, C. Prolonged administration of recombinant human erythropoietin increases submaximal performance more than maximal aerobic capacity. Eur. J. Appl. Physiol. 2007, 101, 481–486. [Google Scholar] [CrossRef]
- Calbet, J.A.; Losa-Reyna, J.; Torres-Peralta, R.; Rasmussen, P.; Ponce-Gonzalez, J.G.; Sheel, A.W.; de la Calle-Herrero, J.; Guadalupe-Grau, A.; Morales-Alamo, D.; Fuentes, T.; et al. Limitations to oxygen transport and utilization during sprint exercise in humans: Evidence for a functional reserve in muscle O2 diffusing capacity. J. Physiol. 2015, 593, 4649–4664. [Google Scholar] [CrossRef]
- Parolin, M.L.; Chesley, A.; Matsos, M.P.; Spriet, L.L.; Jones, N.L.; Heigenhauser, G.J. Regulation of skeletal muscle glycogen phosphorylase and pdh during maximal intermittent exercise. Am. J. Physiol. 1999, 277, E890–E900. [Google Scholar] [CrossRef]
- Gaitanos, G.C.; Williams, C.; Boobis, L.H.; Brooks, S. Human muscle metabolism during intermittent maximal exercise. J. Appl. Physiol. (1985) 1993, 75, 712–719. [Google Scholar] [CrossRef]
- Bogdanis, G.C.; Nevill, M.E.; Boobis, L.H.; Lakomy, H.K. Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J. Appl. Physiol. 1996, 80, 876–884. [Google Scholar] [CrossRef]
- Zinner, C.; Morales-Alamo, D.; Ortenblad, N.; Larsen, F.J.; Schiffer, T.A.; Willis, S.J.; Gelabert-Rebato, M.; Perez-Valera, M.; Boushel, R.; Calbet, J.A.; et al. The physiological mechanisms of performance enhancement with sprint interval training differ between the upper and lower extremities in humans. Front. Physiol. 2016, 7, 426. [Google Scholar] [CrossRef]
- Calbet, J.A.; Lundby, C.; Sander, M.; Robach, P.; Saltin, B.; Boushel, R. Effects of atp-induced leg vasodilation on vo2peak and leg O2 extraction during maximal exercise in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R447–R453. [Google Scholar] [CrossRef]
- Calbet, J.A.; Holmberg, H.C.; Rosdahl, H.; van Hall, G.; Jensen-Urstad, M.; Saltin, B. Why do arms extract less oxygen than legs during exercise? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1448–R1458. [Google Scholar] [CrossRef]
- Liu, Z.; Apontes, P.; Fomenko, E.V.; Chi, N.; Schuster, V.L.; Kurland, I.J.; Pessin, J.E.; Chi, Y. Mangiferin accelerates glycolysis and enhances mitochondrial bioenergetics. Int. J. Mol. Sci. 2018, 19, 201. [Google Scholar] [CrossRef]
- Ryan, T.E.; Schmidt, C.A.; Alleman, R.J.; Tsang, A.M.; Green, T.D.; Neufer, P.D.; Brown, D.A.; McClung, J.M. Mitochondrial therapy improves limb perfusion and myopathy following hindlimb ischemia. J. Mol. Cell. Cardiol. 2016, 97, 191–196. [Google Scholar] [CrossRef]
- Krustrup, P.; Gonzalez-Alonso, J.; Quistorff, B.; Bangsbo, J. Muscle heat production and anaerobic energy turnover during repeated intense dynamic exercise in humans. J. Physiol. 2001, 536, 947–956. [Google Scholar] [CrossRef]
- Heinonen, I.H.; Kemppainen, J.; Kaskinoro, K.; Peltonen, J.E.; Borra, R.; Lindroos, M.; Oikonen, V.; Nuutila, P.; Knuuti, J.; Boushel, R.; et al. Regulation of human skeletal muscle perfusion and its heterogeneity during exercise in moderate hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R72–R79. [Google Scholar] [CrossRef]
- Karakas, B.R.; Davran, F.; Elpek, G.O.; Akbas, S.H.; Gulkesen, K.H.; Bulbuller, N. The effects of luteolin on the intestinal ischemia/reperfusion injury in mice. J. Investig. Surg. 2014, 27, 249–255. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, B.; Li, Y.; Zhang, H. Protective effect of luteolin against renal ischemia/reperfusion injury via modulation of pro-inflammatory cytokines, oxidative stress and apoptosis for possible benefit in kidney transplant. Med. Sci. Monit. 2017, 23, 5720–5727. [Google Scholar] [CrossRef]
- Du, Y.; Liu, P.; Xu, T.; Pan, D.; Zhu, H.; Zhai, N.; Zhang, Y.; Li, D. Luteolin modulates serca2a leading to attenuation of myocardial ischemia/reperfusion injury via sumoylation at lysine 585 in mice. Cell. Physiol. Biochem. 2018, 45, 883–898. [Google Scholar] [CrossRef]
- Hong, X.; Zhao, X.; Wang, G.; Zhang, Z.; Pei, H.; Liu, Z. Luteolin treatment protects against renal ischemia-reperfusion injury in rats. Mediat. Inflamm. 2017, 2017, 9783893. [Google Scholar] [CrossRef]
- Wei, B.; Lin, Q.; Ji, Y.G.; Zhao, Y.C.; Ding, L.N.; Zhou, W.J.; Zhang, L.H.; Gao, C.Y.; Zhao, W. Luteolin ameliorates rat myocardial ischaemia-reperfusion injury through activation of peroxiredoxin II. Br. J. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Kumari, S.; Ojha, S.; Arya, D.S. Protective effect of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: Role of age-rage/mapk pathways. Sci. Rep. 2017, 7, 42027. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Li, C.; Jackson, R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol. Cell Physiol. 2002, 282, C227–C241. [Google Scholar] [CrossRef]
- Hendgen-Cotta, U.B.; Merx, M.W.; Shiva, S.; Schmitz, J.; Becher, S.; Klare, J.P.; Steinhoff, H.J.; Goedecke, A.; Schrader, J.; Gladwin, M.T.; et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2008, 105, 10256–10261. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Kim-Shapiro, D.B. The functional nitrite reductase activity of the heme-globins. Blood 2008, 112, 2636–2647. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- Pacher, P.; Nivorozhkin, A.; Szabo, C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol. Rev. 2006, 58, 87–114. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Ponce-Gonzalez, J.G.; Guadalupe-Grau, A.; Rodriguez-Garcia, L.; Santana, A.; Cusso, M.R.; Guerrero, M.; Guerra, B.; Dorado, C.; Calbet, J.A. Increased oxidative stress and anaerobic energy release, but blunted thr172-ampkalpha phosphorylation, in response to sprint exercise in severe acute hypoxia in humans. J. Appl. Physiol. (1985) 2012, 113, 917–928. [Google Scholar] [CrossRef]
- Shiva, S.; Huang, Z.; Grubina, R.; Sun, J.; Ringwood, L.A.; MacArthur, P.H.; Xu, X.; Murphy, E.; Darley-Usmar, V.M.; Gladwin, M.T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007, 100, 654–661. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Guerra, B.; Ponce-Gonzalez, J.G.; Guadalupe-Grau, A.; Santana, A.; Martin-Rincon, M.; Gelabert-Rebato, M.; Cadefau, J.A.; Cusso, R.; Dorado, C.; et al. Skeletal muscle signaling, metabolism, and performance during sprint exercise in severe acute hypoxia after the ingestion of antioxidants. J. Appl. Physiol. (1985) 2017, 123, 1235–1245. [Google Scholar] [CrossRef]
- Rossen, R.; Kabat, H.; Anderson, J.P. Acute arrest of cerebral circulation in man. Arch. Neurol. Psychiatry 1943, 50, 510–528. [Google Scholar]
- Radak, Z.; Suzuki, K.; Higuchi, M.; Balogh, L.; Boldogh, I.; Koltai, E. Physical exercise, reactive oxygen species and neuroprotection. Free Radic. Biol. Med. 2016, 98, 187–196. [Google Scholar] [CrossRef]
- Racinais, S.; Wilson, M.G.; Gaoua, N.; Periard, J.D. Heat acclimation has a protective effect on the central but not peripheral nervous system. J. Appl. Physiol. (1985) 2017, 123, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Labelle, V.; Bosquet, L.; Mekary, S.; Bherer, L. Decline in executive control during acute bouts of exercise as a function of exercise intensity and fitness level. Brain Cogn. 2013, 81, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Billaut, F. Influence of cerebral and muscle oxygenation on repeated-sprint ability. Eur. J. Appl. Physiol. 2010, 109, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Torres-Peralta, R.; Losa-Reyna, J.; Morales-Alamo, D.; Gonzalez-Izal, M.; Perez-Suarez, I.; Ponce-Gonzalez, J.G.; Izquierdo, M.; Calbet, J.A. Increased pio2 at exhaustion in hypoxia enhances muscle activation and swiftly relieves fatigue: A placebo or a pio2 dependent effect? Front. Physiol. 2016, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Billaut, F. Tissue oxygenation in men and women during repeated-sprint exercise. Int. J. Sports Physiol. Perform. 2012, 7, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Hou, F.; Wang, X.; Liu, B. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/nlrp3 inflammasome activation with regulation of ampk in endothelial cells. Metabolism 2015, 64, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Kety, S.S.; Schmidt, C.F. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J. Clin. Investig. 1948, 27, 484–492. [Google Scholar] [CrossRef]
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Benvenuti, L.; Duranti, E.; Masi, S.; Carpi, S.; Nieri, P.; Nericcio, A.; et al. Luteolin prevents cardiometabolic alterations and vascular dysfunction in mice with hfd-induced obesity. Front. Pharmacol. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Greer, F.; McLean, C.; Graham, T.E. Caffeine, performance, and metabolism during repeated wingate exercise tests. J. Appl. Physiol. 1998, 85, 1502–1508. [Google Scholar] [CrossRef]
- Paton, C.D.; Hopkins, W.G. Tests of cycling performance. Sports Med. 2001, 31, 489–496. [Google Scholar] [CrossRef]
- Thompson, C.; Vanhatalo, A.; Jell, H.; Fulford, J.; Carter, J.; Nyman, L.; Bailey, S.J.; Jones, A.M. Dietary nitrate supplementation improves sprint and high-intensity intermittent running performance. Nitric Oxide 2016, 61, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef] [PubMed]





| (a) | |||||||||||||||||
| Placebo (48 h) | Placebo (15 Days) | MA + Luteolin (48 h) | MA + Luteolin (15 Days) | Treatment | Pre-Post | T × t | T × t × d | ||||||||||
| Delta Efficiency (%) | L | 27.0 | ± | 2.5 | 29.2 | ± | 4.6 | 30.0 | ± | 2.1 | 27.2 | ± | 1.9 | 0.74 | 0.73 | 0.46 | <0.001 |
| H | 28.9 | ± | 2.1 | 28.0 | ± | 1.9 | 27.1 | ± | 2.1 | 29.7 | ± | 3.4 | |||||
| MFO (mg/min) | L | 392.2 | ± | 40.0 | 347.3 | ± | 53.6 | 393.7 | ± | 100.9 | 370.9 | ± | 52.9 | 0.81 | 0.35 | 0.50 | 0.84 |
| H | 399.8 | ± | 129.0 | 367.3 | ± | 107.4 | 377.7 | ± | 143.6 | 385.8 | ± | 178.4 | |||||
| MFO VO2 (mL/min) | L | 1377 | ± | 282 | 1260 | ± | 136 | 1260 | ± | 173 | 1313 | ± | 205 | 0.58 | 0.17 | 0.75 | 0.33 |
| H | 1455 | ± | 406 | 1389 | ± | 301 | 1478 | ± | 385 | 1387 | ± | 455 | |||||
| Wmax (W) | L | 277 | ± | 30 | 282 | ± | 25 | 288 | ± | 25 | 271 | ± | 24 | 0.87 | 0.11 | 0.16 | 0.03 |
| H | 291 | ± | 48 | 286 | ± | 42 | 291 | ± | 48 | 291 | ± | 47 | |||||
| HRmax (beats/min) | L | 192 | ± | 8 | 187 | ± | 14 | 187 | ± | 12 | 192 | ± | 8 | 0.20 | 0.33 | 0.13 | 0.08 |
| H | 193 | ± | 8 | 189 | ± | 10 | 198 | ± | 10 | 194 | ± | 12 | |||||
| VO2max (mL/min) | L | 3568 | ± | 513 | 3660 | ± | 318 | 3649 | ± | 387 | 3623 | ± | 240 | 0.87 | 0.11 | 0.16 | 0.026 |
| H | 3821 | ± | 456 | 3742 | ± | 566 | 3770 | ± | 590 | 3681 | ± | 567 | |||||
| RERmax | L | 1.17 | ± | 0.09 | 1.16 | ± | 0.05 | 1.18 | ± | 0.06 | 1.14 | ± | 0.03 | 0.2 | 0.33 | 0.13 | 0.08 |
| H | 1.11 | ± | 0.03 | 1.14 | ± | 0.04 | 1.13 | ± | 0.07 | 1.12 | ± | 0.05 | |||||
| VEmax (L/min) | L | 148 | ± | 35 | 161 | ± | 24 | 153 | ± | 27 | 167 | ± | 38 | 0.78 | 0.61 | 0.47 | 0.54 |
| H | 161 | ± | 21 | 167 | ± | 25 | 164 | ± | 20 | 160 | ± | 17 | |||||
| BFmax (breaths/min) | L | 56 | ± | 13 | 63 | ± | 11 | 60 | ± | 10 | 64 | ± | 15 | 0.90 | 0.67 | 0.026 | 0.86 |
| H | 62 | ± | 9 | 64 | ± | 11 | 63 | ± | 8 | 64 | ± | 8 | |||||
| PETCO2 (mmHg) | L | 37.1 | ± | 2.9 | 33.3 | ± | 4.8 | 37.3 | ± | 3.3 | 34.2 | ± | 3.5 | 0.69 | 0.07 | 0.63 | 0.57 |
| H | 33.5 | ± | 2.6 | 31.8 | ± | 4.6 | 32.8 | ± | 2.7 | 33.1 | ± | 2.2 | |||||
| PETO2 (mmHg) | L | 117 | ± | 5 | 119 | ± | 4 | 117 | ± | 4 | 120 | ± | 6 | 0.47 | 0.08 | 0.61 | 0.91 |
| H | 119 | ± | 3 | 119 | ± | 3 | 119 | ± | 3 | 118 | ± | 2 | |||||
| (b) | |||||||||||||||||
| Placebo (48 h) | Placebo (15 days) | MA + Luteolin (48 h) | MA + Luteolin (15 days) | Treatment | Pre-Post | T × t | T × t × d | ||||||||||
| Lact at 100 W (mM) | L | 1.9 | ± | 0.5 | 1.8 | ± | 0.5 | 1.7 | ± | 0.3 | 1.9 | ± | 0.4 | 0.55 | 0.94 | 0.41 | 0.92 |
| H | 2.1 | ± | 1.1 | 2.1 | ± | 1.3 | 2.1 | ± | 1.0 | 2.1 | ± | 1.6 | |||||
| Lact at 200 W (mM) | L | 5.8 | ± | 2.6 | 6.0 | ± | 1.6 | 5.2 | ± | 1.2 | 5.7 | ± | 0.5 | 0.11 | 0.59 | 0.44 | 0.69 |
| H | 6.4 | ± | 3.9 | 5.9 | ± | 3.2 | 5.0 | ± | 1.9 | 5.4 | ± | 3.7 | |||||
| LT 4 mM (W) | L | 177 | ± | 29 | 173 | ± | 26 | 181 | ± | 19 | 170 | ± | 8 | 0.78 | 0.40 | 0.84 | 0.39 |
| H | 180 | ± | 58 | 177 | ± | 63 | 181 | ± | 48 | 182 | ± | 68 | |||||
| Lact Peak Post-Ischemia (mM) | L | 9.1 | ± | 2.2 | 10.2 | ± | 1.5 | 8.6 | ± | 2.4 | 11.2 | ± | 1.2 | 0.53 | 0.02 | 0.29 | 0.88 |
| H | 10.5 | ± | 3.2 | 10.6 | ± | 2.7 | 10.4 | ± | 2.4 | 11.7 | ± | 2.3 | |||||
| RPE (post Incremental exercise) | L | 7.5 | ± | 0.6 | 7.8 | ± | 1.0 | 6.8 | ± | 2.2 | 7.8 | ± | 1.9 | 0.89 | 0.12 | 0.60 | 0.88 |
| H | 7.3 | ± | 1.6 | 7.3 | ± | 2.3 | 7.7 | ± | 1.5 | 8.1 | ± | 0.5 | |||||
| Time trial total work (kJ) | L | 81.7 | ± | 54.9 | 124.5 | ± | 73.6 | 96.1 | ± | 48.2 | 124.1 | ± | 74.3 | 0.78 | 0.07 | 0.99 | 0.60 |
| H | 94.5 | ± | 63.8 | 118.8 | ± | 71.7 | 88.5 | ± | 83.5 | 126.5 | ± | 100.0 | |||||
| First 15 s Sprint | Second 15 s Sprint | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (48 h) | Placebo (15 Days) | MA + Luteolin (48 h) | MA + Luteolin (15 Days) | Placebo (48 h) | Placebo (15 Days) | MA + Luteolin (48 h) | MA + Luteolin (15 Days) | Sprint | Treat | Pre-Post | Sprint × Treat | ||||||||||||||||||
| HR (beats/min) | L | 170 | ± | 12 | 171 | ± | 13 | 168 | ± | 12 | 170 | ± | 13 | 168 | ± | 12 | 172 | ± | 11 | 165 | ± | 11 | 170 | ± | 14 | 0.36 | 0.79 | 0.011 | 0.74 |
| H | 180 | ± | 15 | 182 | ± | 10 | 180 | ± | 14 | 184 | ± | 13 * | 178 | ± | 16 | 182 | ± | 9 | 177 | ± | 14 | 186 | ± | 14 * | |||||
| VO2 (mL) | L | 530 | ± | 121 | 542 | ± | 101 | 515 | ± | 105 | 554 | ± | 127 | 728 | ± | 160 | 749 | ± | 98 | 655 | ± | 76 | 729 | ± | 94 | <0.001 | 0.010 | 0.038 | 0.99 |
| H | 585 | ± | 85 | 577 | ± | 93 | 461 | ± | 124 | 553 | ± | 69 | 823 | ± | 112 | 799 | ± | 131 | 740 | ± | 85 | 821 | ± | 120 | |||||
| O2 Deficit (mL) | L | 164 | ± | 163 | 219 | ± | 233 | 130 | ± | 70 | 195 | ± | 74 | 28 | ± | 158 | 51 | ± | 136 | 38 | ± | 119 | 41 | ± | 121 | <0.001 | 0.19 | 0.42 | 0.94 |
| H | 306 | ± | 87 | 338 | ± | 106 | 399 | ± | 175 | 466 | ± | 58 | 129 | ± | 142 | 139 | ± | 90 | 232 | ± | 249 | 184 | ± | 53 | |||||
| VE (L/min) | L | 95 | ± | 36 | 102 | ± | 39 | 101 | ± | 25 | 107 | ± | 42 | 121 | ± | 39 | 123 | ± | 35 | 115 | ± | 32 | 126 | ± | 49 | <0.001 | 0.74 | 0.025 | 0.74 |
| H | 119 | ± | 32 | 122 | ± | 18 | 104 | ± | 24 | 119 | ± | 18 | 138 | ± | 23 | 150 | ± | 21 | 134 | ± | 13 | 155 | ± | 11 | |||||
| BF (breaths/min) | 48 | ± | 13 | 50 | ± | 14 | 49 | ± | 8 | 42 | ± | 9 | 53 | ± | 13 | 55 | ± | 13 | 53 | ± | 11 | 49 | ± | 11 | 0.029 | 0.61 | 0.52 | 0.72 | |
| 52 | ± | 11 | 58 | ± | 11 | 55 | ± | 12 | 55 | ± | 13 | 57 | ± | 11 | 61 | ± | 7 * | 59 | ± | 10 | 62 | ± | 8 | ||||||
| PETCO2 (mmHg) | L | 29 | ± | 3 | 30 | ± | 7 | 28 | ± | 4 | 29 | ± | 8 | 31 | ± | 6 | 24 | ± | 10 | 30 | ± | 5 | 28 | ± | 9 | 0.77 | 0.91 | 0.046 | 0.25 |
| H | 27 | ± | 5 | 25 | ± | 6 | 25 | ± | 4 | 25 | ± | 5 | 29 | ± | 3 | 26 | ± | 4 * | 27 | ± | 4 | 27 | ± | 3 | |||||
| PETO2 (mmHg) | L | 119 | ± | 4 | 118 | ± | 7 | 121 | ± | 4 | 119 | ± | 8 | 116 | ± | 6 | 122 | ± | 8 | 116 | ± | 5 | 117 | ± | 10 | 0.057 | 0.72 | 0.101 | 0.178 |
| H | 121 | ± | 4 | 122 | ± | 6 | 124 | ± | 5 | 123 | ± | 6 | 118 | ± | 3 | 120 | ± | 3 | 120 | ± | 4 | 120 | ± | 4 | |||||
| First 30 s Sprint | Second 30 s Sprint | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (48 h) | Placebo (15 Days) | MA + Luteolin (48 h) | MA + Luteolin (15 Days) | Placebo (48 h) | Placebo (15 Days) | MA + Luteolin (48 h) | MA+ Luteolin (15 Days) | Sprint | Treat | Pre-Post | Sprint × Treat | ||||||||||||||||||
| HR (beats/min) | L | 164 | ± | 6 | 164 | ± | 7 | 161 | ± | 2 | 162 | ± | 6 | 165 | ± | 6 | 165 | ± | 7 | 164 | ± | 4 | 166 | ± | 8 | 0.011 | 0.92 | 0.62 | 0.058 |
| H | 169 | ± | 10 | 170 | ± | 9 | 170 | ± | 9 | 171 | ± | 13 | 171 | ± | 8 | 171 | ± | 9 | 172 | ± | 8 | 172 | ± | 10 | |||||
| VO2 (mL) | L | 1321 | ± | 238 | 1292 | ± | 239 | 1182 | ± | 181 | 1337 | ± | 240 | 1393 | ± | 196 | 1392 | ± | 293 | 1379 | ± | 183 | 1442 | ± | 203 | <0.001 | 0.59 | 0.58 | 0.27 |
| H | 1230 | ± | 246 | 1287 | ± | 143 | 1264 | ± | 209 | 1226 | ± | 181 | 1435 | ± | 234 | 1414 | ± | 202 | 1428 | ± | 257 | 1377 | ± | 177 | |||||
| O2 Deficit (mL) | L | 1566 | ± | 307 | 1415 | ± | 275 | 1573 | ± | 358 | 1421 | ± | 348 | 1212 | ± | 332 | 1091 | ± | 269 | 1141 | ± | 256 | 1167 | ± | 290 | <0.001 | 0.55 | 0.58 | 0.55 |
| H | 1783 | ± | 358 | 1683 | ± | 276 | 1794 | ± | 314 | 1610 | ± | 362 | 1211 | ± | 295 | 1291 | ± | 228 | 1364 | ± | 229 | 1258 | ± | 277 | |||||
| VE (L/min) | L | 86 | ± | 23 | 83 | ± | 26 | 78 | ± | 19 | 91 | ± | 40 | 115 | ± | 33 | 124 | ± | 44 | 119 | ± | 29 | 122 | ± | 34 | <0.001 | 0.82 | 0.49 | 0.71 |
| H | 98 | ± | 23 | 102 | ± | 24 | 101 | ± | 24 | 99 | ± | 18 | 134 | ± | 16 | 135 | ± | 20 | 137 | ± | 10 | 136 | ± | 15 | |||||
| BF (breaths/min) | L | 49 | ± | 11 | 49 | ± | 12 | 48 | ± | 8 | 43 | ± | 10 | 52 | ± | 12 | 51 | ± | 9 | 53 | ± | 10 | 49 | ± | 12 | 0.001 | 0.88 | 0.34 | 0.108 |
| H | 49 | ± | 10 | 50 | ± | 11 | 48 | ± | 15 | 52 | ± | 16 | 56 | ± | 11 | 55 | ± | 9 | 61 | ± | 11 | 58 | ± | 10 | |||||
| PETCO2 (mmHg) | L | 28 | ± | 2 | 26 | ± | 4 | 26 | ± | 6 | 27 | ± | 5 | 26 | ± | 4 | 24 | ± | 6 | 25 | ± | 5 | 26 | ± | 5 | 0.101 | 0.73 | 0.71 | 0.57 |
| H | 23 | ± | 7 | 23 | ± | 5 | 24 | ± | 7 | 24 | ± | 5 | 23 | ± | 5 | 23 | ± | 5 | 22 | ± | 5 | 23 | ± | 4 | |||||
| PETO2 (mmHg) | L | 112 | ± | 4 | 113 | ± | 5 | 113 | ± | 9 | 111 | ± | 8 | 117 | ± | 5 | 120 | ± | 6 | 119 | ± | 5 | 117 | ± | 6 | <0.001 | 0.626 | 0.783 | 0.548 |
| H | 119 | ± | 8 | 118 | ± | 8 | 117 | ± | 11 | 117 | ± | 8 | 122 | ± | 6 | 122 | ± | 4 | 122 | ± | 6 | 122 | ± | 4 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Galvan-Alvarez, V.; Curtelin, D.; Perez-Valera, M.; Juan Habib, J.; Pérez-López, A.; Vega, T.; Morales-Alamo, D.; et al. Enhancement of Exercise Performance by 48 Hours, and 15-Day Supplementation with Mangiferin and Luteolin in Men. Nutrients 2019, 11, 344. https://doi.org/10.3390/nu11020344
Gelabert-Rebato M, Wiebe JC, Martin-Rincon M, Galvan-Alvarez V, Curtelin D, Perez-Valera M, Juan Habib J, Pérez-López A, Vega T, Morales-Alamo D, et al. Enhancement of Exercise Performance by 48 Hours, and 15-Day Supplementation with Mangiferin and Luteolin in Men. Nutrients. 2019; 11(2):344. https://doi.org/10.3390/nu11020344
Chicago/Turabian StyleGelabert-Rebato, Miriam, Julia C. Wiebe, Marcos Martin-Rincon, Victor Galvan-Alvarez, David Curtelin, Mario Perez-Valera, Julian Juan Habib, Alberto Pérez-López, Tanausú Vega, David Morales-Alamo, and et al. 2019. "Enhancement of Exercise Performance by 48 Hours, and 15-Day Supplementation with Mangiferin and Luteolin in Men" Nutrients 11, no. 2: 344. https://doi.org/10.3390/nu11020344
APA StyleGelabert-Rebato, M., Wiebe, J. C., Martin-Rincon, M., Galvan-Alvarez, V., Curtelin, D., Perez-Valera, M., Juan Habib, J., Pérez-López, A., Vega, T., Morales-Alamo, D., & Calbet, J. A. L. (2019). Enhancement of Exercise Performance by 48 Hours, and 15-Day Supplementation with Mangiferin and Luteolin in Men. Nutrients, 11(2), 344. https://doi.org/10.3390/nu11020344