Glucose Tolerance Test and Pharmacokinetic Study of Kaempferia parviflora Extract in Healthy Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Test Product Description
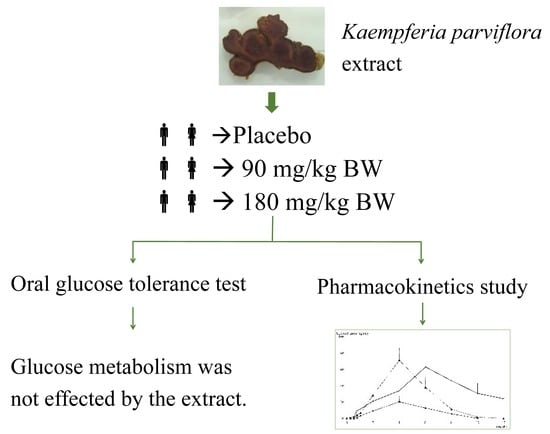
2.2. Study Design
2.3. Analysis of Blood Biochemical Parameters
2.4. Quantization of Methoxyflavones by HPLC Method
2.4.1. Sample Preparation
2.4.2. HPLC System
2.5. Data Analysis
3. Results
3.1. Subject Characteristics
3.2. Oral Glucose Tolerance Test
3.3. Pharmacokinetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sutthanut, K.; Sripanidkulchai, B.; Yenjai, C.; Jay, M. Simultaneous Identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J. Chromatogr. A 2007, 1143, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kessomboon, N.; Sripanidkulchai, B. Situation and trend in consumption of Krachai-dam products. Isan J. Pharm. Sci. 2008, 4, 81–92. [Google Scholar]
- Sripanidkulchai, B. Kaempferia parviflora: Research and Product Development, 2nd ed.; Klungnanavittaya: Khon Kaen, Thailand, 2014. [Google Scholar]
- Chaturapanich, G.; Chaiyakul, S.; Verawatnapakul, V.; Pholpramool, C. Effects of Kaempferia parviflora extracts on reproductive parameters and spermatic blood flow in male rats. Reproduction 2008, 136, 515–522. [Google Scholar] [CrossRef]
- Sudwan, P.; Saenphet, K.; Saenphet, S.; Suwansirikul, S. Effect of Kaempferia parviflora Wall. Ex. Baker on sexual activity of male rats and its toxicity. Southeast Asian J. Trop. Med. Public Health 2006, 37, 210–215. [Google Scholar] [PubMed]
- Wattanathorn, J.; Pangpookiew, P.; Muchimapura, S.; Sripanidkuchai, K.; Sripanidkulchai, B. Aphrodisiac activity of Kaempferia parviflora. Am. J. Agric. Biol. Sci. 2012, 7, 114–120. [Google Scholar] [CrossRef]
- Lert-Amornpat, T.; Maketon, C.; Fungfuang, W. Effect of Kaempferia parviflora on sexual performance in streptozotocin-induced diabetic male rats. Andrologia 2017, 49, e12770. [Google Scholar] [CrossRef] [PubMed]
- Sae-wong, C.; Tansakul, P.; Tewtrakul, S. Anti-inflammatory mechanism of Kaempferia parviflora in murine macrophage cells (RAW 264.7) and in experimental animals. J. Ethnopharmacol. 2009, 124, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Banjerdpongchai, R.; Suwannachot, K.; Rattanapanone, V.; Sripanidkulchai, B. Ethanolic rhizome extract from Kaempferia parviflora Wall. Ex. Baker induced apoptosis in HL-60 cells. Asian Pac. J. Cancer Prev. 2008, 9, 595–600. [Google Scholar]
- Leardkamolkarn, V.; Tiamyuyen, S.; Sripanidkulchai, B. Pharmacological activity of Kaempferia parviflora extract against human bile duct cancer cell lines. Asian Pac. J. Cancer Prev. 2009, 10, 695–698. [Google Scholar] [PubMed]
- Malakul, W.; Ingkaninan, K.; Sawasdee, P.; Woodman, O.L. The ethanolic extract of Kaempferia parviflora reduces ischemic injury in rat isolated hearts. J. Ethnopharmacol. 2011, 137, 184–191. [Google Scholar] [CrossRef]
- Malakul, W.; Thirawarapan, S.; Ingkaninan, K.; Sawasdee, P. Effects of Kaempferia parviflora wall. ex baker in endothelial dysfunction in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2011, 133, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Rujjanawate, C.; Kanjanapothi, D.; Amornlerdpison, D.; Pojanagaroon, S. Antigastric ulcer effect of Kaempferia parviflora. J. Ethnopharmacol. 2005, 102, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Kummee, S.; Tewtrakul, S.; Subhadhirasakul, S. Anitimicrobial activity of the ethanol extract and compounds from the rhizomes of Kaempferia parviflora. Songklanakarin J. Sci. Technol. 2008, 30, 463–466. [Google Scholar]
- Tewtrakul, S.; Subhadhirasakul, S.; Kummee, S. Anti-allergic activity of compounds from Kaempferia parviflora. J. Ethnopharmacol. 2008, 116, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Kayano, S.I.; Matsumura, Y.; Konishi, Y.; Tanaka, Y.; Kikuzaki, H. Antimutagenic and α-glucosidase inhibitory effects of constituents from Kaempferia parviflora. Food Chem. 2011, 125, 471–475. [Google Scholar] [CrossRef]
- Hawiset, T.; Muchimapura, S.; Wattanathorn, J.; Sripanidkulchai, B. Screening neuropharmacological activities of Kaempferia parviflora (Krachai Dam) in healthy adult male rats. Am. J. Appl. Sci. 2011, 8, 695–702. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Pangpookiew, P.; Sripanidkulchai, K.; Muchimapura, S.; Sripanidkuchai, B. Evaluation of the anxiolytic and antidepressant effects of alcoholic extract of Kaempferia parviflora in aged rats. Am. J. Agric. Biol. Sci. 2007, 2, 94–98. [Google Scholar] [CrossRef]
- Welbat, J.U.; Chaisawang, P.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Sripanidkulchai, B.; Wigmore, P. Kaempferia parviflora extract ameliorates the cognitive impairments and the reduction in cell proliferation induced by valproic acid treatment in rats. Ann. Anat. 2016, 206, 7–13. [Google Scholar] [CrossRef]
- Park, J.E.; Woo, S.W.; Kim, M.B.; Kim, C.; Hwang, J.K. Standardized Kaempferia parviflora extract inhibits intrinsic aging process in human dermal fibroblasts and hairless mice by inhibiting cellular senescence and mitochondrial dysfunction. Evid. Based Complement. Altern. Med. 2017, 6861085. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.; Lee, S.H.; Jang, H.D.; Kim, Y.H. Anti-osteoporotic and antioxidant activities by rhizomes of Kaempferia parviflora Wall. ex baker. Nat. Prof. Sci. 2016, 22, 13–19. [Google Scholar] [CrossRef]
- Kobayashi, H.; Suzuki, R.; Sato, K.; Ogami, T.; Tomozawa, H.; Tsubata, M.; Ichinose, K.; Aburada, M.; Ochiai, W.; Sugiyama, K.; et al. Effect of Kaempferia parviflora extract on knee osteoarthritis. J. Nat. Med. 2018, 72, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Akase, T.; Shimada, T.; Terabayashi, S.; Ikeya, Y.; Sanada, H.; Adurada, M. Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J. Natl. Med. 2011, 65, 73–80. [Google Scholar] [CrossRef]
- Hidaka, M.; Horikawa, K.; Akase, T. Efficacy of Kaempferia parviflora in a mouse model of obesity-induced dermatopathy. J. Natl. Med. 2017, 71, 59–67. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.; Kwon, D.; Kim, M.B.; Hwang, J.K. Standardized Kaempferia parviflora Wall. ex Baker (Zingiberaceae) extract inhibits fat accumulation and muscle atrophy in ob/ob mice. Evid. Based Complement. Altern. Med. 2018, 28, 8161042. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Yoneshiro, T.; Aita, S.; Kamiya, T.; Kusaba, N.; Yamaguchi, K.; Takagaki, K.; Kameya, T.; Sugie, H.; Saito, M. Kaempferia parviflora extract increases whole-body energy expenditure in humans: Roles of brown adipose tissue. J. Nutr. Sci. Vitaminol. (Tokyo) 2015, 61, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Takeuchi, T.; Nozaki, T.; Ishihara, K.O.; Matsuo, T. Kaempferia parviflora ethanol extract, a peroxisome proliferator-activated receptor γ ligand-binding agonist, improves glucose tolerance and suppresses fat accumulation in diabetic NSY mice. J. Food Sci. 2019, 84, 339–348. [Google Scholar] [CrossRef]
- Yoshino, S.; Kim, M.; Awa, R.; Kuwahara, H.; Kano, Y.; Kawada, T. Kaempferia parviflora extract increases energy consumption through activation of BAT in mice. Food Sci. Nutr. 2014, 2, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Horiguchi-Babamoto, E.; Suzuki, M.; Makihara, H.; Tomozawa, H.; Tsubata, M.; Shimada, T.; Sugiyama, K.; Aburada, M. Effects of ethyl acetate extract of Kaempferia parviflora on brown adipose tissue. J. Natl. Med. 2016, 70, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Pariwatthanakun, C.; Sripanidkulchai, K.; Sripanidkulchai, B. The Effect of Kaempferia parviflora extract on blood glucose level in normal and diabetic rats. In Proceedings of the 3rd Sino-Thai International Conference on Traditional Medicine and Natural Health Products, Guangxi Traditional Chinese Medical University, Nanning, China, 29–31 October 2008. [Google Scholar]
- Shimada, T.; Horikawa, T.; Ikeya, Y.; Matsuo, H.; Kinoshita, K.; Taguchi, T.; Ichinose, K.; Takahashi, K.; Aburada, M. Preventive effect of Kaempferia parviflora ethyl acetate extract and its major components polymethoxyflavonoid on metabolic diseases. Fitoterapia 2011, 82, 1272–1278. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Muchimapura, S.; Tong-un, T.; Saenghong, N.; Thukhum-Mee, W.; Sripanidkulchai, B. Positive modulation effect of 8-week consumption of Kaempferia parviflora on health-related physical fitness and oxidative status in healthy elderly volunteers. Evid. Based Complement. Altern. Med. 2012, 2012, 732816. [Google Scholar] [CrossRef]
- Promthep, K.; Eungpinichpong, W.; Sripanidkulchai, B.; Chatchawan, U. Effect of Kaempferia parviflora extract on physical fitness of soccer players: A randomized double-blind placebo-controlled trial. Med. Sci. Monit. Basic Res. 2015, 21, 100–108. [Google Scholar] [CrossRef]
- Yoshino, S.; Awa, R.; Miyake, Y.; Fukuhara, I.; Sato, H.; Ashino, T.; Tomita, S.; Kuwahara, H. Daily intake of Kaempferia parviflora extract decreases abdominal fat in overweight and preobese subjects: A randomized, double-blind, placebo-controlled clinical study. Diabetes Metab. Syndr. Obes. 2018, 11, 447–458. [Google Scholar] [CrossRef]
- Stein, R.A.; Schmid, K.; Bolivar, J.; Swick, A.G.; Joyal, S.V.; Hirsh, S.P. Kaempferia parviflora ethanol extract improves self-assessed sexual health in men: A pilot study. J Integr. Med. 2018, 16, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Eungpinichpong, W.; Chatchawan, U.; Sripanidkulchai, B.; Arunpongpaisal, S.; Chompoopan, W. Effects of Kaempferia parviflora on physical and psychological stresses in adults. Int. J. GEOMATE 2018, 15, 26–31. [Google Scholar] [CrossRef]
- Chivapat, S.; Chavalittumrog, P.; Phadungpat, S.; Pama, K.K.; Chansuvanich, N.; Attawish, A. Acute and chronic toxicity study of Kaempferia parviflora Wall ex. Bak powder. J. Thai Tradit. Altern. Med. 2004, 2, 3–16. [Google Scholar]
- Mekjaruskul, C.; Jay, M.; Sripanidkulchai, B. Pharmacokinetics, bioavailability, tissue distribution, excretion, and metabolite identification of methoxyflavones in Kaempferia parviflora extract in rats. Drug Met. Dispos. 2012, 40, 2342–2353. [Google Scholar] [CrossRef]
- Gopi, S.; Jacob, J.; Varma, K.; Amalraj, A.; Sreeraj, T.R.; Kunnumakkara, A.B.; Divya, C. Natural sports supplement formulation for physical endurance: A randomized, double-blind, placebo-controlled study. Sport Sci. Health 2017, 13, 183–194. [Google Scholar] [CrossRef]


 : PMF concentration in KP extract 90 mg/day group (n = 5), ▲: PMF concentration in KP extract 180 mg/day group (n = 9),
: PMF concentration in KP extract 90 mg/day group (n = 5), ▲: PMF concentration in KP extract 180 mg/day group (n = 9),  : TMF concentration in KP extract 180 mg/day group (n = 4)). PMF: 3,5,7,3′,4′-pentamethoxyflavone, TMF: 5,7,4′-trimethoxyflavone, KP: Kaempferia parviflora.
: TMF concentration in KP extract 180 mg/day group (n = 4)). PMF: 3,5,7,3′,4′-pentamethoxyflavone, TMF: 5,7,4′-trimethoxyflavone, KP: Kaempferia parviflora.
 : PMF concentration in KP extract 90 mg/day group (n = 5), ▲: PMF concentration in KP extract 180 mg/day group (n = 9),
: PMF concentration in KP extract 90 mg/day group (n = 5), ▲: PMF concentration in KP extract 180 mg/day group (n = 9),  : TMF concentration in KP extract 180 mg/day group (n = 4)). PMF: 3,5,7,3′,4′-pentamethoxyflavone, TMF: 5,7,4′-trimethoxyflavone, KP: Kaempferia parviflora.
: TMF concentration in KP extract 180 mg/day group (n = 4)). PMF: 3,5,7,3′,4′-pentamethoxyflavone, TMF: 5,7,4′-trimethoxyflavone, KP: Kaempferia parviflora.
| Composition (mg) | Serving Size: 300 mg/Capsule | |
|---|---|---|
| Placebo | KP Extract | |
| KP extract | 0 | 90 |
| Microcrystalline cellulose | 282 | 192 |
| Silica dioxide | 15 | 15 |
| Magnesium stearate | 3 | 3 |
| Group | FPG (mg/dL, mean ± SD) | |||
|---|---|---|---|---|
| 0 min | 30 min | 60 min | 120 min | |
| Placebo (n = 15) | 82.6 ± 7.0 | 134.7 ± 23.5 | 120.9 ± 37.9 | 83.7 ± 18.8 |
| p-value * | - | <0.001 | 0.002 | 0.847 |
| KP 90 mg (n = 15) | 81.9 ± 6.9 | 128.5 ± 28.4 | 113.9 ± 39.3 | 82.5 ± 14.7 |
| p-value * | - | <0.001 | 0.010 | 0.902 |
| p-value ** | 0.940 | 0.491 | 0.432 | 0.893 |
| KP 180 mg (n = 14) | 84.7 ± 7.7 | 131.6 ± 18.9 | 123.6 ± 30.3 | 83.0 ± 17.3 |
| p-value * | - | <0.001 | 0.001 | 0.711 |
| p-value ** | 0.816 | 0.730 | 0.764 | 0.940 |
| p-value *** | 0.759 | 0.730 | 0.278 | 0.952 |
| Group | FPG (mg/dL, mean ± SD) | |||
|---|---|---|---|---|
| 0 min | 30 min | 60 min | 120 min | |
| Placebo (n = 15) | 86.7 ± 6.9 | 126.9 ± 25.4 | 122.0 ± 41.3 | 79.5 ± 15.2 |
| p-value * | - | <0.001 | 0.004 | 0.088 |
| KP 90 mg (n = 15) | 87.5 ± 6.1 | 120.6 ± 13.7 | 115.1 ± 31.7 | 91.3 ± 19.7 |
| p-value * | - | <0.001 | 0.005 | 0.502 |
| p-value ** | 0.949 | 0.588 | 0.549 | 0.312 |
| KP 180 mg (n = 14) | 89.2 ± 10.2 | 142.5 ± 43.9 | 129.4 ± 61.9 | 94.2 ± 36.5 |
| p-value * | - | <0.001 | 0.018 | 0.542 |
| p-value ** | 0.833 | 0.186 | 0.533 | 0.214 |
| p-value *** | 0.882 | 0.064 | 0.226 | 0.802 |
| Group | AUC of FPG at 0–120 min | p-Value* (Baseline vs. Day 28) | |
|---|---|---|---|
| Baseline | Day 28 | ||
| Placebo | 2224.1 ± 1122.0 | 2255.0 ± 1459.6 | 0.880 |
| KP 90 mg | 2014.0 ± 1309.1 | 1676.0 ± 678.5 | 0.281 |
| KP 180 mg | 2186.0 ± 1119.7 | 2220.0 ± 1512.3 | 0.884 |
| p-value ** (Placebo vs. KP 90 mg) | 1.000 | 1.000 | |
| p-value ** (Placebo vs. KP 180 mg) | 1.000 | 1.000 | |
| p-value ** (KP 90 mg vs. KP 180 mg) | 1.000 | 1.000 | |
| Parameters * | PMF | TMF | |
|---|---|---|---|
| KP Extract 90 mg/day (n = 9) | KP Extract 180 mg/day (n = 9) | KP Extract 180 mg/day (n = 4) | |
| AUC (ng∙h/mL) | 86.7 ± 18.8 | 291.9 ± 48.2 | 412.2 ± 203.7 |
| Cmax (ng/mL) | 26.0 ± 4.7 | 71.2 ± 11.3 | 63.0 ± 18.0 |
| Tmax (h) | 4.02 ± 0.54 | 4.02 ± 0.37 | 6.03 ± 0.96 |
| Ke (h−1) | 0.75 ± 0.26 | 0.66 ± 0.13 | 0.32 ± 0.13 |
| T1/2 (h) | 2.51 ± 0.88 | 1.83 ± 0.36 | 3.30 ± 0.96 |
| Vd (L) | 218.1 ± 99.3 | 199.0 ± 48.6 | 88.3 ± 24.3 |
| Cl (L/h) | 57.8 ± 20.8 | 54.7 ± 11.2 | 13.0 ± 6.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sripanidkulchai, B.; Mekjaruskul, C.; Areemit, R.; Cheawchanwattana, A.; Sithithaworn, J. Glucose Tolerance Test and Pharmacokinetic Study of Kaempferia parviflora Extract in Healthy Subjects. Nutrients 2019, 11, 1176. https://doi.org/10.3390/nu11051176
Sripanidkulchai B, Mekjaruskul C, Areemit R, Cheawchanwattana A, Sithithaworn J. Glucose Tolerance Test and Pharmacokinetic Study of Kaempferia parviflora Extract in Healthy Subjects. Nutrients. 2019; 11(5):1176. https://doi.org/10.3390/nu11051176
Chicago/Turabian StyleSripanidkulchai, Bungorn, Catheleeya Mekjaruskul, Rosawan Areemit, Areewan Cheawchanwattana, and Jiraporn Sithithaworn. 2019. "Glucose Tolerance Test and Pharmacokinetic Study of Kaempferia parviflora Extract in Healthy Subjects" Nutrients 11, no. 5: 1176. https://doi.org/10.3390/nu11051176
APA StyleSripanidkulchai, B., Mekjaruskul, C., Areemit, R., Cheawchanwattana, A., & Sithithaworn, J. (2019). Glucose Tolerance Test and Pharmacokinetic Study of Kaempferia parviflora Extract in Healthy Subjects. Nutrients, 11(5), 1176. https://doi.org/10.3390/nu11051176