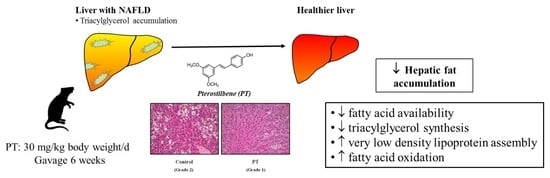
Pterostilbene Reduces Liver Steatosis and Modifies Hepatic Fatty Acid Profile in Obese Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Diet, and Experimental Design
2.2. Serum Parameters
2.3. Steatosis Assessment
2.4. Enzyme Activities
2.5. Liver RNA Extraction and Real Time RT-PCR
2.6. Liver Total Protein Extraction and Western Blot
2.7. Fatty Acid Profile of Hepatic Triacylglycerols and Phospholipids
2.8. Statistical Analysis
3. Results
3.1. Liver Weight and Hepatic Steatosis
3.2. Enzyme Activities
3.3. Gene Expression
3.4. Protein Expression
3.5. Fatty Acid Profile
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dongiovanni, P.; Lanti, C.; Riso, P.; Valenti, L. Nutritional therapy for nonalcoholic fatty liver disease. J. Nutr. Biochem. 2016, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Goceri, E.; Shah, Z.K.; Layman, R.; Jiang, X.; Gurcan, M.N. Quantification of liver fat: A comprehensive review. Comput. Biol. Med. 2016, 71, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef]
- Sanches, S.C.; Ramalho, L.N.; Augusto, M.J.; da Silva, D.M.; Ramalho, F.S. Nonalcoholic Steatohepatitis: A Search for Factual Animal Models. Biomed. Res. Int. 2015, 2015, 574832. [Google Scholar] [CrossRef]
- Argilés, J.M. The obese Zucker rat: A choice for fat metabolism 1968–1988: Twenty years of research on the insights of the Zucker mutation. Prog. Lipid Res. 1989, 28, 53–66. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Canale, R.E.; Marshall, K.E.; Kabir, M.M.; Bloomer, R.J. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: A summary of available findings. Nutr. J. 2011, 10, 107. [Google Scholar] [CrossRef]
- Papandreou, D.; Andreou, E. Role of diet on non-alcoholic fatty liver disease: An updated narrative review. World J. Hepatol. 2015, 7, 575–582. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Macarulla, M.T.; Alberdi, G.; Gómez, S.; Tueros, I.; Bald, C.; Rodríguez, V.M.; Martínez, J.A.; Portillo, M.P. Effects of different doses of resveratrol on body fat and serum parameters in rats fed a hypercaloric diet. J. Physiol. Biochem. 2009, 65, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Hekmatdoost, A.; Adibi, P. Resveratrol and liver: A systematic review. J. Res. Med. Sci. 2015, 20, 797–810. [Google Scholar] [CrossRef]
- Aguirre, L.; Portillo, M.P.; Hijona, E.; Bujanda, L. Effects of resveratrol and other polyphenols in hepatic steatosis. World J. Gastroenterol. 2014, 20, 7366–7380. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Joseph, J.A.; Fisher, D.R.; Cheng, V.; Rimando, A.M.; Shukitt-Hale, B. Cellular and behavioral effects of stilbene resveratrol analogues: Implications for reducing the deleterious effects of aging. J. Agric. Food Chem. 2008, 56, 10544–10551. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Fernández-Quintela, A.; Churruca, I.; Rodríguez, V.; Simón, E.; Portillo, M. Hepatomegaly induced by trans-10,cis-12 conjugated linoleic acid in adult hamsters fed an atherogenic diet is not associated with steatosis. J. Am. Coll. Nutr. 2009, 28, 43–49. [Google Scholar] [CrossRef]
- Zabala, A.; Churruca, I.; Macarulla, M.T.; Rodríguez, V.M.; Fernández-Quintela, A.; Martínez, J.A.; Portillo, M.P. The trans-10,cis-12 isomer of conjugated linoleic acid reduces hepatic triacylglycerol content without affecting lipogenic enzymes in hamsters. Br. J. Nutr. 2004, 92, 383–389. [Google Scholar] [CrossRef]
- Bieber, L.L.; Abraham, T.; Helmrath, T. A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal. Biochem. 1972, 50, 509–518. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Srere, P. Citrate synthase. In Methods Enzymology; Academic Press: Cambridge, MA, USA, 1969; Volume 3, pp. 3–11. [Google Scholar]
- Athar, H.; Iqbal, J.; Jiang, X.C.; Hussain, M.M. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 2004, 45, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Satheesh, M.; Pari, L. Effects of pterostilbene on lipids and lipid profiles in streptozotocin-nicotinamide induced type 2 diabetes mellitus. J. Appl. Biomed. 2008, 6, 31–37. [Google Scholar] [CrossRef]
- Ahmed, M. Non-alcoholic fatty liver disease in 2015. World J. Hepatol. 2015, 7, 1450–1459. [Google Scholar] [CrossRef]
- Lückhoff, H.K.; Kruger, F.C.; Kotze, M.J. Composite prognostic models across the non-alcoholic fatty liver disease spectrum: Clinical application in developing countries. World J. Hepatol. 2015, 7, 1192–1208. [Google Scholar] [CrossRef]
- Crespillo, A.; Alonso, M.; Vida, M.; Pavón, F.J.; Serrano, A.; Rivera, P.; Romero-Zerbo, Y.; Fernández-Llebrez, P.; Martínez, A.; Pérez-Valero, V.; et al. Reduction of body weight, liver steatosis and expression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br. J. Pharmacol. 2011, 164, 1899–1915. [Google Scholar] [CrossRef]
- Sadi, G.; Ergin, V.; Yilmaz, G.; Pektas, M.B.; Yildirim, O.G.; Menevse, A.; Akar, F. High-fructose corn syrup-induced hepatic dysfunction in rats: Improving effect of resveratrol. Eur. J. Nutr. 2015, 54, 895–904. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Den Boer, M.; Voshol, P.J.; Kuipers, F.; Havekes, L.M.; Romijn, J.A. Hepatic steatosis: A mediator of the metabolic syndrome. Lessons from animal models. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 644–649. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Heebøll, S.; Thomsen, K.L.; Clouston, A.; Sundelin, E.I.; Radko, Y.; Christensen, L.P.; Ramezani-Moghadam, M.; Kreutzfeldt, M.; Pedersen, S.B.; Jessen, N.; et al. Effect of resveratrol on experimental non-alcoholic steatohepatitis. Pharmacol. Res. 2015, 95–96, 34–41. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Fernández-Quintela, A.; Lasa, A.; Aguirre, L.; Rimando, A.M.; Portillo, M.P. Pterostilbene, a dimethyl ether derivative of resveratrol, reduces fat accumulation in rats fed an obesogenic diet. J. Agric. Food Chem. 2014, 62, 8371–8378. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Trepiana, J.; Fernández-Quintela, A.; González, M.; Portillo, M.P. Resveratrol and Pterostilbene, Two Analogue Phenolic Compounds, Affect Aquaglyceroporin Expression in a Different Manner in Adipose Tissue. Int. J. Mol. Sci. 2018, 19, 2654. [Google Scholar] [CrossRef]
- Yu, X.X.; Murray, S.F.; Pandey, S.K.; Booten, S.L.; Bao, D.; Song, X.Z.; Kelly, S.; Chen, S.; McKay, R.; Monia, B.P.; et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology 2005, 42, 362–371. [Google Scholar] [CrossRef]
- Choi, C.S.; Savage, D.B.; Kulkarni, A.; Yu, X.X.; Liu, Z.X.; Morino, K.; Kim, S.; Distefano, A.; Samuel, V.T.; Neschen, S.; et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 2007, 282, 22678–22688. [Google Scholar] [CrossRef]
- Chavin, K.D.; Yang, S.; Lin, H.Z.; Chatham, J.; Chacko, V.P.; Hoek, J.B.; Walajtys-Rode, E.; Rashid, A.; Chen, C.H.; Huang, C.C.; et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J. Biol. Chem. 1999, 274, 5692–5700. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Wu, T.C.; Huang, C.C.; Chen, C.H.; Lin, H.Z.; Yang, S.Q.; Lee, F.Y.; Diehl, A.M. Mitochondrial proteins that regulate apoptosis and necrosis are induced in mouse fatty liver. Hepatology 1999, 29, 1131–1138. [Google Scholar] [CrossRef]
- Baffy, G.; Zhang, C.Y.; Glickman, J.N.; Lowell, B.B. Obesity-related fatty liver is unchanged in mice deficient for mitochondrial uncoupling protein 2. Hepatology 2002, 35, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Stärkel, P.; Sempoux, C.; Leclercq, I.; Herin, M.; Deby, C.; Desager, J.P.; Horsmans, Y. Oxidative stress, KLF6 and transforming growth factor-beta up-regulation differentiate non-alcoholic steatohepatitis progressing to fibrosis from uncomplicated steatosis in rats. J. Hepatol. 2003, 39, 538–546. [Google Scholar] [CrossRef]
- Sheets, A.R.; Fülöp, P.; Derdák, Z.; Kassai, A.; Sabo, E.; Mark, N.M.; Paragh, G.; Wands, J.R.; Baffy, G. Uncoupling protein-2 modulates the lipid metabolic response to fasting in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1017–G1024. [Google Scholar] [CrossRef] [PubMed]
- Baena, M.; Sangüesa, G.; Dávalos, A.; Latasa, M.J.; Sala-Vila, A.; Sánchez, R.M.; Roglans, N.; Laguna, J.C.; Alegret, M. Fructose, but not glucose, impairs insulin signaling in the three major insulin-sensitive tissues. Sci. Rep. 2016, 6, 26149. [Google Scholar] [CrossRef] [PubMed]
- Pettinelli, P.; Obregón, A.M.; Videla, L.A. Molecular mechanisms of steatosis in nonalcoholic fatty liver disease. Nutr. Hosp. 2011, 26, 441–450. [Google Scholar] [CrossRef] [PubMed]







| Sense Primer | Antisense Primer | |
|---|---|---|
| CD36 | 5′-GGT GTG CTC AAC AGC CTT ATC-3′ | 5′-TTA TGG CAA CCT TGC TTA TG-3′ |
| Cox-2 | 5′-AAC AAT TCT CCC AGC TGT CAT TC-3′ | 5′-AGT CAA AGC ATA GGT CTT CAT AGT C-3′ |
| β-actin | 5′-ACG AGG CCC AGA GCA AGA-3′ | 5′-GGT GTG GTG CCA GAT CTT CTC-3′ |
| Control | PT | Effect Size a | ||
|---|---|---|---|---|
| Initial body weight (g) | 203 ± 4 | 204 ± 6 | ||
| Body weight (g) | 372 ± 10 | 347 ± 12 | NS | |
| Food intake (g/day) | 24.7 ± 1.8 | 22.6 ± 1.2 | NS | |
| Adipose tissue weight (g) | 49.4 ± 1.8 | 43.5 ± 1.9 | p = 0.04 | −1.01 |
| Liver weight (g) | 18.4 ± 0.5 | 16.9 ± 0.7 | NS | |
| Hepatic triacylglycerol (mg/g) | 60.0 ± 3.6 | 49.8 ± 6.9 | NS | |
| Serum glucose (mg/dL) | 139 ± 11 | 108 ± 5 | p = 0.03 | −1.16 |
| Serum insulin (mU/L) | 54.1 ± 15.1 | 19.0 ± 4.4 | p = 0.03 | −1.01 |
| HOMA-IR | 18.7 ± 4.8 | 4.5 ± 1.5 | p = 0.02 | −1.28 |
| Serum triacylglycerols (mg/dL) | 277.7 ± 36.2 | 274.8 ± 50.8 | NS | |
| Serum NEFAs (mmol/L) | 2.3 ± 0.4 | 2.1 ± 0.1 | NS | |
| Serum AST (U/L) | 154 ± 22 | 143 ± 17 | NS | |
| Serum ALT (U/L) | 102 ± 8 | 90 ± 7 | NS |
| Fatty Acid | Control | PT | Effect Size a | |
|---|---|---|---|---|
| C14:0 | 1.58 ± 0.27 | 1.67 ± 0.04 | NS | |
| C16:0 | 35.02 ± 1.44 | 33.56 ± 0.87 | NS | |
| C16:1 cis-9 n-7 | 10.56 ± 0.68 | 9.45 ± 0.49 | NS | |
| C18:0 | 1.99 ± 0.17 | 2.41 ± 0.28 | NS | |
| C18:1 cis-9 n-9 | 22.82 ± 1.86 | 22.11 ± 1.27 | NS | |
| C18:1 cis-11 n-7 | 7.63 ± 1.40 | 6.89 ± 0.79 | NS | |
| C18:2 n-6 | 14.59 ± 1.36 | 18.85 ± 1.17 | p = 0.04 | 1.07 |
| C18:3 n-3 | 0.45 ± 0.07 | 0.68 ± 0.08 | p = 0.05 | 0.97 |
| C20:0 | 4.39 ± 2.14 | 3.21 ± 0.42 | NS | |
| C22:0 | 0.04 ± 0.02 | 0.04 ± 0.02 | NS | |
| C20:4 n-6 | 0.93 ± 0.18 | 1.14 ± 0.27 | NS | |
| ∑SFA | 43.02 ± 1.15 | 40.89 ± 0.82 | NS | |
| ∑MUFA | 41.00 ± 1.59 | 38.44 ± 1.13 | NS | |
| ∑PUFA | 15.98 ± 1.50 | 20.67 ± 1.36 | p = 0.04 | 1.04 |
| Fatty Acid | Control | PT | Effect Size a | |
|---|---|---|---|---|
| C14:0 | 0.77 ± 0.12 | 0.50 ± 0.04 | NS | |
| C16:0 | 23.34 ± 0.49 | 21.42 ± 0.25 | NS | |
| C16:1 cis-9 n-7 | 3.64 ± 0.65 | 2.13 ± 0.25 | p = 0.06 | −0.98 |
| C18:0 | 19.11 ± 1.13 | 20.93 ± 0.77 | NS | |
| C18:1 cis-9 n-9 | 7.06 ± 1.16 | 4.65 ± 0.37 | NS | |
| C18:1 cis-11 n-7 | 4.00 ± 0.45 | 2.83 ± 0.16 | p = 0.04 | −1.11 |
| C18:2 n-6 | 7.06 ± 0.29 | 8.20 ± 1.03 | NS | |
| C18:3 n-3 | 0.13 ± 0.04 | 0.11 ± 0.04 | NS | |
| C20:4 n-6 | 28.04 ± 1.82 | 30.89 ± 0.78 | NS | |
| C20:5 n-3 | 0.03 ± 0.01 | 0.03 ± 0.01 | NS | |
| C22:6 n-3 | 6.81 ± 0.57 | 8.31 ± 0.35 | p = 0.05 | 1.01 |
| ∑SFA | 43.22 ± 0.14 | 42.85 ± 0.54 | NS | |
| ∑MUFA | 14.71 ± 2.25 | 9.61 ± 0.74 | p = 0.06 | −0.97 |
| ∑PUFA | 42.07 ± 2.23 | 47.53 ± 0.91 | p = 0.05 | 1.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguirre, L.; Palacios-Ortega, S.; Fernández-Quintela, A.; Hijona, E.; Bujanda, L.; Portillo, M.P. Pterostilbene Reduces Liver Steatosis and Modifies Hepatic Fatty Acid Profile in Obese Rats. Nutrients 2019, 11, 961. https://doi.org/10.3390/nu11050961
Aguirre L, Palacios-Ortega S, Fernández-Quintela A, Hijona E, Bujanda L, Portillo MP. Pterostilbene Reduces Liver Steatosis and Modifies Hepatic Fatty Acid Profile in Obese Rats. Nutrients. 2019; 11(5):961. https://doi.org/10.3390/nu11050961
Chicago/Turabian StyleAguirre, Leixuri, Sara Palacios-Ortega, Alfredo Fernández-Quintela, Elizabeth Hijona, Luis Bujanda, and María P. Portillo. 2019. "Pterostilbene Reduces Liver Steatosis and Modifies Hepatic Fatty Acid Profile in Obese Rats" Nutrients 11, no. 5: 961. https://doi.org/10.3390/nu11050961
APA StyleAguirre, L., Palacios-Ortega, S., Fernández-Quintela, A., Hijona, E., Bujanda, L., & Portillo, M. P. (2019). Pterostilbene Reduces Liver Steatosis and Modifies Hepatic Fatty Acid Profile in Obese Rats. Nutrients, 11(5), 961. https://doi.org/10.3390/nu11050961