Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies
Abstract
:1. Introduction
2. Methods
2.1. Systematic Review
2.1.1. Literature Sources and Searches
2.1.2. Inclusions
2.1.3. Exclusions
2.1.4. Selection
2.1.5. Data Extraction and Calculation
- GI and GL by quantiles (means or medians or calculated as shown in the footnotes to Tables S1 and S7).
- The reference standard used (glucose or white bread)—so to adjust to a common reference standard of glucose (GI of white bread = 100 on the bread scale, and 70 on the glucose scale).
- Relative risks for T2D (risk ratios, hazard ratios or odds ratios) and their 95% confidence intervals (95% CI) by quantiles or by risk relations (increment in RR per units of increased exposure).
- Energy intake by quantiles and the intake of energy to which GL was adjusted.
- Whether RR values from the prospective cohort studies were adjusted for protein intake simultaneously with adjustments for energy, fats, and fiber intakes (See endnote b after the Discussion for a hypothesis of when, in original studies, adjusting for protein intake might make an adjustment for carbohydrate intake, and why it might not).
- Whether study-level adjustments were made for fiber, magnesium, red meat, macronutrients, and family history of diabetes (FHD).
- Study population’s average alcohol (ALC) consumption and fiber (including type) intakes.
- Sex, as a fraction of the men in the sampled population (SEX).
- Ethnicity (ETH).
- Population region.
- The person-years of follow-up, the number of cases, and the number of persons followed up.
- Duration of follow-up in years (FUY).
- Whether the dietary instruments used were validated and the level of validity for carbohydrate as indicated by the validity correlation coefficient for carbohydrate (CORR).
- The number of repeated uses of the dietary instrument over the duration of the follow-up.
- The method used for the ascertainment of T2D (self-report or clinical report).
- Body mass index (BMI) values (kg/m2) for subcohorts differing by BMI.
- Adverse events of any kind reported in the original studies.
- Whether the authors of the prospective cohort studies were potentially conflicted by sources of funding.
2.1.6. Study Quality
2.1.7. Additional Analyses
2.2. Data Synthesis and Meta-Analysis
2.2.1. Hypotheses and Statistics
- (i)
- (ii)
- Ascertainment of T2D by self-report and or by clinical record [9].
- (iii)
- Study-level adjustment for protein intake simultaneously with energy and other macronutrient intakes except carbohydrate (see endnote b after the Discussion for a hypothesis of when an adjustment for carbohydrate may have been made and why it may have not) [10].
- (iv)
- Population’s average ALC consumption [40].
- (v)
- (vi)
- (vii)
- Number of dietary assessments [10].
- (viii)
- (ix)
- Specific nutrient adjustments at the study level (a posteriori).
- (x)
- (xi)
- (xii)
- Geographical region (a posteriori).
2.2.2. Global Dose-Response Meta-Analysis
2.2.3. Statistical Tests
2.2.4. Data and Terminology
2.2.5. Presentation
3. Results
3.1. The Literature Search
3.2. The T2D–GI Risk Relation
3.2.1. Study Characteristics
3.2.2. Combined Observations
3.2.3. Clinical Versus Self-Report of Diabetes
3.2.4. Funnel, Trim-and-Fill, and Galbraith-Like Plot Asymmetries
3.2.5. Sensitivity to Individual Studies
3.2.6. Mixed-Sex Studies
3.2.7. Single-Sex Studies
3.2.8. Duration of Follow-Up
3.2.9. Number of Dietary Assessments
3.2.10. Observations by Body Mass Index
3.2.11. Studies not Eligible for the Primary Analysis
3.2.12. Specific Nutrient and Non-Nutrient Factors
3.2.13. Family History of Diabetes and CORR
3.2.14. Alcohol
3.2.15. Study Size
3.2.16. Geographical Region and Ethnicity
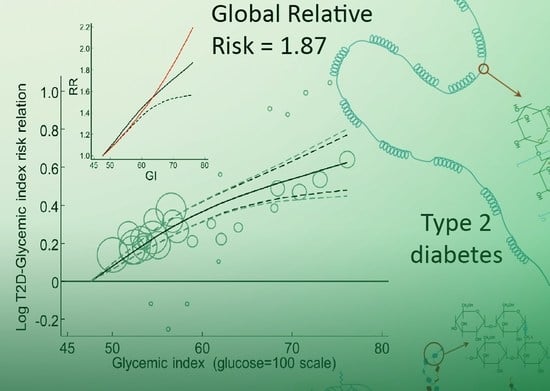
3.2.17. Global Dose-Response Meta-Analysis
3.3. The T2D–GL Risk Relation
3.3.1. Study Characteristics
3.3.2. Combined Observations
3.3.3. Clinical Versus Self-Report of Diabetes
3.3.4. Sensitivity to Individual Studies with CORR > 0.55
3.3.5. Dietary Instruments, a Determinant of RR
3.3.6. Funnel, Trim-and-Fill, and Galbraith-Like Plot Asymmetry
3.3.7. Specific Nutrient and Non-Nutrient Factors
3.3.8. Adjustment for Multiple Covariates
3.3.9. Sensitivity of the Fully Adjusted T2D–GL Relative Risk to Study Selections
3.3.10. Sensitivity to Health Professionals’ Studies
3.3.11. Observations by the Sex of Participants
3.3.12. Observations by Body Mass Index
3.3.13. Observations by Alcohol Intake
3.3.14. Sensitivities of RR when Using the Multi-Covariate Adjustments Including Alcohol
3.3.15. Protein (When a Surrogate for Carbohydrate)
3.3.16. Family History of Diabetes
3.3.17. Studies not Eligible for the Primary Analyses
3.3.18. Geographical Region or Ethnicity
3.3.19. Global Dose-Response Meta-Analysis
4. Discussion
5. Conclusions
6. Endnotes
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Registration
References
- International Diabetes Federation. IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015; Available online: http://www.idf.org/diabetesatlas (accessed on 21 March 2017).
- Bazzano, L.A.; Serdula, M.; Liu, S. Prevention of type 2 diabetes by diet and lifestyle modification. J. Am. Coll. Nutr. 2005, 24, 310–319. [Google Scholar] [CrossRef] [PubMed]
- AlEssa, H.B.; Bhupathiraju, S.N.; Malik, V.S.; Wedick, N.M.; Campos, H.; Rosner, B.; Willett, W.C.; Hu, F.B. Carbohydrate quality and quantity and risk of type 2 diabetes in US women 2. Am. J. Clin. Nutr. 2015, 102, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Halton, T.L.; Liu, S.; Manson, J.E.; Hu, F.B. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2008, 87, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: Systematic review and dose-response meta-analysis of prospective studies. Diabetes Care 2013, 36, 4166–4171. [Google Scholar] [CrossRef] [PubMed]
- Scientific Advisory Committee on Nutrition. Carbohydrates and Health, Chapter 4 Diabetes/Starch; TSO: London, UK, 2015. Available online: https://www.gov.uk/government/publications/sacn-carbohydrates-and-health-report (accessed on 21 March 2017).
- Scientific Advisory Committee on Nutrition. Carbohydrates and Health, Chapter 4 Diabetes/Sugars; TSO: London, UK, 2015. Available online: https://www.gov.uk/government/publications/sacn-carbohydrates-and-health-report (accessed on 21 March 2017).
- Bazzano, L.A.; Li, T.Y.; Joshipura, K.J.; Hu, F.B. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008, 31, 1311–1317. [Google Scholar] [CrossRef]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk—A meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Tobias, D.K.; Malik, V.S.; Pan, A.; Hruby, A.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and risk of type 2 diabetes: Results from 3 large US cohorts and an updated meta-analysis. Am. J. Clin. Nutr. 2014, 100, 218–232. [Google Scholar] [CrossRef]
- Dong, J.Y.; Zhang, L.; Zhang, Y.H.; Qin, L.Q. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Br. J. Nutr. 2011, 106, 1649–1654. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Wunters, N.; Mate, E.; Morenga, L.T. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 10170, 434–445. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Livesey, H.; Liu, S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2013, 97, 584–596. [Google Scholar] [CrossRef] [Green Version]
- Livesey, G.; Taylor, R.; Livesey, H.; Liu, S. Is There a Dose-Response Relation of Dietary Glycemic Load to Risk of Type 2 Diabetes? Meta-Analysis of Prospective Cohort Studies: Supplementary Discussion. 2013. Available online: http://biomedfrontiers.org/diabetes-2013-may-2-1 (accessed on 21 November 2016).
- Livesey, G.; Taylor, R.; Livesey, H.; Buyken, A.; Jenkins, D.; Augustin, L.; Sievenpiper, J.L.; BarLiu, S.; Barclay, A.; Wolever, T.; et al. Dietary glycemic index and load and the risk of type 2 diabetes: Assessment of causal relations. Nutrients 2019. submitted for publication. [Google Scholar]
- Riccardi, G.; Costabile, G. Carbohydrate quality is key for a healthy and sustainable diet. Nat. Rev. Endocrinol. 2019, 15, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Stallone, D.; Juneja, M.; Bingham, S.; Marmot, M. Dietary assessment in Whitehall II: Comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. Br. J. Nutr. 2001, 86, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Livesey, H. Coronary heart disease and dietary carbohydrate, glycemic index and glycemic load: Dose-response meta-analyses of prospective cohort studies. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Longnecker, M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Orsini, N.; Bellocco, R.; Greenland, S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006, 6, 40–57. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology; Oxford University Press: New York, NY, USA; Oxford, UK, 1998. [Google Scholar]
- Smith, G.D.; Egger, M. Meta-analyses of observational data should be done with due care. BMJ 1999, 318, 56. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M. Systematic reviews of observational studies. In Systematic Reviews in Healthcare-Meta-Analysis in Context; Egger, M., Smith, G.D., Altman, D.G., Eds.; BMJ Books: London, UK, 2007; pp. 211–227. [Google Scholar]
- Hill, B.A. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Hu, F.B.; Pereira, M.A.; Liu, S.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Whole-grain intake and the risk of type 2 diabetes: A prospective study in men. Am. J. Clin. Nutr. 2002, 76, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Dhawan, A.; Liese, A.D.; Teff, K.; Schulz, M. Towards understanding of glycaemic index and glycaemic load in habitual diet: Associations with measures of glycaemia in the insulin resistance atherosclerosis study. Br. J. Nutr. 2006, 95, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Liese, A.; Fang, F.; Gillard, T.; Karter, A. Is the association between dietary glycemic index and type 2 diabetes modifed by waist circumference? Dabetes Care 2006, 29, 1102–1104. [Google Scholar] [CrossRef]
- Mohan, V.; Radhika, G.; Sathya, R.M.; Tamil, S.R.; Ganesan, A.; Sudha, V. Dietary carbohydrates, glycaemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59). Br. J. Nutr. 2009, 102, 1498–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, R.; Woo, J.; Chan, R.; Sham, A.; Ho, S.; Tso, A.; Cheung, B.; Lam, T.H.; Lam, K. Relationship between dietary intake and the development of type 2 diabetes in a Chinese population: The Hong Kong dietary survey. Public Health Nutr. 2011, 14, 1133–1141. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0. 2008. Available online: http://handbook.cochrane.org/ (accessed on 21 November 2016).
- Wells, G.; Shea, S.; O’Connell, D.; Robertson, J.; Peterson, P.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2009. Available online: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf (accessed on 21 November 2016).
- Lin, L.; Cho, H.; Hodges, J.S. Alternative measures of between-studies heterogeneity in Meta-analysis: Reducing the impact of outlying studies. Biometrics 2017, 73, 1560166. [Google Scholar] [CrossRef]
- Galbraith, R.F. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat. Med. 1988, 7, 889–894. [Google Scholar] [CrossRef]
- Tang, J.L.; Liu, J.L. Misleading funnel plot for detection of bias in meta-analysis. J. Clin. Epidemiol. 2000, 53, 477–484. [Google Scholar] [CrossRef]
- Egger, M.; Schneider, M.; Smith, G.D. Meta-analysis spurious precision? Meta-analysis of observational studies. BMJ 1998, 316, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Mekary, R.A.; Rimm, E.B.; Giovannucci, E.; Stampfer, M.J.; Willett, W.C.; Ludwig, D.S.; Hu, F.B. Joint association of glycemic load and alcohol intake with type 2 diabetes incidence in women. Am. J. Clin. Nutr. 2011, 94, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Simila, M.E.; Valsta, L.M.; Kontto, J.P.; Albanes, D.; Virtamo, J. Low-, medium- and high-glycaemic index carbohydrates and risk of type 2 diabetes in men. Br. J. Nutr. 2011, 105, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Hopping, B.N.; Erber, E.; Grandinetti, A.; Verheus, M.; Kolonel, L.N.; Maskarinec, G. Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. J. Nutr. 2010, 140, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Rosenberg, L.; Singer, M.; Hu, F.B.; Djousse, L.; Cupples, L.A.; Palmer, J.R. Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Arch. Intern. Med. 2007, 167, 2304–2309. [Google Scholar] [CrossRef]
- Oba, S.; Nanri, A.; Kurotani, K.; Goto, A.; Kato, M.; Mizoue, T.; Noda, M.; Inoue, M.; Tsugane, S. Dietary glycemic index, glycemic load and incidence of type 2 diabetes in Japanese men and women: The Japan public health center-based prospective study. Nutr. J. 2013, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Liu, S.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am. J. Clin. Nutr. 2004, 80, 348–356. [Google Scholar] [CrossRef]
- Villegas, R.; Liu, S.; Gao, Y.T.; Yang, G.; Li, H.; Zheng, W.; Shu, X.O. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch. Intern. Med. 2007, 167, 2310–2316. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Peto, R.; Awasthi, S.; Read, S.; Clark, S.; Bundy, D. Vitamin A supplementation in Indian children—Authors’ reply. Lancet 2013, 382, 594–596. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Peto, R. Interpreting Large-Scale Randomised Evidence. John Snow Lecture. 2013. Available online: http://www.youtube.com/watch?v=vybc0PsZ718&list=PL3oyPcbygtxuqmYps-aEj9Sa-ScThzMLh (accessed on 25 November 2016).
- Sterne, J.; Newton, H.J.; Cox, N.J. Meta-Analysis in Stata: An Updated Collection from the Stata Journal; Stata Press: Colledge Station, TX, USA, 2009; pp. 1–257. [Google Scholar]
- Orsini, N.; Bellocco, R.; Greenland, S. Generalized least squares for trend estimation of summarized dose-response data. In Meta-Analysis in Stata: An Updated Collection from the Stata Journal; Sterne, J., Ed.; Stata Press: College Station, TX, USA, 2009; pp. 200–217. [Google Scholar]
- Hoaglin, D.C. Misunderstandings about Q and ‘Cochran’s Q test’ in meta-analysis. Stat. Med. 2016, 35, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Crippa, A.; Discacciati, A.; Bottai, M.; Sppiegelmann, D.; Orcini, N. One-stage dose—Response meta-analysis for aggregated data. Stat. Methods Med. Res. 2019, 28, 1579–1596. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A. Dietary glycemic index and glycemic load in diabetes prevention—What can we learn from observational studies? Nat. Rev. Endocrinol. 2008, 4, 430–431. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Solomon, C.G.; Hu, F.B. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 2006, 29, 2223–2230. [Google Scholar] [CrossRef]
- Feskens, E.; Sluik, D.; Mikkilä, V.; Poppitt, S.; Silvestre, M.; Tremblay, A.; Bouchard, C.; Brand-Miller, J.; Raben, A. The preview population studies: Role of lifestyle factors (EG protein, glycemic index) in relation to pre-diabetes and diabetes risk. Ann. Nutr. Metab. 2017, 71, 123. [Google Scholar]
- Salmeron, J.; Ascherio, A.; Rimm, E.B.; Colditz, G.A.; Spiegelman, D.; Jenkins, D.J.; Stampfer, M.J.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997, 20, 545–550. [Google Scholar] [CrossRef]
- Patel, A.V.; McCullough, M.L.; Pavluck, A.L.; Jacobs, E.J.; Thun, M.J.; Calle, E.E. Glycemic load, glycemic index, and carbohydrate intake in relation to pancreatic cancer risk in a large US cohort. Cancer Causes Control 2007, 18, 287–294. [Google Scholar] [CrossRef]
- Sluijs, I.; van der Schouw, Y.T.; van der, A.D.; Spijkerman, A.M.; Hu, F.B.; Grobbee, D.E.; Beulens, J.W. Carbohydrate quantity and quality and risk of type 2 diabetes in the European prospective investigation into cancer and nutrition-netherlands (EPIC-NL) study. Am. J. Clin. Nutr. 2010, 92, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Rimm, E.B.; Willett, W.C. Dietary fat intake and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2001, 73, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R.J.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosdol, A.; Witte, D.R.; Frost, G.; Marmot, M.G.; Brunner, E.J. Dietary glycemic index and glycemic load are associated with high-density-lipoprotein cholesterol at baseline but not with increased risk of diabetes in the Whitehall II study. Am. J. Clin. Nutr. 2007, 86, 988–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, J.; Ahn, K.; Juhaeri; Houston, D.; Steffan, L.; Couper, D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: The ARIC study. Diabetes Care 2002, 25, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Beulens, J.W.; van der Schouw, Y.T.; van der, A.D.; Buckland, G.; Kuijsten, A.; Schulze, M.B.; Amiano, P.; Ardanaz, E.; Balkau, B.; et al. Dietary glycemic index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European countries. J. Nutr. 2013, 143, 93–99. [Google Scholar] [PubMed]
- Barclay, A.W.; Flood, V.M.; Rochtchina, E.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, dietary fiber, and risk of type 2 diabetes in a cohort of older Australians. Diabetes Care 2007, 30, 2811–2813. [Google Scholar] [CrossRef]
- Hodge, A.M.; English, D.R.; O’Dea, K.; Giles, G.G. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004, 27, 2701–2706. [Google Scholar] [CrossRef]
- Sahyoun, N.R.; Anderson, A.L.; Tylavsky, F.A.; Lee, J.S.; Sellmeyer, D.E.; Harris, T.B. Dietary glycemic index and glycemic load and the risk of type 2 diabetes in older adults. Am. J. Clin. Nutr. 2008, 87, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, M.; Nakamura, K.; Miura, K.; Takamura, T.; Yoshita, K.; Morikawa, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; Suwazono, Y.; et al. Dietary glycemic index and risk of type 2 diabetes mellitus in middle-aged Japanese men. Metabolism 2011, 61, 47–55. [Google Scholar] [CrossRef]
- Van Woudenbergh, G.J.; Kuijsten, A.; Sijbrands, E.J.; Hofman, A.; Witteman, J.C.; Feskens, E.J. Glycemic index and glycemic load and their association with C-reactive protein and incident type 2 diabetes. J. Nutr. Metab. 2011, 2011, 623076. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, N.R.; Anderson, A.L.; Kanaya, A.M.; Koh-Banerjee, P.; Kritchevsky, S.B.; de Rekeneire, N.; Tylavsky, F.A.; Schwartz, A.V.; Lee, J.S.; Harris, T.B. Dietary glycemic index and load, measures of glucose metabolism, and body fat distribution in older adults. Am. J. Clin. Nutr. 2005, 82, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, J.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997, 277, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Similä, M.; Valsta, L.; Kontto, J.; Virtamo, J. Dietary glycemic index and risk of type 2 diabetes: Foods with other effects opposite to their glycemic risks complicate the results. J. Diabetes 2009, S11, A73. [Google Scholar]
- Rossi, M.; Turati, F.; Lagiou, P.; Trichopoulos, D.; Augustin, L.S.; La Vecchia, C.; Trichopoulou, A. Mediterranean diet and glycaemic load in relation to incidence of type 2 diabetes: Results from the Greek cohort of the population-based European Prospective Investigation into Cancer and Nutrition (EPIC). Diabetologia 2013, 56, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Gnardellis, C.; Trichopoulou, A.; Katsouyanni, K.; Polychronopoulos, E.; Rimm, E.B.; Trichopoulos, D. Reproducibility and validity of an extensive semiquantitative food frequency questionnaire among Greek school teachers. Epidemiology 1995, 6, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G. Glycemic response and toleration. In Sweeteners and Sugar Alternatives in Food Technology, 2nd ed.; O’Donnell, K., Kearsley, M.W., Eds.; Wiley-Blackwell: Oxford, UK, 2012; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781118373941 (accessed on 30 May 2018).
- WHO/FAO. Diet, Nutrition and the Prevention of Chronic Diseases. Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Unwin, J.D.; Haslam, D.; Livesey, G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: The glycaemic index revisited. J. Insul. Resist. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Barclay, A.W.; Flood, V.M.; Brand-Miller, J.C.; Mitchell, P. Validity of carbohydrate, glycaemic index and glycaemic load data obtained using a semi-quantitative food-frequency questionnaire. Public Health Nutr. 2008, 11, 573–580. [Google Scholar] [CrossRef]
- Wolever, T.M.; Augustin, L.S.; Brand-Miller, J.C.; Delport, E.; Livesey, G.; Ludwig, D.S.; Sievenpiper, J.L. Glycemic index is as reliable as macronutrients on food labels. Am. J. Clin. Nutr. 2017, 105, 768–769. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Lyon, J.L.; Egger, M.J.; Robison, L.M.; French, T.K.; Gao, R. Misclassification of exposure in a case-control study: The effects of different types of exposure and different proxy respondents in a study of pancreatic cancer. Epidemiology 1992, 3, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Copeland, K.T.; Checkoway, H.; McMichael, A.J.; Holbrook, R.H. Bias due to misclassification in the estimation of relative risk. Am. J. Epidemiol. 1977, 105, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Jurek, A.M.; Greenland, S.; Maldonado, G.; Church, T.R. Proper interpretation of non-differential misclassification effects: Expectations vs. observations. Int. J. Epidemiol. 2005, 34, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G. Joint association of glycemic load and alcohol intake with type 2 diabetes incidence in women. Am. J. Clin. Nutr. 2012, 95, 983. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An international scientific consensus summit from the international carbohydrate quality consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2014, 25, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.H.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [Green Version]
- Wolever, T.M.S. The Glycaemic Index: A Physiological Classification of Dietary Carbohydrates; CABI: Oxford, UK, 2006. [Google Scholar]
- Augustin, L.S.; Franceschi, S.; Jenkins, D.J.; Kendall, C.W.; La Vecchia, C. Glycemic index in chronic disease: A review. Eur. J. Clin. Nutr. 2002, 56, 1049–1071. [Google Scholar] [CrossRef]
- Smith, J.D.; Hou, T.; Ludwig, D.S.; Rimm, E.B.; Willett, W.; Hu, F.B.; Mozaffarian, D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: Results from 3 prospective cohorts. Am. J. Clin. Nutr. 2015, 101, 1216–1224. [Google Scholar] [CrossRef]
- Larsen, T.M.; Dalskov, S.-M.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.H.; Martinez, J.A.; Handjieva-Darlenska, T.; KuneÅ¡ová, M.; PihlsgÃ¥rd, M.; et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med. 2010, 363, 2102–2113. [Google Scholar] [CrossRef]










| Number of Studies | Model | Mean Relative Risks (95% CI) | p-Value for RR | Inconsistency (I2%) | Heterogeneity (τ 2) | p-Value for τ2 and I2 | ||
|---|---|---|---|---|---|---|---|---|
| Per 10 Units GI a | ||||||||
| (1) Studies with CORR ≤ 0.55: | ||||||||
| All such eligible studies b | 7 | Fixed | 1.02 | (0.98–1.06) | 0.377 | - | - | - |
| Random | 1.05 | (0.97–1.14) | 0.271 | 65 | 0.007 | 0.009 | ||
| (2) Studies with CORR > 0.55: | ||||||||
| All such eligible studies c | 10 | Fixed | 1.26 | (1.21–1.31) | <0.001 | (0) d | - | |
| Random | 1.27 | (1.15–1.40) | <0.001 | 68 | 0.013 | <0.001 | ||
| Men-only and | 6 | Fixed | 1.26 | (1.21–1.31) | <0.001 | (0) | - | |
| women-only combined e | Random | 1.29 | (1.15–1.45) | <0.001 | 79 | 0.014 | <0.001 | |
| Women-only | 3 | Fixed | 1.25 | (1.20–1.30) | <0.001 | (0) | - | |
| studies f | Random | 1.29 | (1.10–1.50) | <0.001 | 90 | 0.017 | <0.001 | |
| Men-only | 3 | Fixed | 1.32 | (1.16–1.51) | <0.001 | (0) | - | |
| Studies g | Random | 1.31 | (1.06–1.63) | 0.013 | 47 | 0.017 | 0.15 | |
| Largest three studies | 3 | Fixed | 1.25 | (1.20–1.30) | <0.001 | (0) | ||
| (China and the USA) h | Random | 1.29 | (1.11–1.50) | 0.002 | 90 | 0.017 | <0.001 | |
| Study-Level Adjustment Made within Studies | Number of Studies | Model a | Mean Combined Relative Risk and (95%CI) | p-Value for RR | Inconsistency I2 | Heterogeneity τ 2 | p-Value for τ 2 and I2 | ||
|---|---|---|---|---|---|---|---|---|---|
| Per 10 units GI | (%) | (Per 10 | |||||||
| units GI)2 | |||||||||
| All studies with CORR > 0.55 bar one outlier [40] b | 10 | Random | 1.27 | (1.15–1.40) | <0.001 | 68 | 0.013 | <0.001 | |
| All fiber types c | 7 | Random | 131 | (1.17–1.47) | <0.001 | 76 | 0.0138 | <0.001 | |
| Cereal fiber d | 3 | Random | 1.39 | (1.26–1.53) | <0.001 | 45 | 0.0034 | 0.161 | |
| Vegetable fiber e | 1 | Random | 1.50 | (0.95–2.36) | 0.080 | - | - | - | |
| Magnesium f | 2 | Random | 1.28 | (0.94–1.79) | <0.001 | 69 | 0.0355 | 0.070 | |
| Protein g | 3 | Random | 1.39 | (1.26–1.53) | <0.001 | 45 | 0.0034 | 0.161 | |
| Red meat h | 1 | Random | 1.47 | (1.35–1.60) | <0.001 | - | - | - | |
| Alcohol i | 9 | Random | 1.26 | (1.13–1.40) | <0.001 | 71 | 0.0132 | <0.001 | |
| Energy j | 8 | Random | 1.27 | (1.14–1.40) | <0.001 | 74 | 0.0135 | <0.001 | |
| Saturated fats k | 3 | Random | 1.28 | (1.07–1.48) | 0.007 | 53 | 0.0110 | 0.012 | |
| Trans fats l | 3 | Random | 1.39 | (1.26–1.53) | <0.001 | 45 | 0.0034 | 0.161 | |
| Region | Number of Studies | Sum Study Weights | Mean Risk Relation | p-Value for RR | I2 | τ 2 | p-Value for I2 and τ 2 | |
|---|---|---|---|---|---|---|---|---|
| Mean | (95% CI) | |||||||
| (%) | Per 10 units GI/d | (%) | ||||||
| 1. Asia, east a | 3 | 33 | 1.17 | (1.08–1.28) | <0.001 | 9 | 0.002 | 0.33 |
| 2. Australia b | 2 | 14 | 1.29 | (1.05–1.58) | 0.017 | 0 | 0.000 | 0.44 |
| 3. Europe c | 1 | 5 | 0.92 | (0.63–1.35) | - | - | - | - |
| 4. Euro-American d | 4 | 49 | 1.38 | (1.24–1.52) | <0.001 | 43 | 0.005 | 0.16 |
| 5. European ancestry e | 7 | 68 | 1.32 | (1.19–1.47) | <0.001 | 45 | 0.007 | 0.093 |
| 6. Eastern ancestry f | 3 | 33 | 1.27 | (1.27–1.34) | <0.001 | 0 | 0.000 | 0.84 |
| 7. Eastern ancestry g | 4 | -- | 1.26 | (1.20–1.33) | <0.001 | 0 | 0.000 | 0.80 |
| Number of Studies | Model a | Mean Relative Risks (95% CI) | p-Value for RR | Inconsist-ency | Heterogeneity | p-Value for τ 2 and I2 | ||
|---|---|---|---|---|---|---|---|---|
| Per 80 Units GL per 2000 kcal b | (I2 %) | (τ 2) | ||||||
| (1) Studies with CORR ≤ 0.55 | ||||||||
| 1. All such eligible studies c | 6 | Fixed | 1.09 | (0.99–1.21) | 0.086 | (0) d | - | - |
| Random | 1.09 | (0.97–1.23) | 0.133 | 19 | 0.0040 | 0.289 | ||
| (2) Studies with CORR > 0.55 | ||||||||
| 2. All such eligible studies e | 15 | Fixed | 1.24 | (1.16–1.32) | <0.001 | (0) | - | |
| Random | 1.26 | (1.15–1.37) | <0.001 | 35 | 0.0089 | 0.091 | ||
| 3. Clinical report of T2D in ≥0.97 of studies f | 9 | Fixed | 1.24 | (1.14–1.36) | <0.001 | (0) | ||
| Random | 1.33 | (1.14–1.55) | <0.001 | 58 | 0.0278 | 0.014 | ||
| 4. Adjusted for CORR (centered on 0.7) g | 15 | Fixed | 1.36 | (1.26–1.48) | <0.001 | (0) | - | - |
| Random | 1.36 | (1.26–1.48) | <0.001 | 0 | 0 | 0.78 | ||
| 5. Adjusted for CORR (centered on 0.7) and FHD (centered on 0.50) h | 21 | Fixed | 1.34 | (1.24–1.46) | <0.001 | (0) | - | - |
| Random | 1.34 | (1.24–1.46) | <0.001 | 1 | –0.0002 | 0.643 | ||
| 6. After 5. Women-only studies i | 8 | Fixed | 1.38 | (1.27–1.51) | <0.001 | (0) | - | - |
| Random | 1.38 | (1.27–1.51) | <0.001 | 0 | 0 | 0.784 | ||
| 7. After 5. Men-only studies, j | 5 | Fixed | 1.30 | (1.16–1.44) | <0.001 | (0) | - | - |
| Random | 1.30 | (1.16–1.44) | <0.001 | 0 | 0 | 0.640 | ||
| 8. Three largest studies (China and the USA) k | 3 | Fixed | 1.29 | (1.19–1.39) | <0.001 | (0) | ||
| Random | 1.29 | (1.19–1.39) | <0.001 | (0) | 0 | 0.706 | ||
| Subjects/Study | Number of Studies | Model | Mean Risk Relation | p-Value for RR | Inconsistency (I2) | p-Value for I2 | |||
|---|---|---|---|---|---|---|---|---|---|
| By Extreme | By Dose (GL) | ||||||||
| Quintiles | Response | (95%CI) | |||||||
| Per 10th to | Per 80 g | Corresponding | % | ||||||
| 90th pctl of | increment in | units, see left | |||||||
| population | GL in a 2000 | ||||||||
| GL | kcal diet | ||||||||
| 1. | All eligible studies | 22 | Random | 1.33 | (1.21–1.45) | <0.001 | 4 | 0.66 | |
| with centered covariates b | |||||||||
| 2. | Women-only studies c | 8 | Random | 1.42 | (1.30–1.54) | <0.001 | 0 d | 0.53 | |
| Men-only studies e | 6 | Random | 1.23 | (1.11–1.37) | <0.001 | 0 | 0.53 | ||
| 3. | Women-only BMI. | 4 | Random | 1.23 | - g | (0.99–1.50) | 0.067 | 0 | 0.91 |
| <25 or <27 f | |||||||||
| Women-only BMI | 4 | Random | 1.33 | - g | (1.16–1.54) | <0.001 | 0 | 0.48 | |
| ≥ 25 or ≥27 f | |||||||||
| 4. | Additional adjustment for | 22 | Random | 1.32 | (1.20–1.44) | <0.001 | 3 | 0.41 | |
| conditional protein intake h | |||||||||
| 5. | Replacement of | 22 | Random | 1.31 | (1.19–1.44) | <0.001 | 3 | 0.42 | |
| SEX as covariate by | |||||||||
| ALC as covariate | |||||||||
| when known (SEX not kept) i | |||||||||
| 6. | Additional adjustment | 22 | Random | 1.31 | (1.20–1.44) | <0.001 | 2 | 0.50 | |
| for ALC intake when | |||||||||
| known (covariate SEX kept) j | |||||||||
| Studies Included in the Meta-Analysis with Covariates a | Number of Studies | Relative Risk (RR) and 95%CI | p-Value for RR | Inconsistency (I2) | p-Value for I2 | |
|---|---|---|---|---|---|---|
| Based on Random Effects | ||||||
| Mean | (95% CI) | |||||
| Per 80 g increment | % | |||||
| in GL in a 2000 kcal (8400 kJ diet) | ||||||
| All included studies a,b | 22 | 1.33 | (1.21–1.45) | <0.001 | 4 | 0.66 |
| Dropping health professionals’ NHS I, NHS II, and HPS studies a,c | 19 | 1.33 | (1.20–1.48) | <0.001 | 12 | 0.31 |
| Dropping health professionals’ and outlier studies from Meyer et al. (2000) and Sluijs et al. (2010) a,d | 17 | 1.34 | (1.22–1.47) | <0.001 | 0 | 0.87 |
| Studies | n | Mean Risk Relation | 95%CI | p-Value | I2 | Person Years | Figure Table or Section | Model Effects | |
|---|---|---|---|---|---|---|---|---|---|
| Per 10 units GI | % | Millions | |||||||
| T2D–Glycemic index relative risk | |||||||||
| 1 | Studies with CORR ≤ 0.55. a | 7 | 1.05 | (0.97–1.14) | 0.271 | 65 | - | Table 1 | Random |
| 2 | Studies with CORR > 0.55. b | 10 | 1.27 | (1.15–1.40) | <0.001 | 68 | 5.0 | Table 1 | Random |
| 3 | As 2 + study-level adjustment for clinical report of T2D. c | 6 | 1.40 | (1.29–1.51) | <0.001 | 14 | 4.2 | 3.2.3 | Random |
| 4 | As 3 + study-level adjustment for FHD. d | 5 | 1.41 | (1.32–1.51) | <0.001 | 5 | 3.8 | 3.2.13 | Random |
| Per 80 g/d GL in 2000 kcal | |||||||||
| T2D–Glycemic load relative risk | (8400 kJ) diet | ||||||||
| 1 | Studies with CORR ≤ 0.55. e | 6 | 1.09 | (0.97–1.23) | 0.133 | 19 | - | Table 4 | Random |
| 2 | Studies with CORR > 0.55. f | 15 | 1.26 | (1.15–1.37) | <0.001 | 35 | 4.4 | Table 4 | Random |
| 3 | As 2 + study-level adjustment for clinical report of T2D. g | 9 | 1.33 | (1.14–1.55) | <0.001 | 58 | 3.8 | Table 4 | Random |
| 4 | As 3 + study-level adjustment for FHD. h | 4 | 1.61 h | (1.18–2.12) | 0.003 | 31 | 3.1 | 3.3.16 | Random |
| n | Mean Risk Relation | 95%CI | p-Value | I2 | Person Years | Figure or Table or Section | ModelEffects | |||
|---|---|---|---|---|---|---|---|---|---|---|
| T2D–glycemic index risk relation | Per 10 units GI | % | Millions | |||||||
| 1 | Meta-analysis-level adjusted for CORR (centered on 0.7) (includes CORR ≤ 0.55 and CORR > 0.55). a | 15 | 1.25 | (1.12–1.41) | <0.001 | 67 | 6.6 | _ a | Random | |
| 2 | As 1 dropping invalid studies (CORR ≤ 0.55). b | 10 | 1.24 | (1.11–1.37) | <0.001 | 57 | 5.0 | – b | Random | |
| 3 | As 1 + meta-analysis level adjusted for studies having | 14 | 1.28 | (1.23–1.34) | <0.001 | 0 | 6.5 | 3.2.13 | Random | |
| made study-level adjustments for FHD | ||||||||||
| (centered on 0.5). c | ||||||||||
| 4 | As 3 + covariate for ALC intake (centered on 7 g/d). d | 15 | 1.26 | (1.21–1.32) | <0.001 | 0 | 6.9 | 3.2.14 | Random | |
| Per 80 g/d GL in a 2000 kcal | ||||||||||
| T2D–glycemic load risk relation | (8400 kJ) diet | |||||||||
| 1 | Meta-analysis-level adjusted for CORR (centered on 0.7) (includes CORR ≤ 0.55 and >0.55). e | 21 | 1.32 | (1.22–1.43) | <0.001 | 1 | 6.3 | 3.3.16 | Random | |
| 2 | As 1 dropping invalid studies (CORR ≤ 0.55). f | 15 | 1.36 | (1.26–1.48) | <0.001 | 0 | 4.4 | 3.3.5 | Random | |
| 3 | As 1 + meta-analysis level adjustment for FHD (centered on 0.5). g | 21 | 1.34 | (1.24–1.46) | <0.001 | 0 | 6.3 | Table 4 | Random | |
| 4 | As 3 + covariate for population average ALC | 21 | 1.35 d | (1.22–1.49) | <0.001 | 0 | 6.3 | 3.3.16 | Random | |
| consumption (centered on 7 g/d). h | ||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies. Nutrients 2019, 11, 1280. https://doi.org/10.3390/nu11061280
Livesey G, Taylor R, Livesey HF, Buyken AE, Jenkins DJA, Augustin LSA, Sievenpiper JL, Barclay AW, Liu S, Wolever TMS, et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies. Nutrients. 2019; 11(6):1280. https://doi.org/10.3390/nu11061280
Chicago/Turabian StyleLivesey, Geoffrey, Richard Taylor, Helen F. Livesey, Anette E. Buyken, David J. A. Jenkins, Livia S. A. Augustin, John L. Sievenpiper, Alan W. Barclay, Simin Liu, Thomas M. S. Wolever, and et al. 2019. "Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies" Nutrients 11, no. 6: 1280. https://doi.org/10.3390/nu11061280
APA StyleLivesey, G., Taylor, R., Livesey, H. F., Buyken, A. E., Jenkins, D. J. A., Augustin, L. S. A., Sievenpiper, J. L., Barclay, A. W., Liu, S., Wolever, T. M. S., Willett, W. C., Brighenti, F., Salas-Salvadó, J., Björck, I., Rizkalla, S. W., Riccardi, G., Vecchia, C. L., Ceriello, A., Trichopoulou, A., ... Brand-Miller, J. C. (2019). Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies. Nutrients, 11(6), 1280. https://doi.org/10.3390/nu11061280