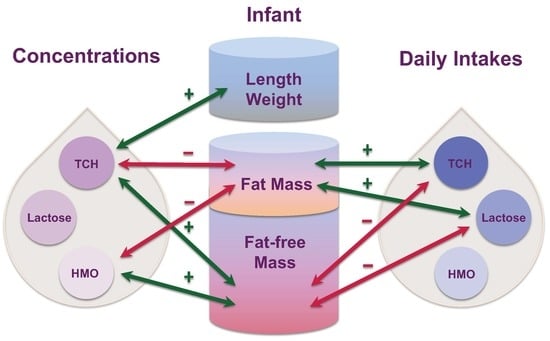
Carbohydrates in Human Milk and Body Composition of Term Infants during the First 12 Months of Lactation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Measurement of Human Milk Carbohydrates
2.3. Calculated Daily Intakes of Carbohydrates
2.4. Statistical Analyses
3. Results
3.1. Subjects
3.2. Changes in Body Composition and Human Milk Carbohydrates during First Year of Lactation
3.3. Maternal Body Composition and Carbohydrates
3.4. Infant Body Composition and Concentrations of Carbohydrates
3.5. Infant Body Composition and Daily Intakes of Carbohydrates
3.6. Associations between Breastfeeding Parameters and Carbohydrates
3.7. Changes in Infant Body Composition and Calculated Daily Intakes of Carbohydrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Maternal Predictor | Between 2 and 5 Months (n = 17) | 9 Months (n = 7) | 12 Months (n = 7) | p-Value (n = 18) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | Predictor | Infant Age (Months) | Interaction | |
| Calculated daily intake of human milk oligosaccharides (g) | |||||||||
| Fat mass (%) | 1.64 (10.50) a | 0.322 (0.315) | 18.60 (16.00) | −0.209 (0.525) | 64.80 (20.10) | −2.01 (0.735) | 0.80 b | 0.90 | 0.014 c |
| FM/FFM | 6.25 (6.75) | 11.80 (12.80) | 16.30 (10.80) | −9.10 (23.70) | 52.40 (15.40) | −112.0 (40.30) | 0.93 | 0.92 | 0.013 |
| Predictor | Between 2 and 5 Months (n = 17) | 9 Months (n = 8) | 12 Months (n = 8) | P-Value (n = 18) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | Predictor | Infant Age (Months) | Interaction | |
| CDI of TCH (g) d | |||||||||
| Concentration of TCH (g) | 21.80 (17.10) a | 0.544 (0.207) | −4.05 (19.30) | 0.544 (0.207) | −5.47 (18.60) | 0.544 (0.207) | 0.009 b | <0.001 | 0.50 c |
| 24-h milk intake (g) d | −5.84 (7.05) | 0.087 (0.008) | 0.92 (5.13) | 0.087 (0.008) | 0.70 (5.05) | 0.087 (0.008) | <0.001 | 0.22 | 0.059 |
| Feeding frequency (24-h MP) d | 21.80 (17.30) | 5.39 (2.07) | 15.80 (12.30) | 5.39 (2.07) | 15.80 (11.20) | 5.39 (2.07) | 0.009 | 0.69 | 0.13 |
| Feeding frequency (SR) d | 77.30 (5.65) | −4.25 (1.35) | 60.50 (7.32) | −4.25 (1.35) | 68.40 (10.40) | −4.25 (1.35) | <0.001 | <0.001 | 0.052 |
| CDI of lactose (g) d | |||||||||
| Concentration of lactose (g) | 17.80 (24.00) | 0.551 (0.366) | −3.56 (25.40) | 0.551 (0.366) | −7.77 (25.70) | 0.551 (0.366) | 0.14 | <0.001 | 0.31 |
| 24-h milk intake (g) | −2.80 (2.54) | 0.069 (0.003) | −0.50 (1.86) | 0.069 (0.003) | −0.78 (1.72) | 0.069 (0.003) | <0.001 | 0.33 | 0.29 |
| Feeding frequency (24-h MP) | 15.10 (11.90) | 4.74 (1.42) | 8.98 (8.45) | 4.74 (1.42) | 9.29 (7.38) | 4.74 (1.42) | <0.001 | 0.51 | 0.12 |
| Feeding frequency (SR) | 62.00 (4.26) | −3.04 (1.04) | 45.70 (5.60) | −3.04 (1.04) | 49.80 (7.71) | −3.04 (1.04) | 0.006 | <0.001 | 0.78 |
| CDI of HMO (g) d | |||||||||
| Concentration of HMO (g) | 2.62 (1.87) | 0.612 (0.105) | 2.47 (2.96) | 0.388 (0.125) | −4.50 (1.91) | 0.799 (0.071) | <0.001 | 0.001 | 0.014 |
| 24-h milk intake (g) | 10.00 (8.24) | 0.003 (0.010) | −0.04 (10.00) | 0.022 (0.019) | −16.10 (8.20) | 0.057 (0.016) | 0.020 | 0.50 | 0.016 |
| Feeding frequency (24-h MP) | 0.42 (9.60) | 1.45 (1.15) | 3.00 (6.99) | 1.45 (1.15) | 4.12 (6.32) | 1.45 (1.15) | 0.22 | 0.82 | 0.76 |
| Feeding frequency (SR) | 15.30 (3.62) | −1.10 (1.00) | 15.10 (5.16) | −1.10 (1.00) | 18.10 (7.54) | −1.10 (1.00) | 0.29 | 0.87 | 0.36 |
| Changes in Infant Characteristic (Response) | Months after Birth | |||||
|---|---|---|---|---|---|---|
| 5 and 2 | 9 and 2 | 12 and 2 | 9 and 5 | 12 and 5 | 12 and 9 | |
| Total carbohydrates CDI (g) between 2 and 5 months (n = 17) c | ||||||
| ΔLength (cm) | −0.006 (0.026) a 0.83 b | 0.041 (0.028) 0.18 | 0.074 (0.026) d 0.023 | 0.003 (0.024) 0.91 | 0.013 (0.030) 0.67 | 0.016 (0.030) 0.59 |
| ΔWeight (kg) | 0.018 (0.008) 0.039 | 0.025 (0.012) 0.067 | 0.025 (0.014) 0.11 | 0.002 (0.007) 0.81 | −0.0002 (0.009) 0.98 | −0.001 (0.003) 0.81 |
| ΔBMI (kg/m2) | 0.040 (0.016) 0.037 | 0.013 (0.025) 0.61 | −0.008 (0.029) 0.79 | −0.009 (0.017) 0.59 | −0.022 (0.021) 0.30 | −0.013 (0.012) 0.31 |
| ΔFFM with US 4SF (kg) | 0.013 (0.012) 0.30 | 0.034 (0.013) 0.038 | 0.045 (0.016) 0.024 | 0.004 (0.007) 0.57 | 0.007 (0.008) 0.39 | 0.004 (0.004) 0.33 |
| ΔFFM with BIS (kg) | 0.009 (0.004) 0.045 | 0.021 (0.013) 0.16 | 0.024 (0.010) 0.051 | 0.003 (0.008) 0.67 | 0.006 (0.010) 0.55 | 0.006 (0.009) 0.54 |
| ΔFM with US 2SF (kg) | 0.024 (0.008) 0.019 | 0.007 (0.008) 0.41 | 0.011 (0.013) 0.42 | −0.003 (0.008) 0.72 | −0.003 (0.007) 0.65 | 0.001 (0.003) 0.85 |
| ΔFMI with US 2SF (kg/m2) | 0.050 (0.018) 0.022 | 0.001 (0.018) 0.94 | −0.004 (0.027) 0.88 | −0.013 (0.017) 0.46 | −0.020 (0.018) 0.28 | −0.006 (0.007) 0.44 |
| ΔFMI with US 4SF (kg/m2) | 0.006 (0.015) 0.73 | −0.043 (0.030) 0.19 | −0.064 (0.033) 0.10 | −0.014 (0.017) 0.41 | −0.029 (0.014) 0.071 | −0.015 (0.006) 0.031 |
| ΔFM with US 4SF (%) | −0.037 (0.092) 0.70 | −0.247 (0.136) 0.11 | −0.371 (0.139) 0.032 | −0.058 (0.075) 0.45 | −0.115 (0.063) 0.089 | −0.060 (0.036) 0.12 |
| ΔFM/FFM with US 4SF | −0.0004 (0.002) 0.83 | −0.004 (0.002) 0.12 | −0.007 (0.003) 0.034 | −0.002 (0.002) 0.13 | −0.002 (0.001) 0.26 | −0.001 (0.001) 0.31 |
| ΔFM/FFM with BIS | 0.001 (0.001) 0.37 | −0.001 (0.002) 0.71 | −0.005 (0.002) 0.040 | −0.002 (0.001) 0.14 | −0.003 (0.002) 0.15 | −0.001 (0.002) 0.58 |
| Total carbohydrates CDI (g) at 9 months (n = 8) c | ||||||
| ΔBMI (kg/m2) | n/a e | −0.198 (0.272) 0.54 | −0.009 (0.238) 0.97 | −0.062 (0.037) 0.16 | −0.081 (0.029) 0.037 | −0.019 (0.028) 0.52 |
| ΔFFM with US 2SF (kg) | n/a | 0.107 (0.021) 0.037 | −0.011 (0.032) 0.76 | −0.006 (0.017) 0.75 | 0.002 (0.019) 0.92 | −0.008 (0.014) 0.62 |
| ΔFM with BIS (kg) | n/a | −0.029 (0.122) 0.84 | −0.095 (0.062) 0.27 | −0.022 (0.019) 0.30 | −0.033 (0.010) 0.018 | −0.011 (0.015) 0.50 |
| ΔFMI with BIS (kg/m2) | n/a | −0.114 (0.315) 0.75 | −0.181 (0.194) 0.45 | −0.051 (0.041) 0.27 | −0.077 (0.028) 0.040 | −0.027 (0.037) 0.51 |
| Total carbohydrates CDI (g) at 12 months (n = 8) c | ||||||
| ΔFFMI with US 2SF (kg/m2) | n/a e | n/a e | −0.093 (0.030) 0.092 | n/a e | −0.038 (0.014) 0.038 | −0.021 (0.007) 0.033 |
| ΔFFMI with US 4SF (kg/m2) | n/a | n/a | −0.089 (0.055) 0.25 | n/a | −0.031 (0.009) 0.029 | −0.022 (0.011) 0.11 |
| Changes in Infant Characteristic (Response) | Months after Birth | |||||
|---|---|---|---|---|---|---|
| 5 and 2 | 9 and 2 | 12 and 2 | 9 and 5 | 12 and 5 | 12 and 9 | |
| HMO CDI (g) between 2 and 5 months (n = 17) c | ||||||
| ΔHead circumference (cm) | 0.083 (0.054) a 0.16 b | −0.007 (0.075) 0.93 | 0.087 (0.063) 0.21 | −0.010 (0.030) 0.74 | 0.047 (0.018) 0.023 | 0.047 (0.028) 0.12 |
| HMO CDI (g) at 9 months (n = 8) c | ||||||
| ΔBMI (kg/m2) | n/a e | −0.425 (0.231) 0.21 | −0.401 (0.082) d 0.040 | −0.247 (0.129) 0.11 | −0.361 (0.053) 0.001 | −0.114 (0.096) 0.29 |
| ΔFFMI with US 4SF (kg/m2) | n/a | −0.130 (0.208) 0.60 | −0.211 (0.295) 0.55 | −0.024 (0.094) 0.81 | −0.185 (0.119) 0.20 | −0.169 (0.058) 0.032 |
| ΔFM with BIS 5 (kg) | n/a | −0.200 (0.058) 0.075 | −0.048 (0.108) 0.70 | −0.115 (0.060) 0.12 | −0.110 (0.043) 0.049 | 0.005 (0.059) 0.94 |
| ΔFMI with BIS (kg/m2) | n/a | −0.513 (0.176) 0.10 | −0.186 (0.257) 0.55 | −0.274 (0.119) 0.069 | −0.294 (0.097) 0.029 | −0.019 (0.143) 0.90 |
| HMO CDI (g) at 12 months (n = 8) c | ||||||
| ΔFMI with BIS (kg/m2) | n/a e | n/a e | −0.336 (0.327) 0.41 | n/a e | −0.052 (0.017) 0.032 | −0.037 (0.024) 0.17 |
| ΔFM with BIS (%) | n/a | n/a | −1.488 (1.901) 0.52 | n/a | −0.184 (0.070) 0.047 | −0.157 (0.124) 0.26 |
| ΔFM/FFM with BIS | n/a | n/a | −0.024 (0.032) 0.53 | n/a | −0.004 (0.001) 0.021 | −0.003 (0.002) 0.24 |
References
- Koletzko, B.; Brands, B.; Chourdakis, M.; Cramer, S.; Grote, V.; Hellmuth, C.; Kirchberg, F.; Prell, C.; Rzehak, P.; Uhl, O.; et al. The power of programming and the EarlyNutrition project: Opportunities for health promotion by nutrition during the first thousand days of life and beyond. Ann. Nutr. Metab. 2014, 64, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.; Chomoto, S.; Fewtrell, M.S. Programming of body composition by early growth and nutrition. Proc. Nutr. Soc. 2007, 66, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; Franca, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, R.; Park, S.; Galuska, D.A.; Sherry, B.; Freedman, D.S. A longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics 2014, 134, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.A.; Hedderley, D.I.; Herath, T.D.; Paturi, G.; Glyn-Jones, S.; Wiens, F.; Stahl, B.; Gopal, P. Human milk composition and dietary intakes of breastfeeding women of different ethnicity from the Manawatu-Wanganui region of New Zealand. Nutrients 2018, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A. Regional variations in the composition of human milk. In Handbook of Milk Composition; Jensen, R.G., Ed.; Academic Press, Inc.: San Diego, CA, USA, 1995; p. 919. [Google Scholar]
- Kugananthan, S.; Gridneva, Z.; Lai, C.T.; Hepworth, A.R.; Mark, P.J.; Kakulas, F.; Geddes, D.T. Associations between maternal body composition and appetite hormones and macronutrients in human milk. Nutrients 2017, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.A.; Sherriff, J.L.; Hartmann, P.E. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br. J. Nutr. 2002, 88, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Arthur, P.G.; Smith, M.; Hartmann, P.E. Milk lactose, citrate, and glucose as markers of lactogenesis in normal and diabetic women. J. Pediatr. Gastroenterol. Nutr. 1989, 9, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Nommsen, L.A.; Lovelady, C.A.; Heinig, M.; Lonnerdal, B.; Dewey, K.G. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: The Darling study. Am. J. Clin. Nutr. 1991, 53, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hepworth, A.R.; Prime, D.K.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Variation in fat, lactose, and protein composition in breast milk over 24 hours: Associations with infant feeding patterns. J. Hum. Lact. 2013, 29, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Rea, A.; Hepworth, A.R.; Ward, L.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Relationships between breastfeeding patterns and maternal and infant body composition over the first 12 months of lactation. Nutrients 2018, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Ninonuevo, M.R.; Park, Y.; Yin, H.; Zhang, J.; Ward, R.E.; Clowers, B.H.; German, J.B.; Freeman, S.L.; Killeen, K.; Grimm, R.; et al. A strategy for annotating the human milk glycome. J. Agric. Food Chem. 2006, 54, 7471–7480. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. The functional biology of human milk oligosaccharides. Early Hum. Dev. 2015, 91, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Alderete, T.A.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.; Fields, D. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.; Wong, W.; Hopkinson, J.; Smith, E.; Ellis, K. Infant feeding mode affects early growth and body composition. Pediatrics 2000, 16, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Prentice, P.; Ong, K.K.; Schoemaker, M.H.; van Tol, E.A.F.; Vervoort, J.; Hughes, I.A.; Acerini, C.L. Breast milk nutrient content and infancy growth. Acta Paediatr. 2016, 105, 641–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goran, M.I.; Martin, A.A.; Alderete, T.A.; Fujiwara, H.; Fields, D. Fructose in breast milk is positively associated with infant body composition at 6 months of age. Nutrients 2017, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.; Demerath, E. Relationship of insulin, glucose, leptin, IL-6 and TNF-a in human breast milk with infant growth and body composition. Pediatr. Obes. 2012, 7, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A.; Hartmann, P.E. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006, 117, e387–e395. [Google Scholar] [CrossRef] [PubMed]
- Binns, C.W.; Fraser, M.L.; Lee, A.H.; Scott, J. Defining exclusive breastfeeding in Australia. J. Paediatr. Child Health 2009, 45, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Kugananthan, S.; Rea, A.; Lai, C.T.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human milk adiponectin and leptin and infant body composition over the first 12 months of lactation. Nutrients 2018, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Tie, W.J.; Rea, A.; Lai, C.T.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human milk casein and whey protein and infant body composition over the first 12 months of lactation. Nutrients 2018, 10, 1332. [Google Scholar] [CrossRef]
- Arthur, P.; Hartmann, P.; Smith, M. Measurement of the milk intake of breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 758–763. [Google Scholar] [CrossRef]
- Gridneva, Z.; Hepworth, A.R.; Ward, L.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Determinants of body composition in breastfed infants using bioimpedance spectroscopy and ultrasound skinfolds-Methods comparison. Pediatr. Res. 2016, 81, 423–433. [Google Scholar] [CrossRef]
- Van Itallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef]
- Wells, J.C.K.; Cole, T.J. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int. J. Obes. 2002, 26, 947–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Purcell, S.A.; Prado, C.M.; Gonzalez, M.C. Fat mass to fat-free mass ratio reference values from NHANES III using bioelectrical impedance analysis. Clin. Nutr. 2018, 37, 2284–2287. [Google Scholar] [CrossRef] [PubMed]
- Euber, J.; Brunner, J. Determination of lactose in milk products by high-performance liquid chromatography. J. Dairy Sci. 1979, 62, 685–690. [Google Scholar] [CrossRef]
- Albalasmeh, A.; Berhe, A.; Ghezzehei, T. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.; Neubauer, S. Carbohydrates in milks: Analysis, quantities, and significance. In Handbook of Milk Composition; Jensen, R., Ed.; Academic Press, Inc.: San Diego, CA, USA, 1995; pp. 273–349. [Google Scholar]
- Khan, S.; Prime, D.K.; Hepworth, A.R.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Investigation of short-term variations in term breast milk composition during repeated breast expression sessions. J. Hum. Lact. 2013, 29, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Curran-Everett, D. Multiple comparisons: Philosophies and illustrations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1–R8. [Google Scholar] [CrossRef] [PubMed]
- Hambraeus, L. Human milk composition. Nutr. Abstr. Rev. Clin. Nutr. 1984, 54, 219–236. [Google Scholar]
- Young, B.E.; Patinkin, Z.W.; Pyle, L.; de la Houssaye, B.; Davidson, B.S.; Geraghty, S.; Morrow, A.L.; Krebs, N. Markers of oxidative stress in human milk do not differ by maternal BMI but are related to infant growth trajectories. Matern. Child Health J. 2017, 21, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Pierce, K.; Callan, J.; McCarthy, P.; O’Doherty, J. Effects of high dietary concentration of lactose and increased soyabean meal inclusion in starter diets for piglets. Anim. Sci. 2004, 79, 445–452. [Google Scholar] [CrossRef]
- Cederlund, A.; Kai-Larsen, Y.; Printz, G.; Yoshio, H.; Alvelius, G.; Lagercrantz, H.; Strömberg, R.; Jörnvall, H.; Gudmundsson, G.H.; Agerberth, B. Lactose in human breast milk, an inducer of innate immunity with implications for a role in intestinal homeostasis. PLoS ONE 2013, 8, e53876:1–e53876:12. [Google Scholar] [CrossRef]
- Koleva, P.T.; Bridgman, S.L.; Kozyrskyj, A.L. The infant gut microbiome: Evidence for obesity risk and dietary intervention. Nutrients 2015, 7, 2237–2260. [Google Scholar] [CrossRef] [PubMed]
- Quinn, E.A.; Largado, F.; Power, M.; Kuzawa, C.W. Predictors of breast milk macronutrient composition in Filipino mothers. Am. J. Hum. Biol. 2012, 24, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.F.; Skafte, L.; Badsberg, J.H.; Jorgensen, M. Variation in macronutrients in human bank milk: Influencing factors and implications for human milk banking. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Coppa, G.V.; Gabrielli, O.; Pierani, P.; Catassi, C.; Carlucci, A.; Giorgi, P.L. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics 1993, 91, 637–641. [Google Scholar] [PubMed]
- Sprenger, N.; Lee, L.Y.; De Castro, C.A.; Steenhout, P.; Thakkar, S.K. Longitudinal change of selected human milk oligosaccharides and association to infants’ growth, an observatory, single center, longitudinal cohort study. PLoS ONE 2017, 12, e0171814:1–e0171814:15. [Google Scholar] [CrossRef] [PubMed]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of infant formula with human milk oligosaccharides on growth and morbidity: A randomized multicenter trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-T.; Chen, C.; Newburg, D.S. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 2013, 23, 1281–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [Green Version]
- Gridneva, Z.; Kugananthan, S.; Hepworth, A.R.; Tie, W.J.; Lai, C.T.; Ward, L.C.; Hartmann, P.E.; Geddes, D.T. Effect of human milk appetite hormones, macronutrients, and infant characteristics on gastric emptying and breastfeeding patterns of term fully breastfed infants. Nutrients 2017, 9, 15. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Aigner, J.; Reiter, B.; Köfeler, H.; Csapo, B.; Desoye, G.; Bode, L.; van Poppel, M.N.M. Evidence of human milk oligosaccharides in maternal circulation already during pregnancy—A pilot study. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E347–E357. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | 2 Months | 5 Months | 9 Months | 12 Months |
|---|---|---|---|---|
| Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | |
| FM/FFM ratios | (n = 14) | (n = 20) | (M = 18/I = 17) | (M = 17/I = 15) |
| Maternal FM/FFM | 0.56 ± 0.14 a (0.35–0.81) | 0.51 ± 0.16 (0.30–0.89) | 0.48 ± 0.16 (0.25–0.80) | 0.43 ± 0.16 (0.24–0.80) |
| Infant FM/FFM with US 2SF | 0.32 ± 0.04 (0.26–0.61) | 0.37 ± 0.09 (0.24–0.64) | 0.35 ± 0.08 (0.18–0.53) | 0.34 ± 0.06 (0.22–0.45) |
| Infant FM/FFM with US 4SF | 0.33 ± 0.05 (0.24–0.44) | 0.36 ± 0.07 (0.26–0.56) | 0.34 ± 0.07 (0.21–0.45) | 0.31 ± 0.06 (0.21–0.41) |
| Infant FM/FFM with BIS | 0.28 ± 0.04 (0.22–0.34) | 0.41 ± 0.07 (0.28–0.56) | 0.34 ± 0.08 (0.19–0.46) | 0.33 ± 0.06 (0.24–0.44) |
| Concentrations | (n = 15) | (n = 20) | (n = 19) | (n = 14) |
| Total carbohydrates (g/L) | 86.7 ± 9.2 (67.1–97.5) | 80.7 ± 7.9 (69.3–94.1) | 87.8 ± 11.1 (60.9–105.6) | 88.4 ± 21.2 (56.9–126.9) |
| Lactose (g/L) | 64.5 ± 4.1 (59.1–77.9) | 64.3 ± 5.9 (53.5–70.6) | 65.3 ± 5.3 (57.6–79.0) | 66.9 ± 4.0 (60.1–79.3) |
| HMO (g/L) | 22.3 ± 10.7 (0–35.8) | 16.4 ± 9.9 (2.3–29.9) | 22.5 ± 9.2 (0–36.9) | 21.4 ± 22.3 (3.0–62.2) |
| CDI b | n/a b | (n = 17) | (n = 8) | (n = 8) |
| Total carbohydrates (g) | n/a | 63.2 ± 15.0 (42.9–97.2) | 44.8 ± 15.2 (21.2–69.6) | 40.7 ± 29.8 (22.2–100.9) |
| Lactose (g) | n/a | 51.2 ± 14.5 (32.6–83.6) | 34.0 ± 11.0 (19.6–51.3) | 28.7 ± 12.1 (18.0–51.4) |
| HMO (g) | n/a | 12.0 ± 6.0 (2.0–21.6) | 10.8 ± 5.4 (0–15.7) | 12.0 ± 18.5 (1.5–49.5) |
| Changes in Characteristics between Time Points | Months after Birth | ||||||
|---|---|---|---|---|---|---|---|
| 5 and 2 Months | 9 and 2 Months | 12 and 2 Months | 9 and 5 Months | 12 and 5 Months | 12 and 9 Months | Overall p Value | |
| FM/FFM ratios | (n = 14) | (n = 12) | (M = 11/I = 10) | (M = 18/I = 17) | (M = 18/I = 17) | (M = 16/I = 13) | (n = 20) |
| Maternal FM/FFM | 0.01 ± 0.01 b (0.98) | −0.02 ± 0.01 (0.63) | −0.04 ± 0.01 d (0.002) | −0.02 ± 0.01 (0.27) | −0.05 ± 0.01 d (<0.001) | −0.03 ± 0.01 d (0.042) | <0.001 c,d |
| Infant FM/FFM with ultrasound 2-skinfolds | 0.03 ± 0.03 (0.69) | 0.01 ± 0.03 (0.96) | −0.003 ± 0.03 (1.00) | −0.01 ± 0.02 (0.93) | −0.03 ± 0.02 (0.61) | −0.02 ± 0.03 (0.93) | 0.59 |
| Infant FM/FFM with ultrasound 4-skinfolds | 0.02 ± 0.02 (0.86) | 0.002 ± 0.02 (1.00) | −0.03 ± 0.02 (0.54) | −0.01 ± 0.02 (0.87) | −0.05 ± 0.02 (0.095) | −0.03 ± 0.02 (0.41) | 0.16 |
| Infant FM/FFM with BIS | 0.12 ± 0.02 (<0.001) | 0.06 ± 0.02 (0.028) | 0.05 ± 0.02 (0.17) | −0.06 ± 0.02 (0.006) | −0.07 ± 0.02 (0.002) | −0.01 ± 0.02 (0.95) | <0.001 |
| Concentrations | (n = 15) | (n = 14) | (n = 8) | (n = 19) | (n = 14) | (n = 14) | (n = 20) |
| Total carbohydrates (g/L) | −3.0 ± 3.7 (0.86) | 1.4 ± 3.8 (0.98) | −1.2 ± 4.1 (0.99) | 4.4 ± 3.5 (0.60) | 1.8 ± 3.8 (0.97) | −2.6 ± 3.9 (0.91) | 0.65 |
| Lactose (g/L) | −1.3 ± 1.8 (0.89) | −1.1 ± 1.8 (0.93) | 0.5 ± 1.9 (0.98) | 0.2 ± 1.6 (1.00) | 1.8 ± 1.8 (0.73) | 1.6 ± 1.8 (0.80) | 0.70 |
| HMO (g/L) | −2.2 ± 3.9 (0.94) | 2.2 ± 3.9 (0.95) | 0.7 ± 4.3 (1.00) | 4.3 ± 3.6 (0.63) | 2.9 ± 3.9 (0.89) | −1.4 ± 4.0 (0.98) | 0.69 |
| CDI | (n = 7) | (n = 6) | (n = 6) | (n = 9) | |||
| Total carbohydrates (g) e | n/a f | n/a f | n/a f | −21.0 ± 5.9 (0.001) | −24.7 ± 6.2 (<0.001) | −3.7 ± 6.6 (0.84) | 0.003 |
| Lactose (g) e | n/a | n/a | n/a | −19.4 ± 4.4 (<0.001) | −23.1 ± 4.4 (<0.001) | −3.8 ± 4.8 (0.71) | <0.001 |
| HMO (g) e | n/a | n/a | n/a | −1.4 ± 4.0 (0.94) | −1.5 ± 4.2 (0.94) | −0.05 ± 4.9 (1.00) | 0.91 |
| Predictor (Concentration, g/L) | 2 Months (n = 15) | 5 Months (n = 20) | 9 Months (n = 19) | 12 Months (n = 14) | P-Value (n = 20) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | Predictor | Infant Age (Months) | Interaction | |
| Infant Length (cm) | |||||||||||
| TCH | 53.90 (1.22) a | 0.047 (0.013) | 60.90 (1.15) | 0.047 (0.013) | 66.20 (1.21) | 0.047 (0.013) | 70.40 (1.17) | 0.047 (0.013) | <0.001 b | <0.001 | 0.62 c |
| Lactose | 53.60 (2.31) | 0.065 (0.034) | 60.50 (2.27) | 0.065 (0.034) | 66.10 (2.26) | 0.065 (0.034) | 70.0 (2.27) | 0.065 (0.034) | 0.047 | <0.001 | 0.093 |
| HMO | 57.40 (0.62) | 0.031 (0.014) | 64.30 (0.55) | 0.031 (0.014) | 69.70 (0.60) | 0.031 (0.014) | 73.70 (0.60) | 0.031 (0.014) | 0.036 | <0.001 | 0.67 |
| Infant Weight (kg) | |||||||||||
| TCH | 4.55 (0.42) | 0.013 (0.004) | 6.39 (0.40) | 0.013 (0.004) | 7.65 (0.41) | 0.013 (0.004) | 8.47 (0.40) | 0.013 (0.004) | 0.003 | <0.001 | 0.54 |
| HMO | 5.46 (0.22) | 0.009 (0.004) | 7.28 (0.21) | 0.009 (0.004) | 8.54 (0.21) | 0.009 (0.004) | 9.42 (0.22) | 0.009 (0.004) | 0.038 | <0.001 | 0.17 |
| Infant Body Mass Index (kg/m2) | |||||||||||
| TCH | 11.30 (2.04) | 0.063 (0.024) | 18.20 (2.38) | −0.007 (0.029) | 19.10 (1.83) | −0.016 (0.021) | 18.30 (1.29) | −0.013 (0.015) | 0.91 | <0.001 | 0.044 |
| HMO | 15.70 (0.51) | 0.049 (0.020) | 17.80 (0.52) | −0.008 (0.026) | 17.70 (0.59) | −0.001 (0.023) | 17.90 (0.47) | −0.026 (0.016) | 0.99 | <0.001 | 0.027 |
| Infant Fat-free Mass with Bioelectrical Impedance Spectroscopy (kg) | |||||||||||
| TCH | 2.63 (0.35) | 0.020 (0.004) | 3.69 (0.33) | 0.020 (0.004) | 4.78 (0.35) | 0.020 (0.004) | 5.53 (0.33) | 0.020 (0.004) | <0.001 | <0.001 | 0.28 |
| HMO | 4.02 (0.17) | 0.015 (0.004) | 5.05 (0.15) | 0.015 (0.004) | 6.17 (0.17) | 0.015 (0.004) | 6.90 (0.17) | 0.015 (0.004) | <0.001 | <0.001 | 0.069 |
| Infant Fat-free Mass with Ultrasound 2-skinfolds (kg) | |||||||||||
| TCH | 3.41 (0.38) | 0.009 (0.004) | 4.66 (0.36) | 0.009 (0.004) | 5.63 (0.38) | 0.009 (0.004) | 6.32 (0.37) | 0.009 (0.004) | 0.032 | <0.001 | 0.82 |
| Infant Fat-free Mass with Ultrasound 4-skinfolds (kg) | |||||||||||
| TCH | 3.35 (0.38) | 0.010 (0.004) | 4.66 (0.36) | 0.010 (0.004) | 5.64 (0.38) | 0.010 (0.004) | 6.46 (0.36) | 0.010 (0.004) | 0.020 | <0.001 | 0.86 |
| Infant Fat-free Mass Index with Bioelectrical Impedance Spectroscopy (kg/m2) | |||||||||||
| TCH | 10.90 (0.62) | 0.022 (0.007) | 10.80 (0.58) | 0.022 (0.007) | 11.20 (0.62) | 0.022 (0.007) | 11.20 (0.59) | 0.022 (0.007) | 0.002 | 0.054 | 0.83 |
| HMO | 12.40 (0.28) | 0.018 (0.007) | 12.30 (0.24) | 0.018 (0.007) | 12.70 (0.27) | 0.018 (0.007) | 12.80 (0.27) | 0.018 (0.007) | 0.008 | 0.030 | 0.30 |
| Infant Fat Mass with Bioelectrical Impedance Spectroscopy (kg) | |||||||||||
| TCH | −0.03 (0.71) | 0.016 (0.008) | 2.91 (0.83) | −0.010 (0.010) | 3.81 (0.63) | −0.018 (0.007) | 3.14 (0.44) | −0.010 (0.005) | 0.051 | <0.001 | 0.016 |
| HMO | 1.09 (0.17) | 0.012 (0.007) | 2.26 (0.17) | −0.009 (0.009) | 2.56 (0.20) | −0.016 (0.008) | 2.57 (0.15) | −0.011 (0.006) | 0.11 | <0.001 | 0.039 |
| Infant Fat Mass with Bioelectrical Impedance Spectroscopy (%) | |||||||||||
| TCH | 9.84 (6.56) | 0.154 (0.078) | 37.20 (7.66) | −0.106 (0.010) | 46.80 (5.87) | −0.244 (0.067) | 35.60 (4.09) | −0.131 (0.049) | 0.002 | <0.001 | 0.001 |
| HMO | 20.50 (1.54) | 0.122 (0.069) | 30.10 (1.56) | −0.100 (0.087) | 30.50 (1.87) | −0.241 (0.080) | 27.00 (1.40) | −0.121 (0.055) | 0.020 | <0.001 | 0.005 |
| Infant Fat Mass Index with Bioelectrical Impedance Spectroscopy (kg/m2) | |||||||||||
| TCH | 0.40 (1.47) | 0.041 (0.017) | 6.81 (1.72) | −0.022 (0.021) | 8.59 (1.32) | −0.046 (0.015) | 6.72 (0.92) | −0.029 (0.011) | 0.008 | <0.001 | 0.001 |
| HMO | 3.23 (0.36) | 0.033 (0.015) | 5.39 (0.36) | −0.021 (0.019) | 5.41 (0.43) | −0.042 (0.018) | 4.93 (0.32) | −0.032 (0.012) | 0.033 | <0.001 | 0.003 |
| Infant Fat Mass to Fat-free Mass Ratio with Bioelectrical Impedance Spectroscopy | |||||||||||
| TCH | 0.09 (0.12) | 0.003 (0.001) | 0.56 (0.14) | −0.002 (0.002) | 0.70 (0.10) | −0.004 (0.001) | 0.54 (0.07) | −0.003 (0.001) | 0.001 | <0.001 | 0.003 |
| HMO | 0.26 (0.03) | 0.002 (0.001) | 0.44 (0.03) | −0.002 (0.002) | 0.44 (0.03) | −0.004 (0.001) | 0.38 (0.03) | −0.002 (0.001) | 0.027 | <0.001 | 0.006 |
| Predictor (CDI d, g) | Between 2 and 5 Months (n = 17) | 9 Months (n = 8) | 12 Months (n = 8) | P-Value (n = 18) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | Predictor | Infant Age (Months) | Interaction | |
| Infant Body Mass Index (kg/m2) | |||||||||
| TCH | 15.60 (1.30) a | 0.033 (0.019) | 18.60 (1.47) | −0.013 (0.029) | 18.50 (0.72) | −0.018 (0.013) | 0.65 b | 0.77 | 0.019 c |
| Lactose | 15.70 (1.00) | 0.038 (0.018) | 17.90 (1.21) | 0.0003 (0.032) | 19.00 (0.88) | −0.040 (0.025) | 0.38 | 0.59 | 0.011 |
| Infant Fat-free Mass Index with Ultrasound 4-skinfolds (kg/m2) | |||||||||
| TCH | 15.20 (0.56) | −0.034 (0.007) | 14.70 (0.47) | −0.034 (0.007) | 15.10 (0.45) | −0.034 (0.007) | <0.001 | 0.035 | 0.37 |
| Lactose | 13.30 (0.73) | −0.006 (0.013) | 12.90 (0.87) | 0.003 (0.023) | 15.40 (0.65) | −0.060 (0.018) | 0.076 | 0.13 | 0.015 |
| Infant Fat Mass with Ultrasound 2-skinfolds (kg) | |||||||||
| TCH | 1.24 (0.29) | 0.011 (0.004) | 1.82 (0.25) | 0.011 (0.004) | 1.94 (0.23) | 0.011 (0.004) | 0.006 | <0.001 | 0.29 |
| Lactose | 1.23 (0.31) | 0.014 (0.006) | 1.93 (0.24) | 0.014 (0.006) | 2.06 (0.21) | 0.014 (0.006) | 0.008 | <0.001 | 0.19 |
| Infant Fat Mass with Ultrasound 4-skinfolds (kg) | |||||||||
| TCH | 1.09 (0.26) | 0.014 (0.004) | 1.73 (0.22) | 0.014 (0.004) | 1.64 (0.20) | 0.014 (0.004) | <0.001 | <0.001 | 0.16 |
| Lactose | 1.16 (0.30) | 0.015 (0.005) | 1.87 (0.23) | 0.015 (0.005) | 1.80 (0.21) | 0.015 (0.005) | 0.004 | <0.001 | 0.21 |
| HMO | 1.73 (0.14) | 0.020 (0.008) | 2.13 (0.18) | 0.020 (0.008) | 1.99 (0.17) | 0.020 (0.008) | 0.010 | 0.061 | 0.77 |
| Infant Fat Mass with Ultrasound 2-skinfolds (%) | |||||||||
| TCH | 20.10 (2.51) | 0.010 (0.036) | 21.80 (2.15) | 0.010 (0.036) | 20.70 (1.98) | 0.010 (0.036) | 0.005 | 0.64 | 0.064 |
| Lactose | 19.80 (2.80) | 0.128 (0.050) | 22.30 (2.18) | 0.128 (0.050) | 21.60 (1.94) | 0.128 (0.050) | 0.019 | 0.59 | 0.11 |
| Infant Fat Mass with Ultrasound 4-skinfolds (%) | |||||||||
| TCH | 14.00 (2.91) | 0.193 (0.043) | 31.10 (4.47) | −0.083 (0.090) | 18.80 (2.05) | 0.103 (0.042) | <0.001 | 0.051 | 0.016 |
| Lactose | 18.30 (2.66) | 0.156 (0.048) | 21.60 (2.10) | 0.156 (0.048) | 18.70 (1.85) | 0.156 (0.048) | 0.001 | 0.079 | 0.069 |
| HMO | 24.60 (1.29) | 0.168 (0.074) | 25.10 (1.64) | 0.168 (0.074) | 21.30 (1.58) | 0.168 (0.074) | 0.025 | 0.095 | 0.51 |
| Infant Fat Mass Index with Bioelectrical Impedance Spectroscopy (kg/m2) | |||||||||
| Lactose | 3.66 (0.74) | 0.027 (0.013) | 3.27 (0.58) | 0.027 (0.013) | 3.55 (0.53) | 0.027 (0.013) | 0.045 | 0.62 | 0.41 |
| HMO | 5.37 (0.32) | −0.024 (0.017) | 4.51 (0.41) | −0.024 (0.017) | 4.64 (0.40) | −0.024 (0.017) | 0.049 | 0.013 | 0.18 |
| Infant Fat Mass Index with Ultrasound 2-skinfolds (kg/m2) | |||||||||
| TCH | 3.10 (0.61) | 0.025 (0.009) | 3.64 (0.52) | 0.025 (0.009) | 3.36 (0.47) | 0.025 (0.009) | 0.003 | 0.32 | 0.10 |
| Lactose | 3.01 (0.67) | 0.032 (0.012) | 3.74 (0.51) | 0.032 (0.012) | 3.47 (0.47) | 0.032 (0.012) | 0.005 | 0.18 | 0.27 |
| Infant Fat Mass Index with Ultrasound 4-skinfolds (kg/m2) | |||||||||
| TCH | 1.64 (0.74) | 0.048 (0.011) | 5.22 (1.12) | −0.007 (0.023) | 3.21 (0.52) | 0.020 (0.011) | <0.001 | 0.078 | 0.038 |
| Lactose | 2.72 (0.64) | 0.038 (0.012) | 3.52 (0.50) | 0.038 (0.012) | 2.84 (0.46) | 0.038 (0.012) | <0.001 | 0.065 | 0.16 |
| HMO | 4.28 (0.32) | 0.040 (0.018) | 4.43 (0.41) | 0.040 (0.018) | 3.62 (0.39) | 0.040 (0.018) | 0.034 | 0.19 | 0.68 |
| Infant Fat Mass to Fat-free Mass Ratio with Bioelectrical Impedance Spectroscopy | |||||||||
| HMO | 0.43 (0.02) | −0.002 (0.001) | 0.34 (0.03) | −0.002 (0.001) | 0.36 (0.03) | −0.002 (0.001) | 0.024 | <0.001 | 0.095 |
| Infant Fat Mass to Fat-free Mass Ratio with Ultrasound 2-skinfolds | |||||||||
| TCH | 0.23 (0.05) | 0.002 (0.001) | 0.27 (0.04) | 0.002 (0.001) | 0.25 (0.04) | 0.002 (0.001) | 0.004 | 0.68 | 0.052 |
| Lactose | 0.22 (0.06) | 0.003 (0.001) | 0.28 (0.04) | 0.003 (0.001) | 0.27 (0.04) | 0.003 (0.001) | 0.012 | 0.50 | 0.14 |
| Infant Fat Mass to Fat-free Mass Ratio with Ultrasound 4-skinfolds | |||||||||
| TCH | 0.12 (0.05) | 0.004 (0.001) | 0.46 (0.08) | −0.002 (0.002) | 0.23 (0.04) | 0.002 (0.001) | <0.001 | 0.058 | 0.007 |
| Lactose | 0.21 (0.05) | 0.003 (0.001) | 0.27 (0.04) | 0.003 (0.001) | 0.22 (0.04) | 0.003 (0.001) | <0.001 | 0.084 | 0.053 |
| HMO | 0.33 (0.02) | 0.003 (0.001) | 0.34 (0.03) | 0.003 (0.001) | 0.27 (0.03) | 0.003 (0.001) | 0.027 | 0.12 | 0.45 |
| Changes in Infant Characteristic (Response) | Months after Birth | |||||
|---|---|---|---|---|---|---|
| 5 and 2 | 9 and 2 | 12 and 2 | 9 and 5 | 12 and 5 | 12 and 9 | |
| Lactose CDI (g) between 2 and 5 months (n = 17) c | ||||||
| ΔLength (cm) | −0.019 (0.031) a 0.56 b | 0.059 (0.032) 0.11 | 0.100 (0.032) d 0.016 | 0.004 (0.026) 0.88 | 0.021 (0.034) 0.54 | 0.028 (0.033) 0.41 |
| ΔBMI (kg/m2) | 0.049 (0.019) 0.035 | 0.015 (0.030) 0.63 | −0.006 (0.038) 0.88 | −0.015 (0.018) 0.44 | −0.033 (0.023) 0.17 | −0.017 (0.013) 0.23 |
| ΔFFM with US 2SF (kg) | −0.013 (0.011) 0.27 | 0.024 (0.009) 0.029 | 0.015 (0.014) 0.32 | 0.003 (0.008) 0.76 | 0.004 (0.010) 0.66 | 0.0002 (0.005) 0.96 |
| ΔFFM with US 4SF (kg) | 0.003 (0.013) 0.84 | 0.043 (0.012) 0.009 | 0.044 (0.019) 0.052 | 0.005 (0.008) 0.51 | 0.008 (0.009) 0.41 | 0.004 (0.005) 0.44 |
| ΔFFM with BIS (kg) | 0.009 (0.005) 0.12 | 0.023 (0.016) 0.19 | 0.035 (0.012) 0.025 | 0.004 (0.009) 0.63 | 0.011 (0.011) 0.32 | 0.012 (0.010) 0.24 |
| ΔFFMI with US 4SF (kg/m2) | 0.041 (0.023) 0.12 | 0.045 (0.026) 0.13 | 0.088 (0.035) 0.044 | 0.006 (0.013) 0.64 | −0.002 (0.018) 0.91 | −0.004 (0.014) 0.77 |
| ΔFM with US 2SF (kg) | 0.029 (0.010) 0.014 | 0.007 (0.010) 0.53 | 0.014 (0.015) 0.38 | −0.004 (0.008) 0.68 | −0.005 (0.008) 0.53 | 0.001 (0.004) 0.87 |
| ΔFMI with US 2SF (kg/m2) | 0.057 (0.023) 0.036 | −0.003 (0.022) 0.91 | −0.009 (0.035) 0.80 | −0.014 (0.018) 0.46 | −0.025 (0.020) 0.23 | −0.007 (0.008) 0.40 |
| ΔFMI with BIS (kg/m2) | 0.026 (0.015) 0.11 | 0.001 (0.029) 0.98 | −0.020 (0.023) 0.42 | −0.017 (0.017) 0.33 | −0.045 (0.017) 0.023 | −0.026 (0.019) 0.19 |
| ΔFM/FFM with US 2SF | 0.007 (0.003) 0.036 | −0.001 (0.002) 0.60 | −0.001 (0.005) 0.93 | −0.002 (0.002) 0.35 | −0.001 (0.001) 0.67 | −0.0004 (0.001) 0.65 |
| ΔFM/FFM with BIS | 0.001 (0.001) 0.40 | −0.001 (0.003) 0.80 | −0.006 (0.002) 0.032 | −0.002 (0.001) 0.12 | −0.004 (0.002) 0.034 | −0.002 (0.002) 0.32 |
| Lactose CDI (g) at 9 months (n = 8) c | ||||||
| ΔFM with BIS (kg) | n/a e | 0.053 (0.081) 0.58 | −0.033 (0.062) 0.65 | −0.020 (0.025) 0.46 | −0.037 (0.014) 0.045 | −0.017 (0.018) 0.39 |
| Lactose CDI (g) at 12 months (n = 8) c | ||||||
| ΔFFMI with US 2SF (kg/m2) | n/a e | n/a e | −0.082 (0.036) 0.15 | n/a e | −0.097 (0.018) 0.003 | −0.041 (0.018) 0.072 |
| ΔFFMI with US 4SF (kg/m2) | n/a | n/a | −0.075 (0.058) 0.33 | n/a | −0.080 (0.007) 0.0004 *** | −0.042 (0.026) 0.17 |
| ΔFM with US 2SF (kg) | n/a | n/a | 0.036 (0.010) 0.032 | n/a | 0.019 (0.011) 0.13 | 0.005 (0.006) 0.48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gridneva, Z.; Rea, A.; Tie, W.J.; Lai, C.T.; Kugananthan, S.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Carbohydrates in Human Milk and Body Composition of Term Infants during the First 12 Months of Lactation. Nutrients 2019, 11, 1472. https://doi.org/10.3390/nu11071472
Gridneva Z, Rea A, Tie WJ, Lai CT, Kugananthan S, Ward LC, Murray K, Hartmann PE, Geddes DT. Carbohydrates in Human Milk and Body Composition of Term Infants during the First 12 Months of Lactation. Nutrients. 2019; 11(7):1472. https://doi.org/10.3390/nu11071472
Chicago/Turabian StyleGridneva, Zoya, Alethea Rea, Wan Jun Tie, Ching Tat Lai, Sambavi Kugananthan, Leigh C. Ward, Kevin Murray, Peter E. Hartmann, and Donna T. Geddes. 2019. "Carbohydrates in Human Milk and Body Composition of Term Infants during the First 12 Months of Lactation" Nutrients 11, no. 7: 1472. https://doi.org/10.3390/nu11071472
APA StyleGridneva, Z., Rea, A., Tie, W. J., Lai, C. T., Kugananthan, S., Ward, L. C., Murray, K., Hartmann, P. E., & Geddes, D. T. (2019). Carbohydrates in Human Milk and Body Composition of Term Infants during the First 12 Months of Lactation. Nutrients, 11(7), 1472. https://doi.org/10.3390/nu11071472