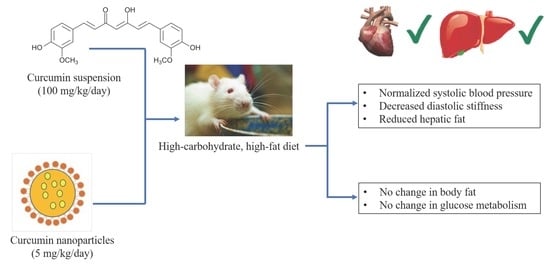
Low-Dose Curcumin Nanoparticles Normalise Blood Pressure in Male Wistar Rats with Diet-Induced Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Curcumin Suspension and Nanoparticles
2.2. Rats and Diets
2.3. Measurements Before Euthanasia
2.4. Measurements After Euthanasia
2.5. Statistical Analysis
3. Results
3.1. Body Parameters and Dietary Intakes
3.2. Metabolic Changes
3.3. Cardiovascular Changes
3.4. Liver Changes
3.5. Gut Structure and Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Sundar Dhilip Kumar, S.; Houreld, N.N.; Abrahamse, H. Therapeutic potential and recent advances of curcumin in the treatment of aging-associated diseases. Molecules 2018, 23, 835. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S. Molecular mechanisms of curcumin action: Gene expression. Biofactors 2013, 39, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanovic-Radic, Z.; Matejic, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Kuanar, A.; Joshi, R.K.; Sandeep, I.S.; Mohanty, S.; Naik, P.K.; Mishra, A.; Nayak, S. Development of prediction model and experimental validation in predicting the curcumin content of turmeric (Curcuma longa L.). Front. Plant Sci. 2016, 7, 1507. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.Y.; Lin, L.C.; Tseng, T.Y.; Wang, S.C.; Tsai, T.H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Munch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, 160287. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Poudyal, H.; Panchal, S.K. Functional foods as potential therapeutic options for metabolic syndrome. Obes. Rev. 2015, 16, 914–941. [Google Scholar] [CrossRef] [PubMed]
- Ganugula, R.; Arora, M.; Jaisamut, P.; Wiwattanapatapee, R.; Jorgensen, H.G.; Venkatpurwar, V.P.; Zhou, B.; Rodrigues Hoffmann, A.; Basu, R.; Guo, S.; et al. Nano-curcumin safely prevents streptozotocin-induced inflammation and apoptosis in pancreatic beta cells for effective management of Type 1 diabetes mellitus. Br. J. Pharmacol. 2017, 174, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; do Nascimento, T.; Casa, D.; Dalmolin, L.; de Mattos, A.; Hoss, I.; Romano, M.; Mainardes, R. Pharmacokinetics of curcumin-loaded PLGA and PLGA–PEG blend nanoparticles after oral administration in rats. Colloids Surf. B Biointerfaces 2013, 101, 353–360. [Google Scholar] [CrossRef]
- Grama, C.N.; Suryanarayana, P.; Patil, M.A.; Raghu, G.; Balakrishna, N.; Kumar, M.N.; Reddy, G.B. Efficacy of biodegradable curcumin nanoparticles in delaying cataract in diabetic rat model. PLoS ONE 2013, 8, e78217. [Google Scholar] [CrossRef]
- Bala, I.; Hariharan, S.; Kumar, M.N. PLGA nanoparticles in drug delivery: The state of the art. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21, 387–422. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the role of the gut microbiome and microbial metabolites in obesity and obesity-associated metabolic disorders: Current evidence and perspectives. Curr. Obes. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Wang, Y.F.; Fan, G.W.; Wang, X.Y.; Xu, S.Y.; Zhu, Y. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front. Microbiol. 2017, 8, 2146. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Shafie, S.R.; Prasadam, I.; Crawford, R.; Panchal, S.K.; Brown, L.; Xiao, Y. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci. Rep. 2017, 7, 46457. [Google Scholar] [CrossRef] [PubMed]
- Wanyonyi, S.; du Preez, R.; Brown, L.; Paul, N.A.; Panchal, S.K. Kappaphycus alvarezii as a food supplement prevents diet-induced metabolic syndrome in rats. Nutrients 2017, 9, 1261. [Google Scholar] [CrossRef]
- Maithilikarpagaselvi, N.; Sridhar, M.G.; Swaminathan, R.P.; Zachariah, B. Curcumin prevents inflammatory response, oxidative stress and insulin resistance in high fructose fed male Wistar rats: Potential role of serine kinases. Chem. Biol. Interact. 2016, 244, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sadeghnia, H.R.; Saberi-Karimian, M.; Safarian, H.; Ferns, G.A.; Ghayour-Mobarhan, M.; Sahebkar, A. Effects of curcumin on serum vitamin E concentrations in individuals with metabolic syndrome. Phytother. Res. 2017, 31, 657–662. [Google Scholar] [CrossRef]
- Tsai, Y.M.; Chang-Liao, W.L.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Effects of polymer molecular weight on relative oral bioavailability of curcumin. Int. J. Nanomed. 2012, 7, 2957–2966. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Karimian, M.S.; Pirro, M.; Johnston, T.P.; Majeed, M.; Sahebkar, A. Curcumin and endothelial function: Evidence and mechanisms of protective effects. Curr. Pharm. Des. 2017, 23, 2462–2473. [Google Scholar] [CrossRef] [PubMed]
- Pierelli, G.; Stanzione, R.; Forte, M.; Migliarino, S.; Perelli, M.; Volpe, M.; Rubattu, S. Uncoupling Protein 2: A key player and a potential therapeutic target in vascular diseases. Oxid. Med. Cell. Longev. 2017, 2017, 7348372. [Google Scholar] [CrossRef] [PubMed]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging 2017, 9, 187–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simental-Mendia, L.E.; Pirro, M.; Gotto, A.M., Jr.; Banach, M.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Lipid-modifying activity of curcuminoids: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.; Haghighatdoost, F. Effect of curcumin on anthropometric measures: A systematic review on randomized clinical trials. J. Am. Coll. Nutr. 2018, 37, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Revelo, X.; Shao, W.; Tian, L.; Zeng, K.; Lei, H.; Sun, H.S.; Woo, M.; Winer, D.; Jin, T. Dietary curcumin intervention targets mouse white adipose tissue inflammation and brown adipose tissue UCP1 expression. Obesity 2018, 26, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lv, Y.; Yao, H.; Yin, L.; Shang, J. Curcumin prevents the non-alcoholic fatty hepatitis via mitochondria protection and apoptosis reduction. Int. J. Clin. Exp. Pathol. 2015, 8, 11503–11509. [Google Scholar] [PubMed]
- Nishikawa, S.; Kamiya, M.; Aoyama, H.; Nomura, M.; Hyodo, T.; Ozeki, A.; Lee, H.; Takahashi, T.; Imaizumi, A.; Tsuda, T. Highly dispersible and bioavailable curcumin but not native curcumin induces brown-like adipocyte formation in mice. Mol. Nutr. Food Res. 2018, 62, 1700731. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Song, Z.; Weng, J.; Fantus, I.G. Curcumin and other dietary polyphenols: Potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E201–E205. [Google Scholar] [CrossRef]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Pagano, E.; Romano, B.; Izzo, A.A.; Borrelli, F. The clinical efficacy of curcumin-containing nutraceuticals: An overview of systematic reviews. Pharmacol. Res. 2018, 134, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef] [PubMed]
- McFadden, R.-M.T.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Ji, H.F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.-F. Bidirectional interactions between dietary curcumin and gut microbiota. Crit. Rev. Food Sci. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.B.; Koh, W.Y.; Parray, H.A.; Paek, W.K.; Lim, J.; Rather, I.A.; Jan, A.T. Gut microbiome: Microflora association with obesity and obesity-related comorbidities. Microb. Pathog. 2018, 124, 266–271. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef]
- Lopresti, A.L. The problem of curcumin and its bioavailability: Could its gastrointestinal influence contribute to its overall health-enhancing effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates Western diet-induced atherosclerosis and glucose intolerance in LDLR−/− mice–Role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, K.; Pardoe, D.; White, D. Scaling basic toxicokinetic parameters from rat to man. Environ. Health Perspect. 1996, 104, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]







| Variables | C | CC5 | CC100 | CCNP | CBNP | H | HC5 | HC100 | HCNP | HBNP |
|---|---|---|---|---|---|---|---|---|---|---|
| Initial body weight, g | 337 ± 1 | 338 ± 1 | 338 ± 1 | 338 ± 1 | 339 ± 1 | 338 ± 1 | 339 ± 1 | 337 ± 1 | 337 ± 1 | 339 ± 1 |
| Final body weight, g | 393 ± 7 c | 388 ± 8 c | 380 ± 8 c | 403 ± 6 c | 380 ± 7 c | 514 ± 10 ab | 492 ± 9 b | 498 ± 9 b | 490 ± 6 b | 538 ± 16 a |
| Body weight gain (weeks 9–16), % | 9.7 ± 1.0 b | 9.0 ± 1.4 b | 9.6 ± 0.9 b | 11.0 ± 1.1 b | 8.9 ± 1.0 b | 23.4 ± 1.0 a | 19.4 ± 1.3 a | 19.8 ± 1.5 a | 21.6 ± 1.3 a | 21.7 ± 1.7 a |
| Final lean mass, g | 295 ± 5 | 289 ± 7 | 291 ± 8 | 288 ± 7 | 286 ± 8 | 320 ± 8 | 289 ± 6 | 291 ± 7 | 309 ± 11 | 303 ± 6 |
| Final fat mass, g | 85 ± 7 c | 70 ± 7 c | 66 ± 6 c | 104 ± 4 c | 77 ± 7 c | 184 ± 10 b | 185 ± 14 b | 202 ± 13 b | 169 ± 9 b | 233 ± 14 a |
| Water intake (weeks 9–16), mL/day | 31.9 ± 2.8 | 24.9 ± 1.2 | 28.5 ± 3.2 | 26.9 ± 1.6 | 30.6 ± 1.8 | 27.8 ± 1.1 | 31.6 ± 1.9 | 26.2 ± 1.2 | 31.3 ± 0.8 | 28.5 ± 1.5 |
| Food intake (weeks 9–16), g/day | 39.1 ± 1.3 c | 39.1 ± 2.2 c | 38.5 ± 1.5 c | 49.7 ± 0.7 a | 44.8 ± 2.1 b | 27.9 ± 0.9 ef | 23.7 ± 0.7 f | 25.0 ± 0.9 f | 33.6 ± 1.3 d | 31.3 ± 1.9 de |
| Energy intake (weeks 9–16), kJ/day | 439 ± 14 c | 432 ± 17 c | 446 ± 15 c | 559 ± 8 b | 512 ± 27 b | 588 ± 15b | 561 ± 15 b | 539 ± 14 b | 714 ± 23 a | 661 ± 37 a |
| Feed efficiency (weeks 9–16), g/kJ | 0.07 ± 0.01 b | 0.07 ± 0.01 b | 0.07 ± 0.01 b | 0.07 ± 0.01 b | 0.06 ± 0.01 b | 0.15 ± 0.01 a | 0.13 ± 0.01 a | 0.15 ± 0.01 a | 0.12 ± 0.01 a | 0.15 ± 0.01 a |
| Retroperitoneal fat, mg/mm | 242 ± 13 d | 208 ± 15 d | 211 ± 10 d | 229 ± 13 d | 179 ± 17 d | 481 ± 27 b | 337 ± 37 c | 436 ± 22 b | 467 ± 22 b | 581 ± 39 a |
| Epididymal fat, mg/mm | 76 ± 8 cd | 79 ± 8 cd | 68 ± 8 d | 68 ± 8 d | 77 ± 6 cd | 171 ± 13 b | 171 ± 17 b | 143 ± 13 b | 126 ± 11 bc | 244 ± 29 a |
| Omental fat, mg/mm | 137 ± 10 c | 137 ± 12 c | 123 ± 7 c | 134 ± 7 c | 161 ± 20 c | 244 ± 14 ab | 224 ± 11 b | 219 ± 11 b | 219 ± 13 b | 278 ± 19 a |
| Total abdominal fat, mg/mm | 455 ± 25 c | 423 ± 34 c | 402 ± 23 c | 432 ± 24 c | 417 ± 38 c | 895 ± 44 b | 827 ± 51 b | 797 ± 39 b | 813 ± 38 b | 1103 ± 70 a |
| Left ventricle + septum weight, mg/mm | 23.8 ± 1.4 | 21.9 ± 0.7 | 21.0 ± 0.8 | 22.7 ± 1.1 | 19.9 ± 0.8 | 23.4 ± 0.8 | 22.5 ± 0.5 | 23.4 ± 1.0 | 23.4 ± 0.8 | 24.2 ± 1.0 |
| Right ventricular weight, mg/mm | 5.1 ± 0.3 abc | 4.1 ± 0.3 cd | 4.7 ± 0.3 bc | 3.4 ± 0.3 d | 4.1 ± 0.5 cd | 5.7 ± 0.2 ab | 4.8 ± 0.3 abc | 4.9 ± 0.2 abc | 4.0 ± 0.2 cd | 5.9 ± 0.1 a |
| Metabolic variables | ||||||||||
| Heat, kcal | 3.87 ± 0.08 ab | 3.86 ± 0.09 ab | 3.48 ± 0.41 b | 2.70 ± 0.18 c | 3.39 ± 0.26 b | 4.34 ± 0.09 a | 4.22± 0.12 a | 4.28 ± 0.10 a | 3.26 ± 0.21 b | 3.45 ± 0.13 b |
| RER | 1.03 ± 0.03 ab | 1.03 ± 0.10 ab | 1.04 ± 0.02 a | 1.03 ± 0.02 ab | 1.02 ± 0.02 ab | 0.91 ± 0.01 ab | 0.92 ± 0.01 ab | 0.90 ± 0.01 ab | 0.87 ± 0.02 b | 0.92 ± 0.01 ab |
| Plasma triglycerides, mmol/L | 0.53 ± 0.06 b | 0.49 ± 0.07 b | 0.41 ± 0.03 b | 0.59 ± 0.06 b | 0.83 ± 0.15 b | 1.71 ± 0.45 a | 1.77 ± 0.56 a | 1.53 ± 0.06 a | 1.52 ± 0.15 a | 1.64 ± 0.21 a |
| Plasma total cholesterol, mmol/L | 1.64 ± 0.08 ab | 1.45 ± 0.06 b | 1.44 ± 0.06 b | 1.60 ± 0.06 ab | 1.73 ± 0.13 ab | 1.53 ± 0.08 b | 1.71 ± 0.10 ab | 1.49 ± 0.09 b | 1.74 ± 0.05 ab | 1.93 ± 0.13 a |
| Plasma non-esterified fatty acids, mmol/L | 1.40 ± 0.20 cd | 1.28 ± 0.09 cd | 0.96 ± 0.08 d | 1.58 ± 0.16 cd | 2.42 ± 0.33 bc | 3.30 ± 0.40 ab | 2.64 ± 0.68 bc | 4.03 ± 0.36 a | 3.73 ± 0.18 ab | 4.50 ± 0.63 a |
| Basal blood glucose, mmol/L | 3.2 ± 0.1 c | 3.6 ± 0.1 bc | 3.2 ± 0.1 c | 3.9 ± 0.1 abc | 3.3 ± 0.2 c | 3.4 ± 0.2 bc | 3.9 ± 0.2 abc | 3.9 ± 0.4 abc | 4.5 ± 0.2 a | 4.2 ± 0.2 ab |
| 120-min blood glucose, mmol/L | 4.6 ± 0.4 bcd | 4.2 ± 0.1 cd | 3.7 ± 0.1 d | 4.5 ± 0.2 bcd | 3.6 ± 0.2 d | 6.0 ± 0.6 a | 5.1 ± 0.2 abc | 5.4 ± 0.3 abc | 4.3 ± 0.2 cd | 5.6 ± 0.4 ab |
| Blood glucose area under the curve, mmol/L×min | 665 ± 8 ab | 591 ± 11 bc | 561 ± 18 c | 664 ± 14 ab | 560 ± 22 c | 739 ± 35 a | 695 ± 15 a | 673 ± 28 ab | 705 ± 14 a | 692 ± 42 a |
| Plasma ALT activity, U/L | 36 ± 2 | 41 ± 4 | 40 ± 5 | 32 ± 3 | 32 ± 3 | 42 ± 2 | 47 ± 5 | 40 ± 3 | 41 ± 3 | 42 ± 4 |
| Plasma AST activity, U/L | 88 ± 2 | 103 ± 6 | 90 ± 5 | 83 ± 3 | 87 ± 4 | 95 ± 2 | 105 ± 10 | 99 ± 9 | 88 ± 4 | 97 ± 10 |
| Plasma curcumin concentrations, ng/ml | - | 97.4 ± 18.0 b | 337.7 ± 84.7 a | 199.7 ± 45.3 b | - | - | 0.0 ± 0.0 c | 146.0 ± 21.2 b | 110.7 ± 17.8 b | - |
| Liver inflammatory cells (cells/200µm2) | 5 ± 1 c | 6 ± 2 c | 5 ± 1 c | 5 ± 2 c | 5 ± 2 c | 23 ± 2 a | 15 ± 3 b | 16 ± 2 b | 15 ± 1 b | 16 ± 1 b |
| Cardiovascular variables | ||||||||||
| 16 week systolic blood pressure, mmHg | 120 ± 4 b | 125 ± 1 b | 122 ± 2 b | 125 ± 2 b | 130 ± 3 b | 143 ± 5 a | 143 ± 4 a | 126 ± 4 b | 128 ± 4 b | 141 ± 3 a |
| Left ventricular diastolic stiffness constant (κ) | 22.0 ± 0.8 b | 21.8 ± 0.6 b | 23.3 ± 0.9 b | 22.8 ± 0.8 b | 21.9 ± 0.5 b | 28.9 ± 0.8 a | 28.5 ± 0.7 a | 23.4 ± 1.1 b | 23.5 ± 0.7 b | 27.9 ± 0.9 a |
| Left ventricle collagen area, % | 11 ± 1 c | 13 ± 1 c | 12 ± 1 c | 15 ± 1 c | 16 ± 1 c | 38 ± 2 a | 22 ± 2 b | 24 ± 2 b | 22 ± 2 b | 27 ± 1 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
du Preez, R.; Pahl, J.; Arora, M.; Ravi Kumar, M.N.V.; Brown, L.; Panchal, S.K. Low-Dose Curcumin Nanoparticles Normalise Blood Pressure in Male Wistar Rats with Diet-Induced Metabolic Syndrome. Nutrients 2019, 11, 1542. https://doi.org/10.3390/nu11071542
du Preez R, Pahl J, Arora M, Ravi Kumar MNV, Brown L, Panchal SK. Low-Dose Curcumin Nanoparticles Normalise Blood Pressure in Male Wistar Rats with Diet-Induced Metabolic Syndrome. Nutrients. 2019; 11(7):1542. https://doi.org/10.3390/nu11071542
Chicago/Turabian Styledu Preez, Ryan, Jessica Pahl, Meenakshi Arora, M. N. V. Ravi Kumar, Lindsay Brown, and Sunil K. Panchal. 2019. "Low-Dose Curcumin Nanoparticles Normalise Blood Pressure in Male Wistar Rats with Diet-Induced Metabolic Syndrome" Nutrients 11, no. 7: 1542. https://doi.org/10.3390/nu11071542
APA Styledu Preez, R., Pahl, J., Arora, M., Ravi Kumar, M. N. V., Brown, L., & Panchal, S. K. (2019). Low-Dose Curcumin Nanoparticles Normalise Blood Pressure in Male Wistar Rats with Diet-Induced Metabolic Syndrome. Nutrients, 11(7), 1542. https://doi.org/10.3390/nu11071542