Exploring the Science behind Bifidobacterium breve M-16V in Infant Health
Abstract
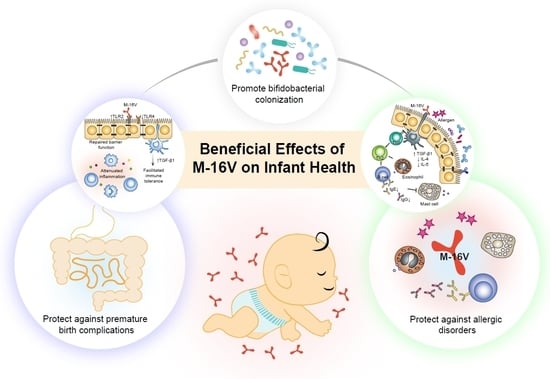
:1. Introduction
2. Probiotics for Infant Health
3. Bifidobacterium breve M-16V as Infant Probiotic
3.1. Origin and Characteristics
3.2. Safety
4. Effects of M-16V on Premature Birth Complications
4.1. Preclinical Studies
4.2. Clinical Studies
4.3. Potential Mechanisms of Action
5. Effects of M-16V on Allergic Disorders
5.1. Preclinical Studies
5.2. Clinical Studies
5.3. Potential Mechanisms of Action
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef]
- Cassir, N.; Benamar, S.; Khalil, J.B.; Croce, O.; Saint-Faust, M.; Jacquot, A.; Million, M.; Azza, S.; Armstrong, N.; Henry, M. Clostridium butyricum strains and dysbiosis linked to necrotizing enterocolitis in preterm neonates. Clin. Infect. Dis. 2015, 61, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.; Bloomfield, F.; O’Sullivan, J. Factors affecting gastrointestinal microbiome development in neonates. Nutrients 2018, 10, 274. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Chen, J.-H.; Chang, J.-H.; Lin, H.-C.; Lin, C.-Y.; Peng, C.-C. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: An updated meta-analysis. PLoS ONE 2017, 12, e0171579. [Google Scholar] [CrossRef] [PubMed]
- Cuello-Garcia, C.A.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schünemann, H.J. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef] [PubMed]
- de Simone, C. The Unregulated Probiotic Market. Clin. Gastroenterol. Hepatol. 2018, 17, 809–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quin, C.; Estaki, M.; Vollman, D.M.; Barnett, J.A.; Gill, S.K.; Gibson, D.L. Probiotic supplementation and associated infant gut microbiome and health: A cautionary retrospective clinical comparison. Sci. Rep. 2018, 8, 8283. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.M.; Hall, N.J.; Fleming, P.; Eaton, S. Probiotics for the prevention of surgical necrotising enterocolitis: Systematic review and meta-analysis. BMJ Paediatr. Open 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- MEDLINE. Available online: https://www.nlm.nih.gov/bsd/medline.html (accessed on 14 June 2019).
- EMBase. Available online: https://www.elsevier.com/solutions/embase-biomedical-research (accessed on 14 June 2019).
- Medical Journal web. Available online: https://www.jamas.or.jp/english/ (accessed on 14 June 2019).
- JDreamIII. Available online: https://jdream3.com/service/search/ (accessed on 14 June 2019). (In Japanese).
- Gritz, E.C.; Bhandari, V. The human neonatal gut microbiome: A brief review. Front. Pediatr. 2015, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Chassard, C.; de Wouters, T.; Lacroix, C. Probiotics tailored to the infant: A window of opportunity. Curr. Opin. Biotechnol. 2014, 26, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Laforest-Lapointe, I.; Arrieta, M.-C. Patterns of early-life gut microbial colonization during human immune development: An ecological perspective. Front. Immunol. 2017, 8, 788. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.B.; Sugahara, H.; Odamaki, T.; Xiao, J.-Z. Different physiological properties of human-residential and non-human-residential bifidobacteria in human health. Benef. Microbes 2018, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Van Sinderen, D.; Ventura, M. Genomics and ecological overview of the genus Bifidobacterium. Int. J. Food Microbiol. 2011, 149, 37–44. [Google Scholar] [CrossRef]
- Katayama, T. Host-derived glycans serve as selected nutrients for the gut microbe: Human milk oligosaccharides and bifidobacteria. Biosci. Biotechnol. Biochem. 2016, 80, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Odamaki, T.; Horigome, A.; Sugahara, H.; Hashikura, N.; Minami, J.; Xiao, J.-Z.; Abe, F. Comparative genomics revealed genetic diversity and species/strain-level differences in carbohydrate metabolism of three probiotic bifidobacterial species. Int. J. Genom. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Minami, J.; Odamaki, T.; Hashikura, N.; Abe, F.; Xiao, J.Z. Lysozyme in breast milk is a selection factor for bifidobacterial colonisation in the infant intestine. Benef. Microbes 2016, 7, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Roger, L.C.; Costabile, A.; Holland, D.T.; Hoyles, L.; McCartney, A.L. Examination of faecal Bifidobacterium populations in breast-and formula-fed infants during the first 18 months of life. Microbiology 2010, 156, 3329–3341. [Google Scholar] [CrossRef] [PubMed]
- Sakata, S.; Tonooka, T.; Ishizeki, S.; Takada, M.; Sakamoto, M.; Fukuyama, M.; Benno, Y. Culture-independent analysis of fecal microbiota in infants, with special reference to Bifidobacterium species. FEMS Microbiol. Lett. 2005, 243, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Foroni, E.; Pizzetti, P.; Giubellini, V.; Ribbera, A.; Merusi, P.; Cagnasso, P.; Bizzarri, B.; de’Angelis, G.L.; Shanahan, F. Exploring the diversity of the bifidobacterial population in the human intestinal tract. Appl. Environ. Microbiol. 2009, 75, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Bozzi Cionci, N.; Baffoni, L.; Gaggìa, F.; Di Gioia, D. Therapeutic Microbiology: The Role of Bifidobacterium breve as Food Supplement for the Prevention/Treatment of Paediatric Diseases. Nutrients 2018, 10, 1723. [Google Scholar] [CrossRef] [PubMed]
- Ishizeki, S.; Sugita, M.; Takata, M.; Yaeshima, T. Effect of administration of bifidobacteria on intestinal microbiota in low-birth-weight infants and transition of administered bifidobacteria: A comparison between one-species and three-species administration. Anaerobe 2013, 23, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Y.; Yang, Z.-Y.; Dai, W.-K.; Huang, J.-Q.; Li, Y.-H.; Zhang, J.; Qiu, C.-Z.; Wei, C.; Zhou, Q.; Sun, X. Protective effect of Bifidobacterium infantis CGMCC313-2 on ovalbumin-induced airway asthma and β-lactoglobulin-induced intestinal food allergy mouse models. World J. Gastroenterol. 2017, 23, 2149. [Google Scholar] [CrossRef]
- Yeşilova, Y.; Çalka, Ö.; Akdeniz, N.; Berktaş, M. Effect of probiotics on the treatment of children with atopic dermatitis. Ann. Dermatol. 2012, 24, 189–193. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J. Strain-specificity and disease-specificity of probiotic efficacy: A systematic review and meta-analysis. Front. Med. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Vecchi, E.; Gabrieli, A.; Zuccotti, G.V.; Drago, L. Probiotic characteristics and in vitro compatibility of a combination of Bifidobacterium breve M-16 V, Bifidobacterium longum subsp. infantis M-63 and Bifidobacterium longum subsp. longum BB536. Ann. Microbiol. 2015, 65, 1079–1086. [Google Scholar] [CrossRef]
- Abe, F.; Miyauchi, H.; Uchijima, A.; Yaeshima, T.; Iwatsuki, K. Stability of bifidobacteria in powdered formula. Int. J. Food Sci. Technol. 2009, 44, 718–724. [Google Scholar] [CrossRef]
- Guidelines for the Evaluation of Probiotics in Food. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 24 April 2019).
- GRAS Notice (GRN) No. 453. Available online: http://wayback.archive-it.org/7993/20171031043458/https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM346877.pdf (accessed on 24 April 2019).
- GRAS Notice (GRN) No. 454. Available online: http://wayback.archive-it.org/7993/20171031043455/https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM346879.pdf (accessed on 24 April 2019).
- List of Authorised Probiotic Strains for Infant’s Food in China. Available online: http://law.foodmate.net/show-188701.html (accessed on 24 April 2019).
- Patole, S.; Keil, A.D.; Chang, A.; Nathan, E.; Doherty, D.; Simmer, K.; Esvaran, M.; Conway, P. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates-a randomised double blind placebo controlled trial. PLoS ONE 2014, 9, e89511. [Google Scholar] [CrossRef]
- Patole, S.K.; Rao, S.C.; Keil, A.D.; Nathan, E.A.; Doherty, D.A.; Simmer, K.N. Benefits of Bifidobacterium breve M-16V supplementation in preterm neonates-a retrospective cohort study. PLoS ONE 2016, 11, e0150775. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Shinohara, K.; Umezaki, H.; Shoji, H.; Satoh, H.; Ohtsuka, Y.; Shiga, S.; Nagata, S.; Shimizu, T.; Yamashiro, Y. Bifidobacteria prevents necrotizing enterocolitis and infection in preterm infants. Int. J. Probiotics Prebiotics 2007, 2, 49. [Google Scholar]
- Abe, F.; Yaeshima, T.; Iwatsuki, K. Safety evaluation of two probiotic bifidobacterial strains, Bifidobacterium breve M-16V and Bifidobacterium infantis M-63, by oral toxicity tests using rats. Biosci. Microflora 2009, 28, 7–15. [Google Scholar] [CrossRef]
- Xiao, J.-Z.; Takahashi, S.; Odamaki, T.; Yaeshima, T.; Iwatsuki, K. Antibiotic susceptibility of bifidobacterial strains distributed in the Japanese market. Biosci. Biotechnol. Biochem. 2010, 74, 336–342. [Google Scholar] [CrossRef]
- Grill, J.P.; Manginot-Dürr, C.; Schneider, F.; Ballongue, J. Bifidobacteria and probiotic effects: Action of Bifidobacterium species on conjugated bile salts. Curr. Microbiol. 1995, 31, 23–27. [Google Scholar] [CrossRef]
- Abe, F.; Muto, M.; Yaeshima, T.; Iwatsuki, K.; Aihara, H.; Ohashi, Y.; Fujisawa, T. Safety evaluation of probiotic bifidobacteria by analysis of mucin degradation activity and translocation ability. Anaerobe 2010, 16, 131–136. [Google Scholar] [CrossRef]
- Shane, A.L.; Deshpande, G.C.; Merenstein, D. Improved neonatal outcomes with probiotics. JAMA Pediatr. 2013, 167, 885–886. [Google Scholar] [CrossRef] [PubMed]
- Berdon, W.E.; Grossman, H.; Baker, D.H.; Mizrahi, A.; Barlow, O.; Blanc, W.A. Necrotizing enterocolitis in the premature infant. Radiology 1964, 83, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Linder, N.; Hammel, N.; Hernandez, A.; Fridman, E.; Dlugy, E.; Herscovici, T.; Klinger, G. Intestinal perforation in very-low-birth-weight infants with necrotizing enterocolitis. J. Pediatr. Surg. 2013, 48, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.W.; Luciano, A.A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.; Gilbert, J.A. Development of the preterm infant gut microbiome: A research priority. Microbiome 2014, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernandez-Barranco, A.; Margolles, A.; de los Reyes-Gavilan, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Sánchez, B.; Milani, C.; Duranti, S.; Solís, G.; Fernández, N.; Clara, G.; Ventura, M.; Margolles, A.; Gueimonde, M. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J. Pediatr. 2015, 166, 538–544. [Google Scholar] [CrossRef]
- Fricke, W.F. The more the merrier? Reduced fecal microbiota diversity in preterm infants treated with antibiotics. J. Pediatr. 2014, 165, 8–10. [Google Scholar] [CrossRef]
- Greenwood, C.; Morrow, A.L.; Lagomarcino, A.J.; Altaye, M.; Taft, D.H.; Yu, Z.; Newburg, D.S.; Ward, D.V.; Schibler, K.R. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J. Pediatr. 2014, 165, 23–29. [Google Scholar] [CrossRef]
- Tanaka, S.; Kobayashi, T.; Songjinda, P.; Tateyama, A.; Tsubouchi, M.; Kiyohara, C.; Shirakawa, T.; Sonomoto, K.; Nakayama, J. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol. Med. Microbiol. 2009, 56, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Rigo-Adrover, M.d.M.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Preclinical immunomodulation by the probiotic Bifidobacterium breve M-16V in early life. PLoS ONE 2016, 11, e0166082. [Google Scholar] [CrossRef]
- Izumi, H.; Minegishi, M.; Sato, Y.; Shimizu, T.; Sekine, K.; Takase, M. Bifidobacterium breve alters immune function and ameliorates DSS-induced inflammation in weanling rats. Pediatr. Res. 2015, 78, 407. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, Y.; Ikegami, T.; Izumi, H.; Namura, M.; Ikeda, T.; Ikuse, T.; Baba, Y.; Kudo, T.; Suzuki, R.; Shimizu, T. Effects of Bifidobacterium breve on inflammatory gene expression in neonatal and weaning rat intestine. Pediatr. Res. 2012, 71, 46. [Google Scholar] [CrossRef] [PubMed]
- Patole, S.K.; Keil, A.D.; Nathan, E.; Doherty, D.; Esvaran, M.; Simmer, K.N.; Conway, P. Effect of Bifidobacterium breve M-16V supplementation on faecal bifidobacteria in growth restricted very preterm infants–analysis from a randomised trial. J. Matern. Fetal Neonatal Med. 2016, 29, 3751–3755. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Hosono, S.; Takahashi, E.; Ishizeki, S.; Takigawa, I.; Imura, S.; Yamauchi, K.; Yaeshima, T.; Hayasawa, H.; Shimamura, S. Effects of oral administration of Bifidobacterium breve on development of intestinal microflora in extremely premature infants. Acta Neonatol. Jpn. 1994, 30, 130–137. [Google Scholar]
- Li, Y.; Shimizu, T.; Hosaka, A.; Kaneko, N.; Ohtsuka, Y.; Yamashiro, Y. Effects of Bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediatr. Int. 2004, 46, 509–515. [Google Scholar] [CrossRef]
- Akiyama, K.; Shimada, M.; Ishizeki, S.; Takigawa, I.; Imura, S.; Yamauchi, K.; Hatano, M.; Abe, N.; Yaeshima, T.; Hayasawa, H. Effects of administration of bifidobacterium in extremely premature infants. Development of intestinal microflora by orally administered bifidobacterium longum (in comparison with bifidobacterium breve). Acta Neonatol. Jpn. 1994, 30, 257–263. [Google Scholar]
- Athalye-Jape, G.; Rao, S.; Simmer, K.; Patole, S. Bifidobacterium breve M-16V as a Probiotic for Preterm Infants: A Strain-Specific Systematic Review. JPEN 2018, 42, 677–688. [Google Scholar] [CrossRef]
- Avcin, S.L.; Pokorn, M.; Kitanovski, L.; Premru, M.M.; Jazbec, J. Bifidobacterium breve sepsis in child with high-risk acute lymphoblastic leukemia. Emerg. Infect. Dis. 2015, 21, 1674. [Google Scholar] [CrossRef]
- Ohishi, A.; Takahashi, S.; Ito, Y.; Ohishi, Y.; Tsukamoto, K.; Nanba, Y.; Ito, N.; Kakiuchi, S.; Saitoh, A.; Morotomi, M. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J. Pediatr. 2010, 156, 679–681. [Google Scholar] [CrossRef]
- Nakazawa, T.; Kaneko, K.-I.; Takahashi, H.; Inoue, S. Neonatal meningitis caused byBifidobacterium breve. Brain Dev. 1996, 18, 160–162. [Google Scholar] [CrossRef]
- van den Akker, C.H.; van Goudoever, J.B.; Szajewska, H.; Embleton, N.D.; Hojsak, I.; Reid, D.; Shamir, R. Probiotics for preterm infants: A strain-specific systematic review and network meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Ohtsuka, Y.; Lee, T.; Kudo, T.; Shoji, H.; Sato, H.; Nagata, S.; Shimizu, T.; Yamashiro, Y. Bifidobacterium breve enhances transforming growth factor β1 signaling by regulating smad7 expression in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shoji, H.; Sato, H.; Nagata, S.; Ohtsuka, Y.; Shimizu, T.; Yamashiro, Y. Effects of oral administration of Bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.W.; Nasr, T.R.; Stoll, B.J. Necrotizing enterocolitis: Recent scientific advances in pathophysiology and prevention. In Semin Perinatol; Elsevier: Amsterdam, The Netherlands, 2008; Volume 32, pp. 70–82. [Google Scholar]
- Luedtke, S.A.; Yang, J.T.; Wild, H.E. Probiotics and necrotizing enterocolitis: Finding the missing pieces of the probiotic puzzle. J. Pediatr. Pharmacol. Ther. 2012, 17, 308–328. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Embleton, N.D.; Marrs, E.C.; Smith, D.P.; Nelson, A.; Abdulkadir, B.; Skeath, T.; Petrosino, J.F.; Perry, J.D.; Berrington, J.E. Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome 2016, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Izumi, H.; Iwabuchi, N.; Odamaki, T.; Namba, K.; Abe, F.; Xiao, J.Z. Bifidobacterium breve prevents necrotising enterocolitis by suppressing inflammatory responses in a preterm rat model. Benef. Microbes 2016, 7, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Hackam, D.J.; Sodhi, C.P. Toll-Like Receptor–Mediated Intestinal Inflammatory Imbalance in the Pathogenesis of Necrotizing Enterocolitis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, Z.; Bolock, A.M.; Good, M. The role of mucosal immunity in the pathogenesis of necrotizing enterocolitis. Front. Pediatr. 2017, 5, 40. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef]
- Hunter, C.J.; Upperman, J.S.; Ford, H.R.; Camerini, V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr. Res. 2008, 63, 117. [Google Scholar] [CrossRef]
- Niño, D.F.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 590. [Google Scholar] [CrossRef] [PubMed]
- Gribar, S.C.; Sodhi, C.P.; Richardson, W.M.; Anand, R.J.; Gittes, G.K.; Branca, M.F.; Jakub, A.; Shi, X.-H.; Shah, S.; Ozolek, J.A. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J. Immunol. 2009, 182, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Villena, J.; Tohno, M.; Fujie, H.; Hosoya, S.; Shimosato, T.; Aso, H.; Suda, Y.; Kawai, Y.; Saito, T. Immunobiotic Lactobacillus jensenii elicits anti-inflammatory activity in porcine intestinal epithelial cells by modulating negative regulators of the Toll-like receptor signaling pathway. Infect. Immun. 2012, 80, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Tomosada, Y.; Villena, J.; Murata, K.; Chiba, E.; Shimazu, T.; Aso, H.; Iwabuchi, N.; Xiao, J.-Z.; Saito, T.; Kitazawa, H. Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression. PLoS ONE 2013, 8, e59259. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, Y.; Sanderson, I.R. Transforming growth factor-β: An important cytokine in the mucosal immune response. Curr. Opin. Gastroenterol. 2000, 16, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.B.; Huh, C.-G.; Becker, D.; Geiser, A.; Lyght, M.; Flanders, K.C.; Roberts, A.B.; Sporn, M.B.; Ward, J.M.; Karlsson, S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 1993, 90, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Osaka, T.; Moriyama, E.; Date, Y.; Kikuchi, J.; Tsuneda, S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef]
- Neu, J.; Pammi, M. Necrotizing enterocolitis: The intestinal microbiome, metabolome and inflammatory mediators. Semin. Fetal Neonatal Med. 2018, 23, 400–405. [Google Scholar] [CrossRef]
- Lin, J.; Nafday, S.M.; Chauvin, S.N.; Magid, M.S.; Pabbatireddy, S.; Holzman, I.R.; Babyatsky, M.W. Variable effects of short chain fatty acids and lactic acid in inducing intestinal mucosal injury in newborn rats. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 545–550. [Google Scholar] [CrossRef]
- Waligora-Dupriet, A.-J.; Dugay, A.; Auzeil, N.; Huerre, M.; Butel, M.-J. Evidence for clostridial implication in necrotizing enterocolitis through bacterial fermentation in a gnotobiotic quail model. Pediatr. Res. 2005, 58, 629. [Google Scholar] [CrossRef]
- Furunyan, R.D.; Ohno, Y.; MacDermott, R.P.; Sanderson, I.R. Short-chain fatty-acids enhance interleukin-1-beta induced secretion of interleukin-8 by Caco-2 cells. In Gastroenterology; WB Saunders Co Independence Square West Curtis Center, Ste 300: Philadelphia, PA, USA, 1995; Volume 108, p. A726. [Google Scholar]
- Lin, J.; Peng, L.; Itzkowitz, S.; Holzman, I.R.; Babyatsky, M.W. Short-chain fatty acid induces intestinal mucosal injury in newborn rats and down-regulates intestinal trefoil factor gene expression in vivo and in vitro. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.; Lærke, H.; Hedemann, M.; Nielsen, T.; Ingerslev, A.; Gundelund Nielsen, D.; Theil, P.; Purup, S.; Hald, S.; Schioldan, A. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ J. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Jenmalm, M.C.; Prescott, S.L. The gut microbiota and its role in the development of allergic disease: A wider perspective. Clin. Exp. Allergy 2015, 45, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Noverr, M.C.; Huffnagle, G.B. The ‘microflora hypothesis’ of allergic diseases. Clin. Exp. Allergy 2005, 35, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Kirjavainen, P.; Eerola, E.; Kero, P.; Salminen, S.; Isolauri, E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 2001, 107, 129–134. [Google Scholar] [CrossRef]
- Melli, L.; do Carmo-Rodrigues, M.S.; Araújo-Filho, H.B.; Solé, D.; De Morais, M.B. Intestinal microbiota and allergic diseases: A systematic review. Allergol. Immunopathol. 2016, 44, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; van den Brandt, P.A.; Kummeling, I.; Snijders, B.; Stelma, F.; Adams, H.; van Ree, R.; Stobberingh, E.E. Gut microbiota composition and development of atopic manifestations in infancy: The KOALA Birth Cohort Study. Gut 2007, 56, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Feng, J.-j.; Yan, D.-y.; Lyu, Y.-j.; Xu, X. Early-life gut microbiome and cow’s milk allergy-a prospective case-control 6-month follow-up study. Saudi. J. Biol. Sci. 2018, 25, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L. Altered fecal microbiota composition for food allergy in infants. Appl. Environ. Microbiol. 2014, 80, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.-J.; Zhang, Q.; Yu, M.; Xu, J.-P.; Zheng, J.; Wang, T.; Xiao, X.-H. Imbalance of fecal microbiota at newly diagnosed type 1 diabetes in Chinese children. Chin. Med. J. 2016, 129, 1298. [Google Scholar] [CrossRef] [PubMed]
- Hougee, S.; Vriesema, A.J.M.; Wijering, S.C.; Knippels, L.M.J.; Folkerts, G.; Nijkamp, F.P.; Knol, J.; Garssen, J. Oral treatment with probiotics reduces allergic symptoms in ovalbumin-sensitized mice: A bacterial strain comparative study. Int. Arch. Allergy Immunol. 2010, 151, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, A.I.; Meulenbroek, L.A.P.M.; van Esch, B.C.A.M.; Hofman, G.A.; Garssen, J.; Willemsen, L.E.M.; Knippels, L.M.J. A Specific Mixture of Fructo-Oligosaccharides and Bifidobacterium breve M-16V Facilitates Partial Non-Responsiveness to Whey Protein in Mice Orally Exposed to β-Lactoglobulin-Derived Peptides. Front. Immunol. 2017, 7, 673. [Google Scholar] [CrossRef]
- Mortaz, E.; Adcock, I.M.; Ricciardolo, F.L.M.; Varahram, M.; Jamaati, H.; Velayati, A.A.; Folkerts, G.; Garssen, J. Anti-inflammatory effects of lactobacillus rahmnosus and bifidobacterium breve on cigarette smoke activated human macrophages. PLoS ONE 2015, 10, e0136455. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Morgan, M.E.; Chen, S.; Vos, A.P.; Garssen, J.; van Bergenhenegouwen, J.; Boon, L.; Georgiou, N.A.; Kraneveld, A.D.; Folkerts, G. Bifidobacterium breve and Lactobacillus rhamnosus treatment is as effective as budesonide at reducing inflammation in a murine model for chronic asthma. Respir. Res. 2014, 15, 46. [Google Scholar] [CrossRef]
- Sagar, S.; Vos, A.P.; Morgan, M.E.; Garssen, J.; Georgiou, N.A.; Boon, L.; Kraneveld, A.D.; Folkerts, G. The combination of Bifidobacterium breve with non-digestible oligosaccharides suppresses airway inflammation in a murine model for chronic asthma. Biochim. Biophys. Acta. 2014, 1842, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Schouten, B.; van Esch, B.C.A.M.; Hofman, G.A.; van Doorn, S.A.C.M.; Knol, J.; Nauta, A.J.; Garssen, J.; Willemsen, L.E.M.; Knippels, L.M.J. Cow milk allergy symptoms are reduced in mice fed dietary synbiotics during oral sensitization with whey. J. Nutr. 2009, 139, 1398–1403. [Google Scholar] [CrossRef]
- Del Giudice, M.M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, K.; Yamamoto, A.; Sasai, M.; Taniuchi, S.; Kojima, T.; Kobayashi, Y.; Iwamoto, H.; Namba, K.; Yaeshima, T. Effects of administration of bifidobacteria on fecal microflora and clinical symptoms in infants with atopic dermatitis. Arerugi 2003, 52, 20–30. [Google Scholar] [PubMed]
- Taniuchi, S.; Hattori, K.; Yamamoto, A.; Sasai, M.; Hatano, Y.; Kojima, T.; Kobayashi, Y.; Iwamoto, H.; Yaeshima, T. Administration of Bifidobacterium to infants with atopic dermatitis: Changes in fecal microflora and clinical symptoms. J. Appl. Res. Clin. Exp. Therapeut. 2005, 5, 387. [Google Scholar]
- Enomoto, T.; Sowa, M.; Nishimori, K.; Shimazu, S.; Yoshida, A.; Yamada, K.; Furukawa, F.; Nakagawa, T.; Yanagisawa, N.; Iwabuchi, N. Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota. Allergol. Int. 2014, 63, 575–585. [Google Scholar] [CrossRef]
- Fiocchi, A.; Pawankar, R.; Cuello-Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Canonica, G.W.; Ebisawa, M. World Allergy Organization-McMaster university guidelines for allergic disease prevention (GLAD-P): Probiotics. World Allergy Organ J. 2015, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Van der Aa, L.B.; Van Aalderen, W.M.C.; Heymans, H.S.A.; Henk Sillevis Smitt, J.; Nauta, A.J.; Knippels, L.M.J.; Ben Amor, K.; Sprikkelman, A.B.; Group, S.S. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 2011, 66, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Iwabuchi, N.; Xiao, J.-Z.; Yaeshima, T.; Iwatsuki, K. Suppressive effects of Bifidobacterium breve strain M-16V on T-helper type 2 immune responses in a murine model. Biol. Pharm. Bull. 2009, 32, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Cousins, D.J. Pinning allergies on pathogenic TH2 cells. Sci. Transl. Med. 2017, 9, eaao0392. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.S.; Mistry, K.J.; Kakade, A.M.; Niphadkar, P.V. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India Off. Organ Indian Chest Soc. 2010, 27, 66. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M. T follicular helper and TH2 cells in allergic responses. Allergol. Int. 2017, 66, 377–381. [Google Scholar] [CrossRef]


| Reference | Study Design | Study Characteristics |
|---|---|---|
| Effect of M-16V on bifidobacterial colonisation | ||
| Patole et al., 2014 [42] | Randomised, double-blinded, placebo-controlled | Study population: Preterm infants (<33 weeks; BW < 1500 g) |
| Country: Australia | ||
| Sample size: n = 159 (Probiotics: 79; Placebo: 80) | ||
| Intervention and dose: 3 × 109 CFU/day in 1.5 mL of sterile water or breast milk. | ||
| Duration of supplementation: Supplementation started when infants were on enteral feeds for <12 h, continued till 37 weeks of corrected gestational age. | ||
| Main outcomes: (1) Significant increase in B. breve faecal counts three weeks after M-16V supplementation. (2) No probiotic sepsis and death in M-16V-supplemented infants. | ||
| Study limitations: Nil | ||
| Patole et al., 2016 [61] | Non-RCT comparative analytical study | Study population: Preterm infants (<33 weeks; BW < 1500 g) |
| Country: Australia | ||
| Sample size: n = 159; subjects were divided into two groups based on their gestational age: (1) SGA due to IUGR (Probiotics: 22; Placebo: 20). (2) Non-SGA (Probiotics: 55; Placebo: 56) | ||
| Intervention and dose: 3 × 109 CFU/day in 1.5 mL of sterile water or breast milk. | ||
| Duration of supplementation: Supplementation started when infants were on enteral feeds for <12 h, continued till 37 weeks of gestational age. | ||
| Main outcomes: (1) B. breve faecal counts did not differ between SGA and non-SGA infants. (2) M-16V-treated SGA infants reached full feeds earlier than SGA controls, after adjustment for age at starting feeds and gestation <28 weeks. | ||
| Study limitations: (1) This was a comparative analytical study that relies on the results obtained from the previous study [42]. (2) Assessment of outcome could not be blinded; however, that was not expected to introduce a major bias because of the objectivity of the outcome. | ||
| Li et al., 2004 [63] | Randomised controlled trial | Study population: Preterm VLBW infants (BW < 1250 g) |
| Country: Japan | ||
| Sample size: n = 30 (A) M-16V given several hours (mean: 7.2 h) after birth; n = 10 (B) M-16V given >24 h (mean: 36.5 h) after birth; n = 10 (C) Control fed normally without probiotic supplement; n = 10 | ||
| Intervention and dose: 1.6 × 108 CFU twice daily in 0.5 mL of 5% glucose sterile water. | ||
| Duration of supplementation: Continued until discharged. | ||
| Main outcomes: (1) Significant increase in bifidobacterial colonisation in both groups A and B. (2) Significant earlier detection of bifidobacteria and a significant decrease in the cell numbers of Enterobacteriaceae were observed in group A. | ||
| Study limitations: (1) This was a non-double-blinded randomised controlled trial which may introduce potential bias. (2) The sample size was relatively small. (3) Assessment of outcome could not be blinded; however, that was not expected to introduce a major bias because of the objectivity of some of the outcomes. | ||
| Ishizeki et al., 2013 [32] | Non-RCT prospective study | Study population: Low birth weight infants (<33 weeks; BW 1000–2000 g) who were ready for feeds within seven days of birth in three cohorts: (1) Control group: October 1999 to June 2000; n = 16 (2) Single-strain M-16V group: December 2000 to June 2001; n = 15 (3) Three-strain probiotics mixture group: April 2002 to April 2003; n = 13 |
| Country: Japan | ||
| Sample size: 44 | ||
| Intervention and dose: (1) Control group: No probiotics (2) Single strain M-16V group: 5 × 108 CFU/day in 1.5 mL sterile water (3) Three-strain probiotics mixture group: B. longum BB536, B. breve M-16V and B. infantis M-63; 5 × 108 CFU/day of each strain in 1.5 mL sterile water | ||
| Duration of supplementation: Six weeks. | ||
| Main outcomes: (1) Both single-strain M-16V and three-strain probiotics mixture groups significantly increased the detection rates and cell numbers of Bifidobacterium in the faeces as compared to the control group. (2) Bifidobacteria proportion was significantly higher in the single-strain M-16V group at weeks one to four and in the three-strain probiotics mixture group at weeks one to six as compared to the control group. (3) The proportion of bifidobacteria in the three-strain probiotics mixture group was significantly higher than that in the single-strain M-16V group at weeks one and six. (4) The detection rates of Clostridium and proportions of Enterobacteriaceae were significantly lower in both probiotic groups. | ||
| Study limitations: (1) This was a retrospective study that relies on the quality of record keeping. (2) The study was conducted at three different timelines which may introduce potential bias. (3) Outcome evaluation could not be blinded; however, that was not expected to introduce a major bias because of the objective of some of the outcomes. | ||
| Akiyama et al., 1994 [62] | Non-RCT | Study population: Preterm LBW infants with mean gestation (range) 32.8 weeks (27.8–37.6 weeks) and BW 1486 g (780–2250 g) |
| Country: Japan | ||
| Sample size: n = 10 (Probiotics: 5; Control: 5) | ||
| Intervention and dose: 5 × 108 CFU/day of M-16V in 1.0 mL of sterile water | ||
| Duration of supplementation: Continued until eight weeks of age | ||
| Main outcomes: (1) Significant increase in bifidobacterial colonisation in M-16V-supplemented infants. | ||
| Study limitations: (1) This was a non-randomised controlled trial which may introduce potential bias. (2) The sample size was very small. (3) Assessment of outcome could not be blinded; however, that was not expected to introduce a major bias because of the objectivity of the outcome. | ||
| Akiyama et al., 1994 [64] | Non-RCT | Study population: Preterm LBW infants with mean gestation (range) 32.8 weeks (27.8–37.6 weeks) and BW 1486 g (780–2250 g) |
| Country: Japan | ||
| Sample size: n = 10 (Probiotics: 5; Control: 5) | ||
| Intervention and dose: 5 × 108 CFU/day of B. longum BB536 in 1.0 mL of sterile water | ||
| Duration of supplementation: Continued until eight weeks of age | ||
| Main outcomes: (1) Significant increase in bifidobacterial colonisation in B. longum BB536-supplemented infants. (2) This study compared the results obtained in the previous study using M-16V and revealed that while M-16V colonised the premature gut as early as week two after birth and remain dominant, the administered strain of B. longum was not detected from week six after birth. | ||
| Study limitations: (1) This was a non-randomised controlled trial comparing the results from the previous study which may introduce potential bias. (2) The sample size was very small. (3) Assessment of outcome could not be blinded; however, that was not expected to introduce a major bias because of the objectivity of the outcome. | ||
| Effect of M-16V on prevention of NEC | ||
| Satoh et al., 2007 [44] | Non-RCT retrospective study | Study population: Preterm VLBW and ELBW infants in two cohorts: (1) Control group: January 1994 to December 1998; n = 226 (ELBW: 101, VLBW: 125) (2) M-16V group: January 1999 to December 2003; n = 338 (ELBW: 220, VLBW: 118) |
| Country: Japan | ||
| Sample size: n = 564 | ||
| Intervention and dose: 1 × 109 CFU/day in milk or mixed with formula | ||
| Duration of supplementation: Commenced within several hours after birth (mean: 7.2 h) and continued till discharge at 37 weeks | ||
| Main outcomes: (1) Significant reduction in the incidence of Stage 1 NEC and infection. (2) Significant reduction in mortality due to infection. (3) Increased survival to discharge: 64.2% (301–600 g), 94% (601–1000 g), and 97.8% (1001–1500 g) | ||
| Study limitations: (1) This was a retrospective study that relies on the quality of record keeping. (2) The study was conducted at two different timelines which may introduce potential bias. (3) Outcome evaluation could not be blinded; however, that was not expected to introduce a major bias because of the objective of some of the outcomes. | ||
| Patole et al., 2016 [43] | Non-RCT retrospective study | Study population: Preterm neonates <34 weeks over two epochs: (1) Before probiotic supplementation: December 2008 to November 2010 (2) After probiotic supplementation: June 2012 to May 2014 |
| Country: Australia | ||
| Sample size: n = 1755 (Epoch 1: 835; Epoch 2: 920) | ||
| Intervention and dose: 3 × 109 CFU/day in 1.5 mL of sterile water or breast milk | ||
| Duration of supplementation: Started when the infant was ready for feeds and continued till 37 weeks corrected gestational age | ||
| Main outcomes: (1) Significant reduction in the incidence of NEC ≥ Stage II in infants supplemented with M-16V. (2) Significant reduction in “NEC ≥ Stage II or all-cause mortality”, late-onset sepsis, and age at full feeds in M-16V group. (3) For the subgroup of neonates <28 weeks, the beneficial effects of M-16V did not reach statistical significance. | ||
| Study limitations: (1) This was a retrospective study that relies on the quality of record keeping. (2) The study was conducted at two different timelines which may introduce potential bias. | ||
| Clinical studies related to the potential mechanisms of action of M-16V | ||
| Fuji et al., 2006 [70] | Randomised controlled trial | Study population: Preterm infants with mean gestation and mean BW of (1) Probiotics group: 31.3 ± 3.16 weeks and 1378 ± 365 g (2) Control group: 31.2 ± 1.98 weeks and 1496 ± 245 g |
| Country: Japan | ||
| Sample size: n = 19 (Probiotics: 11; Control: 8) | ||
| Intervention and dose: 1 × 109 CFU/day twice daily in 0.5 mL of 5% glucose solution | ||
| Duration of supplementation: Commenced within several hours after birth and continued till discharge. | ||
| Main outcomes: (1) Significant increase in the expression of serum TGF-β1 level and expression of TGF-β signalling molecule (Smad3) on day 28 in M-16V group. (2) Serum cytokine levels were not different in the two groups. | ||
| Study limitations: (1) The sample size was small. (2) Assessment of outcome could not be blinded; however, that was not expected to introduce a major bias because of the objectivity of the outcome. | ||
| Wang et al., 2007 [71] | Randomised controlled trial | Study population: Preterm LBW, VLBW, and ELBW infants (gestation: 23–36 weeks, BW: 414–2124 g) |
| Country: Japan | ||
| Sample size: n = 66 (ELBW, <1000 g: n =22; VLBW, <1500 g: n = 22; LBW, <2500 g: n = 22). The infants were divided into two groups: Probiotics and Control, 11 each). | ||
| Intervention and dose: 1.6 × 108 CFU/day twice daily in 0.5 mL of 5% glucose sterile distilled water. | ||
| Duration of supplementation: From birth till discharge | ||
| Main outcomes: (1) Significant increase in the ratio of acetate to total SCFAs in all M-16V-supplemented infants. (2) Significant reduction in faecal butyrate levels in ELBW and VLBW infants supplemented with M-16V. | ||
| Study limitations: (1) The sample size was small. (2) Assessment of outcome could not be blinded; however, that was not expected to introduce a major bias because of the objectivity of the outcome. | ||
| Reference | Type of Allergy | Study Design | Study Characteristics |
|---|---|---|---|
| Hattori et al., 2003 [112] | Atopic dermatitis (eczema) | Randomised controlled trial | Study population: Infants aged 8.6 ± 4.5 months |
| Country: Japan | |||
| Sample size: n = 15 (Probiotics: 8; Control: 7) | |||
| Intervention and dose: 5 × 109 CFU/day | |||
| Duration of supplementation: One month | |||
| Main outcomes: (1) Significant increase in the proportion of Bifidobacterium in the faecal microflora in M-16V group. (2) Significant reduction in the proportion of total aerobes in M-16V group. (3) Significant improvement in the allergic symptoms (cutaneous symptom score and total allergic score) in M-16V group. (4) No significant correlation between the changes in allergic symptoms and changes in intestinal microflora. | |||
| Study limitations: (1) The sample size was small. (2) Assessment of outcome could not be blinded; however, that was not expected to introduce a major bias because of the objectivity of some of the outcomes. | |||
| Taniuchi et al., 2005 [113] | Food allergy | Randomised controlled trial | Study population: Infants aged 3.1–18.5 months with cow’s milk hypersensitivity and atopic dermatitis |
| Country: Japan | |||
| Sample size: n = 17 (Probiotics: 10; Control: 7) | |||
| Intervention and dose: 5 × 109 CFU/day | |||
| Duration of supplementation: Three months | |||
| Main outcomes: (1) Significant increase in the proportion of Bifidobacterium in the faecal microflora in M-16V group. (2) Significant reduction in the proportion of total aerobic bacteria in M-16V group. (3) Significant improvement in the allergic symptoms in M-16V group as compared to the beginning of the study. (4) In the control group, no changes to the overall faecal microflora and total allergic score during the entire study period. | |||
| Study limitations: (1) The sample size was small. (2) Assessment of outcome could not be blinded; however, that was not expected to introduce a major bias because of the objectivity of some of the outcomes. (3) Outcomes were compared with the baseline but not the control group. | |||
| Del Giudice et al., 2017 [111] | Allergic rhinitis | Randomised, double-blinded, placebo-controlled | Study population: Children aged 9 ± 2.2 years with pollen-induced IgE-mediated allergic rhinitis and intermittent asthma |
| Country: Italy | |||
| Sample size: n = 40 (Probiotics: 20; Placebo: 20) | |||
| Intervention and dose: one sachet/day B. breve M-16V: 1 × 109 CFU B. longum BB536: 3 × 109 CFU B. infantis M-63: 1 × 109 CFU | |||
| Duration of supplementation: Four weeks | |||
| Main outcomes: (1) Significant improvement of allergic symptoms and quality of life in children treated with the probiotics mixture. (2) The intergroup analysis showed that probiotics mixture was significantly more superior to the placebo for all parameters. | |||
| Study limitations: The sample size was relatively small. | |||
| Enomoto et al., 2014 [114] | Atopic dermatitis (eczema) | Non-RCT open trial | Study population: Mother–infant pairs; maternal age: (1) Probiotics group: 22–41 years; (2) Control group: 21–38 years. |
| Country: Japan | |||
| Sample size: n = 166 (Probiotics: 130; Control: 36) | |||
| Intervention and dose: Pregnant women: 2 sachets/day; infants: 1 sachet/day B. breve M-16V: 5 × 109 CFU B. longum BB536: 5 × 109 CFU | |||
| Duration of supplementation: One month before the expected date of delivery and postnatally to the infants for six months. | |||
| Main outcomes: (1) Significant reduction in the risk of developing eczema/atopic dermatitis during the first 18 months of life in the probiotics group. (2) The proportion of Proteobacteria was significantly lower in mothers at the time of delivery who received probiotics supplementation when compared with the control group and was positively correlated with that of infants at four months of age. (3) No adverse effects were related to the use of probiotics. | |||
| Study limitations: (1) This was a non-randomised trial which may introduce potential bias. (2) Assessment of outcome could not be blinded; however, that was not expected to introduce a major bias because of the objectivity of some of the outcomes. | |||
| Van der Aa et al., 2011 [116] | Atopic dermatitis (eczema) | Double-blinded, placebo-controlled multicentre trial | Study population: Full-term infants aged <7 months with atopic dermatitis. |
| Country: Netherlands | |||
| Sample size: n = 90 (Synbiotics: 46; Placebo: 44) | |||
| Intervention and dose: Synbiotics consisted of B. breve M-16V at a dose of 1.3 × 109 CFU/100 mL and a mixture of 90% scGOS and 10% lcFOS (Immunofortis®), 0.8 g/100 mL. | |||
| Duration of supplementation: 12 weeks | |||
| Main outcomes: (1) Of the 75 children (mean age 17.3 months) completed the one-year follow-up evaluation, the prevalence of “frequent wheezing” and “wheezing and/or noisy breathing apart from colds” was significantly lower in the synbiotic than in the placebo. (2) Significantly fewer children in the synbiotic than in the placebo group had started to use asthma medication after baseline. (3) Total IgE levels did not differ between the two groups. | |||
| Study limitations: The study tested the effect of M-16V in a synbiotic formulation. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.B.; Iwabuchi, N.; Xiao, J.-z. Exploring the Science behind Bifidobacterium breve M-16V in Infant Health. Nutrients 2019, 11, 1724. https://doi.org/10.3390/nu11081724
Wong CB, Iwabuchi N, Xiao J-z. Exploring the Science behind Bifidobacterium breve M-16V in Infant Health. Nutrients. 2019; 11(8):1724. https://doi.org/10.3390/nu11081724
Chicago/Turabian StyleWong, Chyn Boon, Noriyuki Iwabuchi, and Jin-zhong Xiao. 2019. "Exploring the Science behind Bifidobacterium breve M-16V in Infant Health" Nutrients 11, no. 8: 1724. https://doi.org/10.3390/nu11081724
APA StyleWong, C. B., Iwabuchi, N., & Xiao, J. -z. (2019). Exploring the Science behind Bifidobacterium breve M-16V in Infant Health. Nutrients, 11(8), 1724. https://doi.org/10.3390/nu11081724