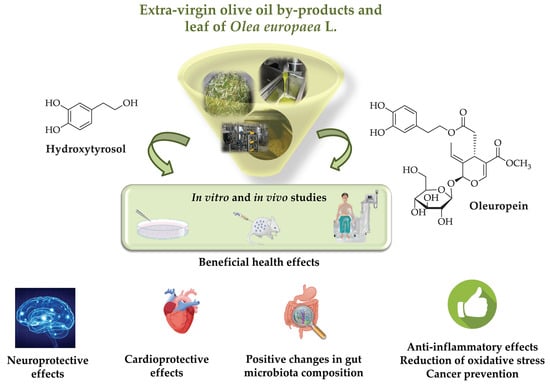
Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L.
Abstract
:1. Introduction
2. Methods
3. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil (EVOO)
Impact of Olive Oil and Its Derivatives on Gut Microbiota Composition
4. Health Effects of Phenolic Compounds Present in Olea By-Products and Waste
5. Health Effects of Phenolic Compounds Present in Olea Leaf and Olea Leaf Extracts
6. Bioaccessibility and Bioavailability of Olea Minor Compounds
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation List
| CKD | Chronic kidney disease |
| COX-1 | Ciclooxygenase-1 |
| COX-2 | Ciclooxygenase-2 |
| CV | Cardiovascular |
| CVD | Cardiovascular disease |
| DXR | Doxorubicin |
| EFSA | European Food Safety Authority |
| EPIC | European Prospective Investigation into Cancer and Nutrition |
| EPICOR | Long-term follow-up of antithrombotic management patterns on acute coronary syndrome patients |
| EU | European Union |
| EVOO | Extra-virgin olive oil |
| Hcy | Homocysteine |
| HDL | High density lipoproteins |
| HPLC-DAD | High performance liquid chromatography-diode array detector |
| HPLC-MS | High performance liquid chromatography-mass detector |
| HT | Hydroxytyrosol |
| HUVEC | Human endothelial cells |
| IMD | Italian Mediterranean diet |
| IMOD | Italian Mediterranean organic diet |
| IOC | International Olive Council |
| K-DOQI | Kidney-Disease Outcomes Quality Initiative |
| LDL | Low density lipoprotein |
| LOX-1 | Lectin-like oxidized LDL receptor-1 |
| MD | Mediterranean diet |
| MDM | Monocyte- derived macrophages |
| MFA | Membrane Filtration Absorption |
| MTHFR | Methylenetetrahydrofolate reductase |
| NCD | Chronic non-communicable disease |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| OLC | Oleocanthal |
| OLE | Oleuropein |
| OO | Olive oil |
| OxLDL | Oxidized low density lipoprotein |
| PMA | Phorbol-myristate acetate |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| RCT | Randomized controlled trial |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated-secretory-phenotype |
| SCSCD | Seven Country Study of Cardiovascular Disease |
| SOP | Stoned Olive Pomace |
| TRPA1 | Transient receptor potential channel, subfamily A, member 1 |
| Tyr | Tyrosol |
| UNESCO | United Nations Educational Scientific and Cultural Organization |
References
- Bosku, D. Olive Oil. In Mediterranean Diets; Simopoulos, A.P., Visioli, F., Eds.; Karger Publishers: Basel, Switzerland, 2000; pp. 56–77. [Google Scholar]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin Olive Oil and Cardiovascular Diseases: Benefits for Human Health. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius-International Food Standards. Available online: http://www.fao.org/fao-who-codexalimentarius/en/ (accessed on 4 April 2019).
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- López-Biedma, A.; Sánchez-Quesada, C.; Delgado-Rodríguez, M.; Gaforio, J.J. The biological activities of natural lignans from olives and virgin olive oils: A review. J. Funct. Foods 2016, 26, 36–47. [Google Scholar] [CrossRef]
- Gambacorta, A.; Tofani, D.; Bernini, R.; Migliorini, A. High-yielding preparation of a stable precursor of hydroxytyrosol by total synthesis and from the natural glycoside oleuropein. J. Agric. Food Chem. 2007, 55, 3386–3391. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, Innovation, and Green Chemistry in the Production and Valorization of Phenolic Extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef]
- Mulinacci, N.; Romani, A.; Galardi, C.; Pinelli, P.; Giaccherini, C.; Vincieri, F.F. Polyphenolic content in olive oil waste waters and related olive samples. J. Agric. Food Chem. 2001, 49, 3509–3514. [Google Scholar] [CrossRef] [PubMed]
- Adriaanse, A.; Bringezu, S.; Hamond, A.; Moriguchi, Y.; Rodenburg, E.; Rogich, D.; Schütz, H. Resource Flows: The Material Base of Industrial Economies; World Resources Institute: Washington, DC, USA, 1997; Available online: http://pdf.wri.org/resourceflows_w.pdf (accessed on 15 March 2019).
- Brunner, P.H.; Rechberger, H. Practical Handbook of Material Flow Analysis; CRC Press LLC.: Boca Raton, FL, USA, 2004. [Google Scholar]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 2013, 248, 185–193. [Google Scholar] [CrossRef]
- Romani, A.; Scardigli, A.; Pinelli, P. An environmentally friendly process for the production of extracts rich in phenolic antioxidants from Olea europaea L. and Cynara scolymus L. matrices. Eur. Food Res. Technol. 2017, 243, 1229–1238. [Google Scholar] [CrossRef]
- Pizzichini, D.; Russo, C.; Vitagliano, M.; Pizzichini, M.; Romani, A.; Ieri, F.; Pinelli, P.; Vignolini, P. Process for producing concentrated and refined actives from tissues and byproducts of Olea europaea with membrane technologies. Patent No. EP2338500A1, 29 June 2011. [Google Scholar]
- Roig, A.; Cayuela, M.L.; Sanchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef]
- Xavier Medina, F. Mediterranean diet, culture and heritage: Challenges for a new conception. Public Health Nutr. 2009, 12, 1618–1620. [Google Scholar] [CrossRef]
- Grosso, G.; Pajak, A.; Mistretta, A.; Marventano, S.; Raciti, T.; Buscemi, S.; Drago, F.; Scalfi, L.; Galvano, F. Protective role of the Mediterranean diet on several cardiovascular risk factors: Evidence from Sicily, southern Italy. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 370–377. [Google Scholar] [CrossRef]
- Grosso, G.; Marventano, S.; Yang, J.; Micek, A.; Pajak, A.; Scalfi, L.; Galvano, F.; Kales, S.N. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit. Rev. Food Sci. Nutr. 2017, 57, 3218–3232. [Google Scholar] [CrossRef]
- Di Daniele, N.; Petramala, L.; Di Renzo, L.; Sarlo, F.; Della Rocca, D.G.; Rizzo, M.; Fondacaro, V.; Iacopino, L.; Pepine, C.J.; De Lorenzo, A. Body composition changes and cardiometabolic benefits of a balanced Italian Mediterranean Diet in obese patients with metabolic syndrome. Acta Diabetol. 2013, 50, 409–416. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Noce, A.; Bigioni, M.; Calabrese, V.; Della Rocca, D.G.; Di Daniele, N.; Tozzo, C.; Di Renzo, L. The effects of Italian Mediterranean organic diet (IMOD) on health status. Curr. Pharm. Des. 2010, 16, 814–824. [Google Scholar] [CrossRef]
- Di Daniele, N.; Di Renzo, L.; Noce, A.; Iacopino, L.; Ferraro, P.M.; Rizzo, M.; Sarlo, F.; Domino, E.; De Lorenzo, A. Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J. Nephrol. 2014, 27, 529–536. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Renaud, S.; Mamelle, N.; Salen, P.; Martin, J.L.; Monjaud, I.; Guidollet, J.; Touboul, P.; Delaye, J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994, 343, 1454–1459. [Google Scholar] [CrossRef]
- McLennan, P.L. Relative effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on cardiac arrhythmias in rats. Am. J. Clin. Nutr. 1993, 57, 207–212. [Google Scholar] [CrossRef]
- Kopple, J.D. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2001, 37, S66–S70. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Jin, H.; Ebin, Z.; Brodsky, S.; Goligorsky, M.S. Effects of homocysteine on endothelial nitric oxide production. Am. J. Physiol. Renal. Physiol. 2000, 279, F671–F678. [Google Scholar] [CrossRef]
- Pastore, A.; Noce, A.; Di Giovamberardino, G.; De Stefano, A.; Calla, C.; Zenobi, R.; Dessi, M.; Di Daniele, N. Homocysteine, cysteine, folate and vitamin B(1)(2) status in type 2 diabetic patients with chronic kidney disease. J. Nephrol. 2015, 28, 571–576. [Google Scholar] [CrossRef]
- EFSA. Panel on Dietetic Products. EFSA J. 2011, 9, 2033–2058. [Google Scholar]
- EFSA. Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2033 (accessed on 5 April 2019).
- Dunn, S.; Vohra, R.S.; Murphy, J.E.; Homer-Vanniasinkam, S.; Walker, J.H.; Ponnambalam, S. The lectin-like oxidized low-density-lipoprotein receptor: A pro-inflammatory factor in vascular disease. Biochem. J. 2008, 409, 349–355. [Google Scholar] [CrossRef]
- Zambonin, L.; Caliceti, C.; Vieceli Dalla Sega, F.; Fiorentini, D.; Hrelia, S.; Landi, L.; Prata, C. Dietary phenolic acids act as effective antioxidants in membrane models and in cultured cells, exhibiting proapoptotic effects in leukaemia cells. Oxidative Med. Cell. Longev. 2012, 2012. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Fagnani, R.; Mitro, N.; Scurati, S.; Masciadri, M.; Mussoni, L.; Galli, G.V.; Bosisio, E.; Crestani, M.; De Fabiani, E.; et al. Minor components of olive oil modulate proatherogenic adhesion molecules involved in endothelial activation. J. Agric. Food Chem. 2006, 54, 3259–3264. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Covas, M.I. Olive oil and the cardiovascular system. Pharmacol. Res. 2007, 55, 175–186. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Incani, A.; Serra, G.; Atzeri, A.; Melis, M.P.; Serreli, G.; Bandino, G.; Sedda, P.; Campus, M.; Tuberoso, C.I.; Deiana, M. Extra virgin olive oil phenolic extracts counteract the pro-oxidant effect of dietary oxidized lipids in human intestinal cells. Food Chem. Toxicol. 2016, 90, 171–180. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef]
- Franconi, F.; Coinu, R.; Carta, S.; Urgeghe, P.P.; Ieri, F.; Mulinacci, N.; Romani, A. Antioxidant effect of two virgin olive oils depends on the concentration and composition of minor polar compounds. J. Agric. Food Chem. 2006, 54, 3121–3125. [Google Scholar] [CrossRef]
- Manna, C.; D’Angelo, S.; Migliardi, V.; Loffredi, E.; Mazzoni, O.; Morrica, P.; Galletti, P.; Zappia, V. Protective effect of the phenolic fraction from virgin olive oils against oxidative stress in human cells. J. Agric. Food Chem. 2002, 50, 6521–6526. [Google Scholar] [CrossRef]
- Salvini, S.; Sera, F.; Caruso, D.; Giovannelli, L.; Visioli, F.; Saieva, C.; Masala, G.; Ceroti, M.; Giovacchini, V.; Pitozzi, V.; et al. Daily consumption of a high-phenol extra-virgin olive oil reduces oxidative DNA damage in postmenopausal women. Br. J. Nutr. 2006, 95, 742–751. [Google Scholar] [CrossRef]
- Covas, M.I.; Nyyssonen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Baumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Int. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Perez-Herrera, A.; Delgado-Lista, J.; Torres-Sanchez, L.A.; Rangel-Zuniga, O.A.; Camargo, A.; Moreno-Navarrete, J.M.; Garcia-Olid, B.; Quintana-Navarro, G.M.; Alcala-Diaz, J.F.; Munoz-Lopez, C.; et al. The postprandial inflammatory response after ingestion of heated oils in obese persons is reduced by the presence of phenol compounds. Mol. Nutr. Food Res. 2012, 56, 510–514. [Google Scholar] [CrossRef]
- Souza, P.A.L.; Marcadenti, A.; Portal, V.L. Effects of Olive Oil Phenolic Compounds on Inflammation in the Prevention and Treatment of Coronary Artery Disease. Nutrients 2017, 9, 1087. [Google Scholar] [CrossRef]
- Tousoulis, D.; Charakida, M.; Stefanadis, C. Endothelial function and inflammation in coronary artery disease. Heart 2006, 92, 441–444. [Google Scholar] [CrossRef]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291–308. [Google Scholar] [CrossRef]
- Brunelleschi, S.; Bardelli, C.; Amoruso, A.; Gunella, G.; Ieri, F.; Romani, A.; Malorni, W.; Franconi, F. Minor polar compounds extra-virgin olive oil extract (MPC-OOE) inhibits NF-kappa B translocation in human monocyte/macrophages. Pharmacol. Res. 2007, 56, 542–549. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C. Olive Oil Phenols and Their Potential Effects on Human Health. J. Agric. Food Chem. 1998, 10, 4292–4296. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Noce, A.; Moriconi, E.; Rampello, T.; Marrone, G.; Di Daniele, N.; Rovella, V. MOSH Syndrome (Male Obesity Secondary Hypogonadism): Clinical Assessment and Possible Therapeutic Approaches. Nutrients 2018, 10, 474. [Google Scholar] [CrossRef]
- Francisco, V.; Ruiz-Fernandez, C.; Lahera, V.; Lago, F.; Pino, J.; Skaltsounis, L.; Gonzalez-Gay, M.A.; Mobasheri, A.; Gomez, R.; Scotece, M.; et al. Natural Molecules for Healthy Lifestyles: Oleocanthal from Extra Virgin Olive Oil. J. Agric. Food Chem. 2019, 67, 3845–3853. [Google Scholar] [CrossRef]
- Cicerale, S.; Breslin, P.A.; Beauchamp, G.K.; Keast, R.S. Sensory characterization of the irritant properties of oleocanthal, a natural anti-inflammatory agent in extra virgin olive oils. Chem. Senses 2009, 34, 333–339. [Google Scholar] [CrossRef]
- Peyrot des Gachons, C.; Uchida, K.; Bryant, B.; Shima, A.; Sperry, J.B.; Dankulich-Nagrudny, L.; Tominaga, M.; Smith, A.B., 3rd; Beauchamp, G.K.; Breslin, P.A. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J. Neurosci. 2011, 31, 999–1009. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Parkinson, L.; Keast, R. Oleocanthal, a phenolic derived from virgin olive oil: A review of the beneficial effects on inflammatory disease. Int. J. Mol. Sci. 2014, 15, 12323–12334. [Google Scholar] [CrossRef]
- Harris, R.E.; Beebe-Donk, J.; Doss, H.; Burr Doss, D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: A critical review of non-selective COX-2 blockade (review). Oncol. Rep. 2005, 13, 559–583. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, Y.; Li, B.; Liu, F.; Ryder, J.W.; Wu, X.; Gonzalez-DeWhitt, P.A.; Gelfanova, V.; Hale, J.E.; May, P.C.; et al. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science 2003, 302, 1215–1217. [Google Scholar] [CrossRef]
- May, A.E.; Seizer, P.; Gawaz, M. Platelets: Inflammatory firebugs of vascular walls. Arterioscler. Thromb. Vasc. Biol. 2008, 28, s5–s10. [Google Scholar] [CrossRef]
- Segura-Carretero, A.; Curiel, J.A. Current Disease-Targets for Oleocanthal as Promising Natural Therapeutic Agent. Int. J. Mol. Sci. 2018, 19, 2899. [Google Scholar] [CrossRef]
- Agrawal, K.; Melliou, E.; Li, X.; Pedersen, T.L.; Wang, S.C.; Magiatis, P.; Newman, J.W.; Holt, R.R. Oleocanthal-rich extra virgin olive oil demonstrates acute anti-platelet effects in healthy men in a randomized trial. J. Funct. Foods 2017, 36, 84–93. [Google Scholar] [CrossRef]
- Priora, R.; Summa, D.; Frosali, S.; Margaritis, A.; Di Giuseppe, D.; Lapucci, C.; Ieri, F.; Pulcinelli, F.M.; Romani, A.; Franconi, F.; et al. Administration of minor polar compound-enriched extra virgin olive oil decreases platelet aggregation and the plasma concentration of reduced homocysteine in rats. J. Nutr. 2008, 138, 36–41. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Del Carlo, M.; Gallina-Toschi, T.; Lercker, G.; Compagnone, D.; Fernandez-Gutierrez, A. Evaluation of the antioxidant capacity of individual phenolic compounds in virgin olive oil. J. Agric. Food Chem. 2005, 53, 8918–8925. [Google Scholar] [CrossRef]
- Vuorela, S.; Kreander, K.; Karonen, M.; Nieminen, R.; Hamalainen, M.; Galkin, A.; Laitinen, L.; Salminen, J.P.; Moilanen, E.; Pihlaja, K.; et al. Preclinical evaluation of rapeseed, raspberry, and pine bark phenolics for health related effects. J. Agric. Food Chem. 2005, 53, 5922–5931. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Analyzing effects of extra-virgin olive oil polyphenols on breast cancer-associated fatty acid synthase protein expression using reverse-phase protein microarrays. Int. J. Mol. Med. 2008, 22, 433–439. [Google Scholar] [CrossRef]
- Fini, L.; Hotchkiss, E.; Fogliano, V.; Graziani, G.; Romano, M.; De Vol, E.B.; Qin, H.; Selgrad, M.; Boland, C.R.; Ricciardiello, L. Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines. Carcinogenesis 2008, 29, 139–146. [Google Scholar] [CrossRef]
- Wongwarawipat, T.; Papageorgiou, N.; Bertsias, D.; Siasos, G.; Tousoulis, D. Olive Oil-related Anti-inflammatory Effects on Atherosclerosis: Potential Clinical Implications. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 51–62. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef]
- Riboli, E.; Hunt, K.J.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondiere, U.R.; Hemon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study populations and data collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef]
- Park, J.Y.; Nicolas, G.; Freisling, H.; Biessy, C.; Scalbert, A.; Romieu, I.; Chajes, V.; Chuang, S.C.; Ericson, U.; Wallstrom, P.; et al. Comparison of standardised dietary folate intake across ten countries participating in the European Prospective Investigation into Cancer and Nutrition. Br. J. Nutr. 2012, 108, 552–569. [Google Scholar] [CrossRef]
- Sieri, S.; Agnoli, C.; Pala, V.; Mattiello, A.; Panico, S.; Masala, G.; Assedi, M.; Tumino, R.; Frasca, G.; Sacerdote, C.; et al. Dietary habits and cancer: The experience of epic-Italy. Epidemiologia e Prevenzione 2015, 39, 333–338. [Google Scholar]
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Wilson Jones, G.; Bernini, R.; Romani, A.; Rovella, V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef]
- Masala, G.; Ceroti, M.; Pala, V.; Krogh, V.; Vineis, P.; Sacerdote, C.; Saieva, C.; Salvini, S.; Sieri, S.; Berrino, F.; et al. A dietary pattern rich in olive oil and raw vegetables is associated with lower mortality in Italian elderly subjects. Br. J. Nutr. 2007, 98, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Bendinelli, B.; Masala, G.; Saieva, C.; Salvini, S.; Calonico, C.; Sacerdote, C.; Agnoli, C.; Grioni, S.; Frasca, G.; Mattiello, A.; et al. Fruit, vegetables, and olive oil and risk of coronary heart disease in Italian women: The EPICOR Study. Am. J. Clin. Nutr. 2011, 93, 275–283. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Gavahian, M.; Khaneghah, A.M.; Lorenzo, J.M.; Munekata, P.E.; Garcia-Mantrana, I.; Carmen Collado, M.; Melendez-Martinez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Pallara, G.; Buccioni, A.; Pastorelli, R.; Minieri, S.; Mele, M.; Rapaccini, S.; Messini, A.; Pauselli, M.; Servili, M.; Giovannetti, L.; et al. Effect of stoned olive pomace on rumen microbial communities and polyunsaturated fatty acid biohydrogenation: An in vitro study. BMC Vet. Res. 2014, 10. [Google Scholar] [CrossRef]
- Martinez, N.; Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martinez-Rodriguez, A.M.; Cobo, A.; Ramirez, M.; Galvez, A.; Martinez-Canamero, M. Refined versus Extra Virgin Olive Oil High-Fat Diet Impact on Intestinal Microbiota of Mice and Its Relation to Different Physiological Variables. Microorganisms 2019, 7, 61. [Google Scholar] [CrossRef]
- Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martinez-Rodriguez, A.M.; Cobo, A.; Ramirez, M.; Abriouel, H.; Galvez, A.; Martinez-Canamero, M. Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS ONE 2018, 13, e0190368. [Google Scholar] [CrossRef]
- Hidalgo, M.; Prieto, I.; Abriouel, H.; Villarejo, A.B.; Ramirez-Sanchez, M.; Cobo, A.; Benomar, N.; Galvez, A.; Martinez-Canamero, M. Changes in Gut Microbiota Linked to a Reduction in Systolic Blood Pressure in Spontaneously Hypertensive Rats Fed an Extra Virgin Olive Oil-Enriched Diet. Plant Foods Hum. Nutr. 2018, 73. [Google Scholar] [CrossRef]
- Martin-Pelaez, S.; Castaner, O.; Sola, R.; Motilva, M.J.; Castell, M.; Perez-Cano, F.J.; Fito, M. Influence of Phenol-Enriched Olive Oils on Human Intestinal Immune Function. Nutrients 2016, 8, 213. [Google Scholar] [CrossRef]
- Fiorentino, A.; Gentili, A.; Isidori, M.; Monaco, P.; Nardelli, A.; Parrella, A.; Temussi, F. Environmental effects caused by olive mill wastewaters: Toxicity comparison of low-molecular-weight phenol components. J. Agric. Food Chem. 2003, 51, 1005–1009. [Google Scholar] [CrossRef]
- Cecchi, L.; Bellumori, M.; Cipriani, C.; Mocali, A.; Innocenti, M.; Mulinacci, N.; Giovannelli, L. A two-phase olive mill by-product (pâté) as a convenient source of phenolic compounds: Content, stability, and antiaging properties in cultured human fibroblasts. J. Funct. Foods 2018, 40, 751–759. [Google Scholar] [CrossRef]
- Bellumori, M.; Cecchi, L.; Romani, A.; Mulinacci, N.; Innocenti, M. Recovery and stability over time of phenolic fractions by an industrial filtration system of olive mill wastewaters: A three-year study. J. Sci. Food Agric. 2018, 98, 2761–2769. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Zanoni, B.; Breschi, C.; Mulinacci, N. An effective HPLC-based approach for the evaluation of the content of total phenolic compounds transferred from olives to virgin olive oil during the olive milling process. J. Sci. Food Agric. 2018, 98, 3636–3643. [Google Scholar] [CrossRef]
- Schaffer, S.; Muller, W.E.; Eckert, G.P. Cytoprotective effects of olive mill wastewater extract and its main constituent hydroxytyrosol in PC12 cells. Pharmacol. Res. 2010, 62, 322–327. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Bioscreening of Australian olive mill waste extracts: Biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem. Toxicol. 2007, 45, 1238–1248. [Google Scholar] [CrossRef]
- Bernini, R.M.E.; Barontini, M.; Crisante, F. Convenient synthesis of hydroxytyrosol and its lipophilic derivatives from tyrosol or homovanillyl alcohol. J. Agric. Food Chem. 2008, 56, 8897–8904. [Google Scholar] [CrossRef]
- Bernini, R.C.S.; Fabrizi, G.; Filisti, E. 2-Arylhydroxytyrosol derivatives via Suzuki-Miyaura cross-coupling. Org. Lett. 2008, 10, 3457–3460. [Google Scholar] [CrossRef]
- Bernini, R.; Crisante, F.; Merendino, N.; Molinari, R.; Soldatelli, M.C.; Velotti, F. Synthesis of a novel ester of hydroxytyrosol and alpha-lipoic acid exhibiting an antiproliferative effect on human colon cancer HT-29 cells. Eur. J. Med. Chem. 2011, 46, 439–446. [Google Scholar] [CrossRef]
- Barontini, M.B.R.; Carastro, R.; Gentili, P.; Romani, A. Synthesis and DPPH radical scavenging activity of novel compounds obtained from tyrosol and cinnamic acid derivatives. New J. Chem. 2014, 38, 809–816. [Google Scholar] [CrossRef]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of Hydroxytyrosol-Enriched Fractions from Olea europaea L. Byproducts and Evaluation of the in Vitro Effects on a Model of Colorectal Cancer Cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef]
- Bernini, R.; Carastro, I.; Santoni, F.; Clemente, M. Synthesis of lipophilic esters of tyrosol, homovanillyl alcohol and hydroxytyrosol. Antioxidants 2019, 8, 174. [Google Scholar] [CrossRef]
- Plastina, P.B.C.; Perri, E.; Fazio, A.; Augimeri, G.; Poland, M.; Witkamp, R.; Meijerink, J. Identification of hydroxytyrosyl oleate, a derivative of hydroxytyrosol with anti-inflammatory properties, in olive oil by-product. Food Chem. 2019, 279, 105–113. [Google Scholar] [CrossRef]
- Visioli, F.W.R.; Richard, D.; Abdullah, M.I.C.B.; Crea, R. Olive phenolics increase glutathione levels in healthy volunteers. J. Agric. Food Chem. 2009, 57, 1793–1796. [Google Scholar] [CrossRef]
- DellaGreca, M.; Previtera, L.; Temussi, F.; Zarrelli, A. Low-molecular-weight components of olive oil mill waste-waters. Phytochem. Anal. 2004, 15, 184–188. [Google Scholar] [CrossRef]
- Massei, G.; Hartley, S.E. Disarmed by domestication? Induced responses to browsing in wild and cultivated olive. Oecologia 2000, 122, 225–231. [Google Scholar] [CrossRef]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017. [Google Scholar] [CrossRef]
- Andrikopoulos, N.K.; Kaliora, A.C.; Assimopoulou, A.N.; Papageorgiou, V.P. Inhibitory activity of minor polyphenolic and nonpolyphenolic constituents of olive oil against in vitro low-density lipoprotein oxidation. J. Med. Food 2002, 5. [Google Scholar] [CrossRef]
- Janahmadi, Z.; Nekooeian, A.A.; Moaref, A.R.; Emamghoreishi, M. Oleuropein attenuates the progression of heart failure in rats by antioxidant and antiinflammatory effects. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 245–252. [Google Scholar] [CrossRef]
- Jemai, H.; El Feki, A.; Sayadi, S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef] [Green Version]
- De Bock, M.; Derraik, J.G.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef]
- Papachristodoulou, A.; Tsoukala, M.; Benaki, D.; Kostidis, S.; Gioti, K.; Aligiannis, N.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.; Mikros, E.; et al. Oleuropein is a Powerful Sensitizer of Doxorubicin-mediated Killing of Prostate Cancer Cells and Exerts Its Action via Induction of Autophagy. J. Cancer Res. Treat 2016, 4, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Elamin, M.H.; Elmahi, A.B.; Daghestani, M.H.; Al-Olayan, E.M.; Al-Ajmi, R.A.; Alkhuriji, A.F.; Hamed, S.S.; Elkhadragy, M.F. Synergistic Anti-Breast-Cancer Effects of Combined Treatment with Oleuropein and Doxorubicin In Vivo. Altern. Ther. 2019, 25, 17–24. [Google Scholar]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef]
- Miceli, C.; Santin, Y.; Manzella, N.; Coppini, R.; Berti, A.; Stefani, M.; Parini, A.; Mialet-Perez, J.; Nediani, C. Oleuropein Aglycone Protects against MAO-A-Induced Autophagy Impairment and Cardiomyocyte Death through Activation of TFEB. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Parri, M.; Nediani, C.; Cerbai, E.; Stefani, M.; Berti, A. Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J. Nutr. Biochem. 2010, 21, 726–735. [Google Scholar] [CrossRef]
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Nichino, D.; Relini, A.; Berti, A.; Stefani, M. Abeta(1-42) aggregates into non-toxic amyloid assemblies in the presence of the natural polyphenol oleuropein aglycon. Curr. Alzheimer Res. 2011, 8, 841–852. [Google Scholar] [CrossRef]
- Rigacci, S.; Miceli, C.; Nediani, C.; Berti, A.; Cascella, R.; Pantano, D.; Nardiello, P.; Luccarini, I.; Casamenti, F.; Stefani, M. Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: A mechanistic insight. Oncotarget 2015, 6, 35344–35357. [Google Scholar] [CrossRef]
- Luccarini, I.; Pantano, D.; Nardiello, P.; Cavone, L.; Lapucci, A.; Miceli, C.; Nediani, C.; Berti, A.; Stefani, M.; Casamenti, F. The Polyphenol Oleuropein Aglycone Modulates the PARP1-SIRT1 Interplay: An In Vitro and In Vivo Study. J. Alzheimers Dis. 2016, 54, 737–750. [Google Scholar] [CrossRef] [Green Version]
- Cordero, J.G.; Garcia-Escudero, R.; Avila, J.; Gargini, R.; Garcia-Escudero, V. Benefit of Oleuropein Aglycone for Alzheimer’s Disease by Promoting Autophagy. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Margheri, F.M.B.; Laurenzana, A.; Del Rosso, M.; Fibbi, G.; Cipolleschi, M.G.; Ruzzolini, J.; Nediani, C.; Mocali, A.; Giovannelli, L. Oleuropein aglycone attenuates the pro-angiogenic phenotype of senescent fibroblasts: A functional study in endothelial cells. J. Funct. Food 2019, 53, 219–226. [Google Scholar] [CrossRef]
- Shamshoum, H.; Vlavcheski, F.; Tsiani, E. Anticancer effects of oleuropein. Biofactors 2017, 43, 517–528. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef] [Green Version]
- Celano, M.; Maggisano, V.; Lepore, S.M.; Russo, D.; Bulotta, S. Secoiridoids of olive and derivatives as potential coadjuvant drugs in cancer: A critical analysis of experimental studies. Pharmacol. Res. 2019, 142, 77–86. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; la Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef]
- Andreadou, I.; Mikros, E.; Ioannidis, K.; Sigala, F.; Naka, K.; Kostidis, S.; Farmakis, D.; Tenta, R.; Kavantzas, N.; Bibli, S.I.; et al. Oleuropein prevents doxorubicin-induced cardiomyopathy interfering with signaling molecules and cardiomyocyte metabolism. J. Mol. Cell. Cardiol. 2014, 69, 4–16. [Google Scholar] [CrossRef]
- Karkovic Markovic, A.; Toric, J.; Barbaric, M.; Jakobusic Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Pastor, A.; Rodriguez-Morato, J.; Olesti, E.; Pujadas, M.; Perez-Mana, C.; Khymenets, O.; Fito, M.; Covas, M.I.; Sola, R.; Motilva, M.J.; et al. Analysis of free hydroxytyrosol in human plasma following the administration of olive oil. J. Chromatogr. A 2016, 1437, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Jilani, H.; Cilla, A.; Barberá, R.; Hamdi, M. Improved bioaccessibility and antioxidant capacity of olive leaf (Olea europaea L.) polyphenols through biosorption on Saccharomyces cerevisiae. Ind. Crops Prod. 2016, 84, 131–138. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Canovas, J.; Barrajon-Catalan, E.; Carreres, J.E.; Micol, V.; Garcia-Perez, J.V. Influence of olive leaf processing on the bioaccessibility of bioactive polyphenols. J. Agric. Food Chem. 2014, 62, 6190–6198. [Google Scholar] [CrossRef]
- De Bock, M.; Thorstensen, E.B.; Derraik, J.G.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef]
- Kendall, M.; Batterham, M.; Callahan, D.L.; Jardine, D.; Prenzler, P.; Robards, K.; Ryan, D. Randomized controlled study of the urinary excretion of biophenols following acute and chronic intake of olive leaf supplements. Food Chem. 2012, 130, 651–659. [Google Scholar] [CrossRef]
- Žugčić, T.; Abdelkebir, R.; Alcantara, C.; Collado, M.C.; García-Pérez, J.V.; Meléndez-Martínez, A.; Jambrak, A.R.; Lorenzo, J.M.; Barba, F.J. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci. Technol. 2019, 83, 63–67. [Google Scholar] [CrossRef]
- Mosele, J.I.; Martin-Pelaez, S.; Macia, A.; Farras, M.; Valls, R.M.; Catalan, U.; Motilva, M.J. Faecal microbial metabolism of olive oil phenolic compounds: In vitro and in vivo approaches. Mol. Nutr. Food Res. 2014, 58, 1809–1819. [Google Scholar] [CrossRef]
- Santos, M.M.; Piccirillo, C.; Castro, P.M.; Kalogerakis, N.; Pintado, M.E. Bioconversion of oleuropein to hydroxytyrosol by lactic acid bacteria. World J. Microbiol. Biotechnol. 2012, 28, 2435–2440. [Google Scholar] [CrossRef]
- Aponte, M.; Ungaro, F.; d’Angelo, I.; De Caro, C.; Russo, R.; Blaiotta, G.; Dal Piaz, F.; Calignano, A.; Miro, A. Improving in vivo conversion of oleuropein into hydroxytyrosol by oral granules containing probiotic Lactobacillus plantarum 299v and an Olea europaea standardized extract. Int. J. Pharm. 2018, 543, 73–82. [Google Scholar] [CrossRef]





| EVOO Minor Polar Components | |
|---|---|
| Secoiridoids | (a) Oleuropein aglycone |
| (b) Deacetoxy oleuropein | |
| (c) Oleocanthal and oleacin | |
| (d) Ligstroside aglycone | |
| Phenolics | (a) Hydroxytyrosol |
| (b) Tyrosol | |
| (c) Hydroxytyrosol glycole | |
| Phenolic acids | (a) Gallic acid |
| (b) Protocatechuic acid | |
| (c) p-Hydroxybenzoic acid | |
| (d) Vanillic acid | |
| (e) Caffeic acid | |
| (f) Syringic acid | |
| (g) p- and o-coumaric acid | |
| (h) Ferulic acid | |
| (i) Cinnamic acid | |
| Flavonoids | (a) Luteolin |
| (b) Apigenin | |
| Lignans | (a) (+) Pinoresinol |
| (b) (+) Acetoxypinoresinol | |
| Extra Virgin Olive Oil | |||||
|---|---|---|---|---|---|
| Type of Study | Reference | Year | Type of Intervention | Primary Outcome | p-Value for Primary Endpoint |
| In vitro cell models | Manna, C. [41] | 2002 | Evaluation of effects of phenolic fraction extract from EVOO on oxidative damage in human erythrocytes and Caco-2 cells | Protective effects of EVOO phenolic fractions | Linear relationship between antioxidant capacity of EVOO phenolic fraction and o-phenolic content. R2 = 0.999 |
| Beauchamp, G.K. [54] | 2005 | Evaluation of effects of oleocanthal as modulator of inflammation and analgesia | Oleocanthal caused dose-dependent inhibition of COX-1 and COX-2 activities. | - N.A. | |
| Carrasco- Pancorbo, A. [62] | 2005 | Electrochemical study on the resistance of oxidative deterioration of VOO correlated to the presence of phenolic compounds | Ability of compounds isolated from VOO by measuring the radical scavenging effect on 1,1-diphenyl-2-picrylhydrazyl radical | - N.A. | |
| Vuorela, S. [63] | 2005 | Phenolic extracts isolated from bioactive sources have been studied for their antioxidant, antimicrobial, anti-inflammatory, and antimutagenic properties. | Phenolic extracts from oils, induced a decrease of proinflammatory mediators (prostaglandin E2). All tested extracts were safe. In fact, they did not stimulate mutagenic nor toxic action on Caco-2 cells or macrophages. | - N.A. | |
| Dell’Agli, M. [34] | 2006 | Evaluation of HT and OleA form EVOO in HUVEC | Expression of
|
| |
| Franconi, F. [40] | 2006 | Whole virgin olive extracts studied to determine whether they maintain the antioxidant activity and whether this last is linked to MPC composition of a single virgin oil | Evaluation of oils derived from Taggiasca and Seggianese olive on human LDL |
| |
| Brunelleschi, S. [48] | 2007 | Evaluation of EVOO extracts rich in minor polar compounds (MPC-OOE) on human cells | NF-kB translocation in monocytes and monocyte-derived macrophages sampled from healthy subjects |
| |
| Menendez, J.A. [64] | 2008 | Evaluation of EVOO phenolic effects on the expression of FASN in human breast cancer epithelial cell lines. | EVOO phenols: lignans, flavonoids, and secoiridoids suppress FASN protein expression in HER2 gene amplificated SKBR3 breast cancer cells |
| |
| Fini, L. [65] | 2008 | Evaluation of anti-cancer effects of EVOO phenolic extracts in cells lines for two EVOOs. (1). EVOO (A) pinoresinol as main phenol (2). EVOO (B) oleocanthal as main phenol | EVOO (A) has powerful chemopreventive actions and upregulates the ATM-p53 cascade | EVOO (A) inhibits cell proliferation in a dependent manner. The comparison between effects of EVOO (A) and (B) demonstrates significant powerful effects of EVOO (A) respect to EVOO (B) p < 0.0001 | |
| Zambonin, L. [33] | 2012 | Evaluation of the antioxidant activity of phenolic acids in HEL cells | Proapoptotic effects in leukemia cells |
In HEL cells:
| |
| Incani, A. [38] | 2016 | Evaluation of two monovarietal EVOO phenolic extracts (Bosana and Nera) on Caco-2 cells | Modulation of enterocyte response to oxidative and inflammatory stimuli after absorption of EVOO |
| |
| Animal | Priora, R. [61] | 2008 | Randomized study in 6 groups for different treatments (10 rats x group). They tested 3 types of oil characterized by different MPC concentration: refined olive oil with trace MPC (control), low-MPC EVOO, and high-MPC EVOO | Effect of EVOO in relation to MPC on platelet aggregation and plasma concentration of Hcy redox form |
|
| Humans | Keys, A. [16] | 1986 | Study among 15 different cohorts (n = 11.579 healthy males) on mortality from all causes, follow-up period 15 years | All cause and coronary disease death during 15-year follow-up was significantly lower in cohorts with olive oil as main fat | - N.A. |
| De Lorgeril, M. [25] | 1994 | MD alpha-linolenic acid rich vs. prudent diet in secondary prevention of CHD patients | Secondary prevention of coronary events and deaths |
| |
| Visioli, F. [35] | 2000 | Six male volunteers 50 mL of olive oil samples accompanied by 40 g of bread, four times | Olive oil phenolics are dose-dependently absorbed in humans | - N.A. | |
| Riboli, E. [68] | 2002 | Multicenter prospective cohort study on 521.000 subjects investigation on the relationship between nutrition and cancer | Evaluation of the possible correlation between the incidence of cancer and nutrition |
| |
| Salvini, S. [42] | 2006 | Randomized Cross over trial 10 postmenopausal women about the effect of high-phenol EVOO vs. low-phenol EVOO on oxidative DNA damage | Two types of olive oil were assumed for 8 weeks (50 g/day) and were tested in peripheral blood lymphocytes |
| |
| Covas, M.I. [43] | 2006 | Evaluated, in 200 healthy male volunteers, the effects of polyphenol content in olive oil on oxidative lipid damage and plasma lipid levels | Crossover study, enrolled subjects assumed randomly 3 types of olive oils daily administration (25 mL/day). One type was low-phenols (2.7 mg/kg of olive oil), medium-phenols (164 mg/kg), or high-phenols (366 mg/kg) content. Intervention periods were 3 weeks. |
| |
| Masala, G. [72] | 2007 | Evaluation of dietary patterns on overall mortality in Italian elderly population (aged > 60 years) | “Olive oil and salad” type is inversely associated with all-cause mortality. While the pasta and meat pattern have an increased mortality for all causes. |
| |
| De Lorenzo, A. [22] | 2010 | IMD and IMOD vs. usual diet in patients with CKD stage II–III | Effect of diet treatment on laboratory and body composition parameters |
| |
| Bendinelli, B. [73] | 2011 | Association between fruit, vegetable, and olive oil consumption and the incidence of CHD in Italian women | 8-year follow-up in which the possible relationships between dietary habits, lifestyle, anthropometric measures, and the development of CHD major events were evaluated. |
H.R. 0.54 (95% CI 0.33–0.90, p = 0.03 Olive oil: H.R. 0.56 (95% CI 0.31–0.99, p = 0.04) | |
| Perez-Herrera, A. [44] | 2012 | Study randomized crossover of 20 obese subjects that received four breakfasts constituted by milk and muffin prepared with one of four different oils: virgin olive oil, sunflower oil, mixture seeds oil with added dimethylpolyxiloxane, or natural antioxidants from olive mill wasterwater alperujo | Evaluations of postprandial inflammatory status in 20 obese subjects by the activation of nuclear NF-kB, the cytoplasmatic concentration of NF-kB inhibitor, the mRNA levels of NF-kB subunits and activators, inflammatory molecules, and LPS levels |
| |
| Di Daniele, N. [23] | 2014 | IMD and IMOD in patients with CKD stage II–III vs. low-protein diet according to MTHFR genotypes | Effect of diet treatment on laboratory and body composition parameters |
| |
| Agrawal, K. [60] | 2017 | Double-blind, randomized controlled crossover study on 9 healthy subjects. They assumed 40 mL/week of tree different phenolic content EVOO. | Evaluation of EVOO assumption on inhibition of platelet aggregation pre and 2 h post-EVOO intake |
| |
| Estruch, R. [24] | 2018 | Mediterranean Diet supplements with EVOO or nuts vs. reduced-fat diet in 7447 Spanish subjects | Major CV events |
| |
| By-Products of EVOO Process | |||||
|---|---|---|---|---|---|
| Type of Study | Reference | Year | Type of Intervention | Primary Outcome | p-Value for Primary Endpoint |
| In vitro cell models | Obied, H.K. [88] | 2007 | Olive mill waste waters tested against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, Aspergillus niger. | Antibacterial activity against S. aureus, B. subtilis, E. coli, and P. aeruginosa | At lower concentrations, the extracts exhibited differential antibacterial action, but at 5 mg/disc extracts were active against all the challenge bacteria |
| Schaffer, S. [87] | 2010 | OMWW extracts and HT were evaluated for their cytoprotective effects in an in vitro model of neuronal-like PC12 cells | Cytoprotective effects in PC12 cells subjected to oxidative or nitrosative stress by adding either ferrous iron or sodium nitroprusside to the cell culture medium for 18 h | Incubating PC12 cells with wastewater extract protect from nitrosative stress. The extract was able to maintain ATP levels but not MMP. | |
| Bernini, R. [93] | 2017 | Lipophilic fractions from Olea by-products were tested on human colon cancer cell line HCT8-β8 engineered to overexpress estrogen receptor β (ERβ) | Antiproliferative effect | HT and lipophilic fractions significantly reduced the proliferation of HCT8-β8-expressing cells in a concentration-dependent manner. HT oleate showed the greater effect. | |
| Plastina, P. [95] | 2019 | Phenolic extracts from OMWW were tested for their ability to reduce NO production by LPS-stimulated RAW-264.7 macrophages | Anti-inflammatory activity | HT stearate and HT oleate decrease NO production in a concentration-dependent manner | |
| Humans | Visioli, F. [96] | 2009 | OMWW extracts were tested on human volunteers 1 h after ingestion | Plasma antioxidant capacity and total reduced glutathione | No difference in plasma antioxidant capacity; a significant increase in total plasma glutathione concentration |
| Olive Leaf Extracts | |||||
|---|---|---|---|---|---|
| Type of Study | Reference | Year | Type of Intervention | Primary Outcome | p-Value for Primary Endpoint |
| In vitro cell models | Andrikopoulos, N.K. [100] | 2002 | Effects against copper ion-induced low-density lipoprotein (LDL) oxidation | LDL mean protection activity | Quercetin, luteolin, and rutin, activities 46.8%, 49.5%, and 53.7% MP, respectively, comparable to oleuropein the 49.0% MP |
| Sudjana, A.N. [107] | 2009 | Antimicrobical activity | Role in regulating the composition of the gastric flora | Specific activity, in reducing levels of H. pylori and C. jejuni. | |
| Rigacci, S. [109,110] | 2010 2011 | Effects on amylin and peptide aggregation and cytotoxicity | Hindering amylin and Aβ-peptide aggregation, preventing their cytotoxicity | Increased viability of β-pancreatic and neuroblastoma cells decreasing caspase-3 activity | |
| Rigacci, S. [111] | 2015 | Neuroprotection effect | Autophagy induction both in vitro in neuronal cells and in in vivo Aβ model deposition (TgCRND8 mice) by Ca2+/CaMKKβ/AMPK/mTOR axis | Cytosolic Ca2+ increase activates CaMKKβ and pAMPK concomitant with increased beclin1/LC3II and decreased phospho-mTOR and phospho-p70S6K expression | |
| Papachristodoulou, A. [105] | 2016 | Anticancer effect and adjuvant to antitumoral therapies | Lowering of the cytotoxic dose in doxorubicin to obtain the same antiproliferative effect in prostate cancer | Remarkable induction of autophagy correlated to significant metabolite alterations | |
| Luccarini, I. [112] | 2016 | Neuroprotection effect | Counteracting neuronal damage through modulation of the PARP1–SIRT1 interplay both in neuronal cells and in TgCRND8 mice | In vitro reduction of PARP1 activation and paralleled overexpression of Sirtuin1. In vivo, (in addition to above reported effects), a decrease of NF-kB and of the pro-apoptotic marker p53 expression | |
| Miceli, C. [108] | 2018 | Cardioprotective effect | Cardioprotection on MAO-A overexpressed cardiomyocytes by restoring the defective autophagic flux due to oxidative stress | Reduction of MAO-induced cardiotoxicity through MTT. Autophagy induction by TFEB nuclear translocation. | |
| Ruzzolini, J. [118] | 2018 | Anticancer effect and adjuvant to antitumoral therapies | Reduction of viability of BRAF melanoma cells. Enhanced effects with chemotherapic drugs (dacarbazina and everolimus) at no toxic dose. | High dose induced cell death by apoptosis, while no toxic dose affected viability through the inhibition of phosphorylation of AKT and the S6 pathway | |
| Margheri, F.M.B. [114] | 2019 | Effect on tumor microenvironment | Anti-angiogenic activity in senescence-associated-secretory-phenotype (SASP) fibroblast cultured media | Decrease of pro-angiogenic factors release in SASP fibroblasts cultured media and inhibition of cell-dependent invasion and of capillary-like structure formation of endothelial cells exposed to the above media | |
| Animals | Jemai, H. [102] | 2009 | Effects in alloxan-diabetic rats | Hypoglycemic and antioxidant activity | |
| Andreadou, I. [119] | 2014 | Effect on chronic doxorubicin induced cardiomyopathy | Prevention of the structural, functional, and histopathological cardiac effects | Activation of AMPK and suppression of iNOS. Reduction of pro-apoptotic mediators and modulation of myocardial metabolism. | |
| Rigacci, S. [111] | 2015 | See above | |||
| Luccarini, I. [112] | 2016 | See above | |||
| Janahmadi, Z. [101] | 2017 | Cardioprotection in rats with heart failure | Antioxidative and anti-inflammatory effects | Increase of SV, EF, FS, and CO (p < 0.05), serum SOD and GRx. Reduction of serum MDA, IL-1β or TNF-α (p < 0.05). | |
| Humans | De Bock, M. [104] | 2013 | 46 Participants (aged 46.465.5 years and BMI 28.062.0 kg/m2) were randomized to receive capsules with olive leaf extract (OLE) or placebo for 12 weeks | Improvement in insulin sensitivity and β-pancreatic cell secretory capacity | Insulin sensitivity (p = 0.024). β-pancreatic cell responsiveness (p = 0.013). |
| Carnevale, R. [103] | 2018 | Twenty healthy subjects were randomized to receive 20 mg oleuropein or 20 mg placebo before lunch | Improvement in postprandial glycemic profile | Lower blood glucose, DPP-4 activity, and higher insulin and glucagon-like peptide-1 vs. placebo | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. https://doi.org/10.3390/nu11081776
Romani A, Ieri F, Urciuoli S, Noce A, Marrone G, Nediani C, Bernini R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients. 2019; 11(8):1776. https://doi.org/10.3390/nu11081776
Chicago/Turabian StyleRomani, Annalisa, Francesca Ieri, Silvia Urciuoli, Annalisa Noce, Giulia Marrone, Chiara Nediani, and Roberta Bernini. 2019. "Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L." Nutrients 11, no. 8: 1776. https://doi.org/10.3390/nu11081776
APA StyleRomani, A., Ieri, F., Urciuoli, S., Noce, A., Marrone, G., Nediani, C., & Bernini, R. (2019). Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients, 11(8), 1776. https://doi.org/10.3390/nu11081776