Therapeutic Potential of β-Caryophyllene: A Dietary Cannabinoid in Diabetes and Associated Complications
Abstract
:1. Introduction
2. Effects and Possible Mechanisms of BCP on Diabetes
2.1. Effect on Pancreatic Islet β-Cells and Insulin Secretion
2.1.1. In Vitro Studies Showing Effects of β-Caryophyllene
2.1.2. In Vivo Studies Showing Antihyperglycemic Effects of β-Caryophyllene
2.2. Effect on Insulin Resistance, Dyslipidemia, and Obesity
2.3. Enhancement of Glucose Uptake in Tissues and Organs
2.4. Inhibition of α-Glucosidase Activity
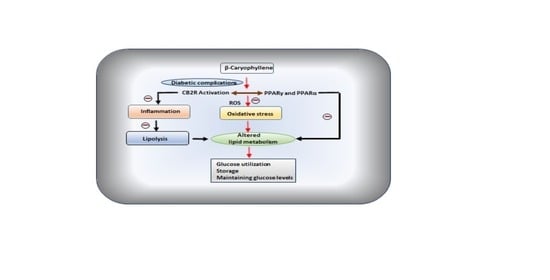
3. β-Caryophyllene in Diabetic Complications
4. β-Caryophyllene in Nonalcoholic Fatty Liver Disease (NAFLD)
5. Safety and Toxicity of β-Caryophyllene
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Diabetes Federation. One Adult in Ten Will Have Diabetes by 2030; International Diabetes Federation: Brussels, Belgium, 2014; Available online: http://www.idf.org/media-events/press-releases/2011/diabetes-atlas-5th-edition (accessed on 25 May 2020).
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Mohammadi, M.T.; Rezaee, R.; Sahebkar, A. Crocin improves renal function by declining Nox-4, IL-18, and p53 expression levels in an experimental model of diabetic nephropathy. J. Cell. Biochem. 2018, 119, 6080–6093. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef] [Green Version]
- Thrasher, J. Pharmacologic Management of Type 2 Diabetes Mellitus: Available Therapies. Am. J. Cardiol. 2017, 120, S4–S16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qaseem, A.; Barry, M.J.; Humphrey, L.L.; Forciea, M.A. Oral Pharmacologic Treatment of Type 2 Diabetes Mellitus: A Clinical Practice Guideline Update From the American College of Physicians. Ann. Intern. Med. 2017, 166, 279–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, B.; Caerels, S.; Mathieu, C. SGLT inhibitors in type 1 diabetes: Weighing efficacy and side effects. Ther. Adv. Endocrinol. Metab. 2020, 11. [Google Scholar] [CrossRef]
- Wu, L.; Gunton, J.E. The Changing Landscape of Pharmacotherapy for Diabetes Mellitus: A Review of Cardiovascular Outcomes. Int. J. Mol. Sci. 2019, 20, 5853. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabet. 2014, 5, 267–281. [Google Scholar] [CrossRef]
- Sikand, G.; Kris-Etherton, P.; Boulos, N.M. Impact of Functional Foods on Prevention of Cardiovascular Disease and Diabetes. Curr. Cardiol. Rep. 2015, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review. Int. J. Mol. Sci. 2019, 21, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assefa, S.T.; Yang, E.Y.; Chae, S.Y.; Song, M.; Lee, J.; Cho, M.C.; Jang, S. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Alkhalidy, H.; Wang, Y.; Liu, D. Dietary Flavonoids in the Prevention of T2D: An Overview. Nutrients 2018, 10, 438. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Sánchez, K.; Garay-Jaramillo, E.; González-Reyes, R.E. Effects of Moringa oleifera on Glycaemia and Insulin Levels: A Review of Animal and Human Studies. Nutrients 2019, 11, 2907. [Google Scholar] [CrossRef] [Green Version]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef] [Green Version]
- Craig, W.J. Phytochemicals: Guardians of our health. J. Am. Diet. Assoc. 1997, 97, S199–S204. [Google Scholar] [CrossRef]
- Sales, D.S.; Carmona, F.; de Azevedo, B.C.; Taleb-Contini, S.H.; Bartolomeu, A.C.; Honorato, F.B.; Martinez, E.Z.; Pereira, A.M. Eugenia punicifolia (Kunth) DC. as an adjuvant treatment for type-2 diabetes mellitus: A non-controlled, pilot study. Phytother. Res. PTR 2014, 28, 1816–1821. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Kamal, M.A.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef] [PubMed]
- Horváth, B.; Mukhopadhyay, P.; Haskó, G.; Pacher, P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am. J. Pathol. 2012, 180, 432–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruden, G.; Barutta, F.; Kunos, G.; Pacher, P. Role of the endocannabinoid system in diabetes and diabetic complications. Br. J. Pharmacol. 2016, 173, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, V.S.; Kaur, G. Therapeutic potential of cannabinoid receptor 2 in the treatment of diabetes mellitus and its complications. Eur. J. Pharmacol. 2019, 862, 172628. [Google Scholar] [CrossRef]
- Veilleux, A.; Di Marzo, V.; Silvestri, C. The Expanded Endocannabinoid System/Endocannabinoidome as a Potential Target for Treating Diabetes Mellitus. Curr. Diabet. Rep. 2019, 19, 117. [Google Scholar] [CrossRef]
- Pauline, S.S.; Thaisa, C.O.; Luís Mário, R.J.; Ana, F.; Lívio, C.C.N. Caryophyllene Delivery Systems: Enhancing the Oral Pharmacokinetic and Stability. Curr. Pharm. Des. 2018, 24, 3440–3453. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. beta-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem.-Biol. Interact. 2016, 245, 50–58. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta 2014, 1840, 2709–2729. [Google Scholar] [CrossRef]
- Horváth, B.; Magid, L.; Mukhopadhyay, P.; Bátkai, S.; Rajesh, M.; Park, O.; Tanchian, G.; Gao, R.Y.; Goodfellow, C.E.; Glass, M.; et al. A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br. J. Pharmacol. 2012, 165, 2462–2478. [Google Scholar] [CrossRef] [Green Version]
- Suijun, W.; Zhen, Y.; Ying, G.; Yanfang, W. A role for trans-caryophyllene in the moderation of insulin secretion. Biochem. Biophys. Res. Commun. 2014, 444, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, A. Small G proteins in islet beta-cell function. Endocr. Rev. 2010, 31, 52–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaram, B.; Syed, I.; Kyathanahalli, C.N.; Rhodes, C.J.; Kowluru, A. Arf nucleotide binding site opener [ARNO] promotes sequential activation of Arf6, Cdc42 and Rac1 and insulin secretion in INS 832/13 β-cells and rat islets. Biochem. Pharmacol. 2011, 81, 1016–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.-Y.; Lai, X.-N.; Qian, X.-L.; Lv, L.-C.; Li, J.; Duan, J.; Xiao, X.-H.; Xiong, L.-X. Cdc42: A Novel Regulator of Insulin Secretion and Diabetes-Associated Diseases. Int. J. Mol. Sci. 2019, 20, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozaki, S.; Ueda, S.; Takenaka, N.; Kataoka, T.; Satoh, T. Role of RalA downstream of Rac1 in insulin-dependent glucose uptake in muscle cells. Cell. Signal. 2012, 24, 2111–2117. [Google Scholar] [CrossRef]
- Kumawat, V.; Kaur, G. Insulinotropic and antidiabetic effects of β-caryophyllene with l -arginine in type 2 diabetic rats. J. Food Biochem. 2020. [Google Scholar] [CrossRef]
- Kaur, G.; Tharappel, L.; Kumawat, V. Evaluation of Safety and in vitro Mechanisms of Anti-diabetic Activity of β-caryophyllene and L-arginine. J. Biol. Sci. 2018, 18, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Geddo, F.; Scandiffio, R.; Antoniotti, S.; Cottone, E.; Querio, G.; Maffei, M.E.; Bovolin, P.; Gallo, M.P. PipeNig((R))-FL, a Fluid Extract of Black Pepper (Piper Nigrum L.) with a High Standardized Content of Trans-beta-Caryophyllene, Reduces Lipid Accumulation in 3T3-L1 Preadipocytes and Improves Glucose Uptake in C2C12 Myotubes. Nutrients 2019, 11, 2788. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Sun, T.; Wang, X. Activation of type 2 cannabinoid receptors (CB2R) promotes fatty acid oxidation through the SIRT1/PGC-1alpha pathway. Biochem. Biophys. Res. Commun. 2013, 436, 377–381. [Google Scholar] [CrossRef]
- Wu, C.; Jia, Y.; Lee, J.H.; Jun, H.J.; Lee, H.S.; Hwang, K.Y.; Lee, S.J. trans-Caryophyllene is a natural agonistic ligand for peroxisome proliferator-activated receptor-alpha. Bioorg. Med. Chem. Lett. 2014, 24, 3168–3174. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. beta-Caryophyllene, a natural sesquiterpene, modulates carbohydrate metabolism in streptozotocin-induced diabetic rats. Acta Histochem. 2014, 116, 1469–1479. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Arulselvan, P.; Muniappan, B.P.; Kandasamy, M. Effect of mangiferin isolated from Salacia chinensis regulates the kidney carbohydrate metabolism in streptozotocin–induced diabetic rats. Asian Pac. J. Trop. BioMed. 2012, 2, S1583–S1587. [Google Scholar] [CrossRef]
- Matschinsky, F.M. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discov. 2009, 8, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Baltrusch, S.; Schmitt, H.; Brix, A.; Langer, S.; Lenzen, S. Additive activation of glucokinase by the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and the chemical activator LY2121260. Biochem. Pharmacol. 2012, 83, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Basha, R.H.; Sankaranarayanan, C. Protective role of β-caryophyllene, a sesquiterpene lactone on plasma and tissue glycoprotein components in streptozotocin-induced hyperglycemic rats. J. Acute Med. 2015, 5, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Duraisamy, G.; Ganesan, R.; Manokaran, K.; Kanakasabapathi, D.; Chandrasekar, U. Protective effect of the whole plant extract of Evolvulus alsinoides on glycoprotein alterations in streptozotocin induced diabetic rats. J. Acute Dis. 2013, 2, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Senthilkumar, G.; Subramanian, S. Biochemical studies on the effect of Terminalia chebula on the levels of glycoproteins in streptozotocin-induced experimental diabetes in rats. J. Appl. BioMed. 2008, 6. [Google Scholar] [CrossRef] [Green Version]
- Zaman Huri, H.; Permalu, V.; Kasim, N.B. Management of Severe/Acute Hyperglycemia in Hospitalised Type 2 Diabetes Mellitus Patients. J. Endocrinol. Diabet. Mellit. 2013. [Google Scholar] [CrossRef]
- Pasupathi, P.; Chandrasekar, V.; Kumar, U. Evaluation of oxidative stress, enzymatic and non-enzymatic antioxidants and metabolic thyroid hormone status in patients with diabetes mellitus. Diabet. Metab. Syndr. Clin. Res. Rev. 2009, 3, 160–165. [Google Scholar] [CrossRef]
- Sirdah, M.M. Protective and therapeutic effectiveness of taurine in diabetes mellitus: A rationale for antioxidant supplementation. Diabet. Metab. Syndr. 2015, 9, 55–64. [Google Scholar] [CrossRef]
- Berg, A.H.; Combs, T.P.; Du, X.; Brownlee, M.; Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001, 7, 947–953. [Google Scholar] [CrossRef]
- Li, C.; Bowe, J.E.; Huang, G.C.; Amiel, S.A.; Jones, P.M.; Persaud, S.J. Cannabinoid receptor agonists and antagonists stimulate insulin secretion from isolated human islets of Langerhans. Diabet. Obes. Metab. 2011, 13, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; Ponery, A.; El-Sharkawy, T.; Parvez, H. L-arginine stimulates insulin secretion from the pancreas of normal and diabetic rats. Amino Acids 2001, 21, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.O.; Santos, I.V.F.d.; Rocha, C.F.d.; Barros, A.S.A.; Faria e Souza, B.S.; Ferreira, I.M.; Bezerra, R.M.; Lima, C.S.; Castro, A.N.; Carvalho, J.C.T. Effect of the treatment of Copaifera duckei oleoresin (copaiba) in streptozotocin-induced diabetic rats. Rev. Brasil. Farmacogn. 2018, 28, 724–731. [Google Scholar] [CrossRef]
- Madagi, S.; Deshmukh, D.S. Identification of Potential Anti-Chronic Disease Targets for Cinnamomum Tamala: An In-Silico Target Screening Approach. Trans Sabinene Hydrate 2014, 113, 6. [Google Scholar] [CrossRef]
- Kumar, S.; Vasudeva, N.; Sharma, S. Pharmacological and pharmacognostical aspects of Cinnamomum tamala Nees & Eberm. J. Pharm. Res. 2012, 5, 580–584. [Google Scholar]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of CB2 and PPAR-gamma receptors. Chem. Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene alleviates diet-induced neurobehavioral changes in rats: The role of CB2 and PPAR-gamma receptors. BioMed. Pharmacother. 2019, 110, 145–154. [Google Scholar] [CrossRef]
- Uddin, N.; Hasan, M.R.; Hossain, M.M.; Sarker, A.; Hasan, A.H.M.N.; Islam, A.F.M.M.; Chowdhury, M.M.H.; Rana, M.S. In vitro α–amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera Montr. fruit. Asian Pac. J. Trop. Biomed. 2014, 4, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Boden, G.; Shulman, G.I. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Investig. 2002, 32 (Suppl. S3), 14–23. [Google Scholar] [CrossRef]
- Pyper, S.R.; Viswakarma, N.; Yu, S.; Reddy, J.K. PPARalpha: Energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recept. Signal. 2010, 8, e002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Gerhart-Hines, Z.; Dominy, J.E., Jr.; Blattler, S.M.; Jedrychowski, M.P.; Banks, A.S.; Lim, J.H.; Chim, H.; Gygi, S.P.; Puigserver, P. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD+. Mol. Cell 2011, 44, 851–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ament, Z.; West, J.A.; Stanley, E.; Ashmore, T.; Roberts, L.D.; Wright, J.; Nicholls, A.W.; Griffin, J.L. PPAR-pan activation induces hepatic oxidative stress and lipidomic remodelling. Free Radic. Biol. Med. 2016, 95, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Kota, B.P.; Huang, T.H.; Roufogalis, B.D. An overview on biological mechanisms of PPARs. Pharmacol. Res. 2005, 51, 85–94. [Google Scholar] [CrossRef]
- D’Aniello, E.; Fellous, T.; Iannotti, F.A.; Gentile, A.; Allarà, M.; Balestrieri, F.; Gray, R.; Amodeo, P.; Vitale, R.M.; Di Marzo, V. Identification and characterization of phytocannabinoids as novel dual PPARα/γ agonists by a computational and in vitro experimental approach. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 586–597. [Google Scholar] [CrossRef]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules 2019, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, S.E.; Kendall, D.A. Cannabinoid activation of peroxisome proliferator-activated receptors: Potential for modulation of inflammatory disease. Immunobiology 2010, 215, 611–616. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, Z.; Liu, S. β-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARγ pathway. Pharmacology 2014, 94, 1–12. [Google Scholar] [CrossRef]
- Seber, S.; Ucak, S.; Basat, O.; Altuntas, Y. The effect of dual PPAR α/γ stimulation with combination of rosiglitazone and fenofibrate on metabolic parameters in type 2 diabetic patients. Diabet. Res. Clin. Prac. 2006, 71, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo-Solleiro, D.; Roldan-Roldan, G.; Diaz, D.; Velasco, M.; Larque, C.; Rico-Rosillo, G.; Vega-Robledo, G.B.; Zambrano, E.; Hiriart, M.; Perez de la Mora, M. Increased anxiety-like behavior is associated with the metabolic syndrome in non-stressed rats. PLoS ONE 2017, 12, e0176554. [Google Scholar] [CrossRef] [Green Version]
- Ashrafian, H.; Harling, L.; Darzi, A.; Athanasiou, T. Neurodegenerative disease and obesity: What is the role of weight loss and bariatric interventions? Metab. Brain Dis. 2013, 28, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyileten, C.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D.; Malek, L.; Postula, M. Antidiabetic Effect of Brain-Derived Neurotrophic Factor and Its Association with Inflammation in Type 2 Diabetes Mellitus. J. Diabet. Res. 2017, 2017, 2823671. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; Sharma, V.; McNeill, J.H. Vanadium increases GLUT4 in diabetic rat skeletal muscle. Mol. Cell. Biochem. 2002, 233, 139–143. [Google Scholar] [CrossRef]
- Fernandes, R.; Carvalho, A.L.; Kumagai, A.; Seica, R.; Hosoya, K.; Terasaki, T.; Murta, J.; Pereira, P.; Faro, C. Downregulation of retinal GLUT1 in diabetes by ubiquitinylation. Mol. Vis. 2004, 10, 618–628. [Google Scholar]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Færch, K.; Vistisen, D.; Pacini, G.; Torekov, S.; Johansen, N.; Witte, D.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; et al. Insulin Resistance Is Accompanied by Increased Fasting Glucagon and Delayed Glucagon Suppression in Individuals With Normal and Impaired Glucose Regulation. Diabetes 2016, 65, db160240. [Google Scholar] [CrossRef] [Green Version]
- Khonsary, S.A. Guyton and Hall: Textbook of Medical Physiology. Surg. Neurol. Int. 2017, 8, 275. [Google Scholar] [CrossRef]
- Kiselyov, V.; Versteyhe, S.; Gauguin, L.; de Meyts, P. Harmonic oscillator model of the insulin and IGF1 receptors’ allosteric binding and activation. Mol. Syst. Biol. 2009, 5, 243. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.K.; Sriram, G.; Dipple, K.M. Insulin sensitivity predictions in individuals with obesity and type II diabetes mellitus using mathematical model of the insulin signal transduction pathway. Mol. Gen. Metab. 2016, 119, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.-H.; Ka, E.H.; Lee, H.S.; Apostolidis, E.; Jang, H.D.; Kwon, Y.I. Comparison of Antioxidant Potential and Rat intestinal a-Glucosidases inhibitory Activities of Quercetin, Rutin, and Isoquercetin. Int. J. Appl. Res. Nat. Prod. 2009, 2, 52–60. [Google Scholar]
- Soltesova-Prnova, M.; Milackova, I.; Stefek, M. 3′-O-(3-Chloropivaloyl)quercetin, α-glucosidase inhibitor with multi-targeted therapeutic potential in relation to diabetic complications. Chem. Pap. 2016, 70, 1439–1444. [Google Scholar] [CrossRef]
- Chiasson, J.L.; Josse, R.G.; Hunt, J.A.; Palmason, C.; Rodger, N.W.; Ross, S.A.; Ryan, E.A.; Tan, M.H.; Wolever, T.M. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann. Intern. Med. 1994, 121, 928–935. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Olasehinde, T.A.; Oboh, G. Essential Oil Composition, Antioxidant, Antidiabetic and Antihypertensive Properties of Two Afromomum Species. J. Oleo Sci. 2017, 66, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Sarikurkcu, C.; Uren, M.C.; Kocak, M.S.; Cengiz, M.; Tepe, B. Chemical composition, antioxidant, and enzyme inhibitory activities of the essential oils of three Phlomis species as well as their fatty acid compositions. Food Sci. Biotechnol. 2016, 25, 687–693. [Google Scholar] [CrossRef]
- Mosbah, H.; Chahdoura, H.; Kammoun, J.; Hlila, M.B.; Louati, H.; Hammami, S.; Flamini, G.; Achour, L.; Selmi, B. Rhaponticum acaule (L.) DC essential oil: Chemical composition, in vitro antioxidant and enzyme inhibition properties. BMC Complement. Altern. Med. 2018, 18, 79. [Google Scholar] [CrossRef] [Green Version]
- Jelassi, A.; Hassine, M.; Besbes Hlila, M.; Ben Jannet, H. Chemical Composition, Antioxidant Properties, α-Glucosidase Inhibitory, and Antimicrobial Activity of Essential Oils from Acacia mollissima and Acacia cyclops Cultivated in Tunisia. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef]
- Jani, N.A.; Sirat, H.M.; Ahmad, F.; Mohamad Ali, N.A.; Jamil, M. Chemical profiling and biological properties of Neolitsea kedahense Gamble essential oils. Nat. Prod. Res. 2017, 31, 2793–2796. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, B.O.; Ojo, O.A.; Fatoba, B.; Afolabi, O.B.; Olayide, I.; Okesola, M.A.; Oyinloye, B.E. In vitro antioxidant and enzyme inhibitory properties of the n-butanol fraction of Senna podocarpa (Guill. and Perr.) leaf. J. Basic Clin. Physiol. Pharmacol. 2019, 31. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.-W.; Huang, C.-D.; Zheng, H.-H.; Zhao, N.; Feng, X.-L.; Ma, S.-J.; Zhang, A.-L.; Zhang, Q. Meroterpene-Like α-Glucosidase Inhibitors Based on Biomimetic Reactions Starting from β-Caryophyllene. Molecules 2020, 25, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Tang, H.Y.; Chen, M.; Yu, J.; Li, H.; Gao, J.M. Natural product driven diversity via skeletal remodeling of caryophyllene β-lactam. Org. Biomol. Chem. 2017, 15, 4456–4463. [Google Scholar] [CrossRef]
- Ma, S.J.; Yu, J.; Yan, D.W.; Wang, D.C.; Gao, J.M.; Zhang, Q. Meroterpene-like compounds derived from β-caryophyllene as potent α-glucosidase inhibitors. Org. Biomol. Chem. 2018, 16, 9454–9460. [Google Scholar] [CrossRef]
- Duarte, A.M.; Guarino, M.P.; Barroso, S.; Gil, M.M. Phytopharmacological Strategies in the Management of Type 2 Diabetes Mellitus. Foods 2020, 9, 271. [Google Scholar] [CrossRef] [Green Version]
- Badalamenti, N.; Ilardi, V.; Rosselli, S.; Bruno, M.; Maggi, F.; Leporini, M.; Falco, T.; Loizzo, M.R.; Tundis, R. Ferulago nodosa Subsp. geniculata (Guss.) Troia & Raimondo from Sicily (Italy): Isolation of Essential Oil and Evaluation of Its Bioactivity. Molecules 2020, 25, 3249. [Google Scholar] [CrossRef]
- Marinas, I.C.; Oprea, E.; Chifiriuc, M.C.; Badea, I.A.; Buleandra, M.; Lazar, V. Chemical Composition and Antipathogenic Activity of Artemisia annua Essential Oil from Romania. Chem. Biodivers. 2015, 12, 1554–1564. [Google Scholar] [CrossRef]
- Singh, P.; Jayaramaiah, R.H.; Sarate, P.; Thulasiram, H.V.; Kulkarni, M.J.; Giri, A.P. Insecticidal potential of defense metabolites from Ocimum kilimandscharicum against Helicoverpa armigera. PLoS ONE 2014, 9, e104377. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Gu, D.; Li, H.; Wang, Q.; Kang, J.; Chu, T.; Guo, H.; Yang, Y.; Tian, J. Rapid prediction and identification of lipase inhibitors in volatile oil from Pinus massoniana L. needles. Phytochemistry 2017, 141, 114–120. [Google Scholar] [CrossRef]
- Shim, H.I.; Song, D.J.; Shin, C.M.; Yoon, H.; Park, Y.S.; Kim, N.; Lee, D.H. Inhibitory Effects of β-caryophyllene on Helicobacter pylori Infection: A Randomized Double-blind, Placebo-controlled Study. Korean J. Gastroenterol. Taehan Sohwagi Hakhoe Chi 2019, 74, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Jiang, C.S.; Gao, L.X.; Gong, J.X.; Wang, Z.H.; Li, J.Y.; Li, J.; Li, X.W.; Guo, Y.W. Design, synthesis and in vitro activity of phidianidine B derivatives as novel PTP1B inhibitors with specific selectivity. Bioorg. Med. Chem. Lett. 2016, 26, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Arellano, E.; Pérez-Vásquez, A.; Rivero-Cruz, I.; Torres-Colin, R.; González-Andrade, M.; Rangel-Grimaldo, M.; Mata, R. Flavonoids and Terpenoids with PTP-1B Inhibitory Properties from the Infusion of Salvia amarissima Ortega. Molecules 2020, 25, 3530. [Google Scholar] [CrossRef] [PubMed]
- Saifudin, A.; Tanaka, K.; Kadota, S.; Tezuka, Y. Chemical constituents of Blumea balsamifera of Indonesia and their protein tyrosine phosphatase 1B inhibitory activity. Nat. Prod. Commun. 2012, 7, 815–818. [Google Scholar] [CrossRef] [Green Version]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. An Off. J. Pol. Physiol. Soc. 2019, 70. [Google Scholar] [CrossRef]
- Martinez, L.C.; Sherling, D.; Holley, A. The Screening and Prevention of Diabetes Mellitus. Primary Care 2019, 46, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.I.; Hong, J.M.; Choi, J.W.; Choi, H.S.; Hwan Kwak, J.; Lee, D.U.; Kook Lee, S.; Lee, S.M. β-Caryophyllene alleviates D-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. Eur. J. Pharmacol. 2015, 764, 613–621. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, Z.; Wang, B.; Guo, S. Trans-caryophyllene inhibits amyloid β (Aβ) oligomer-induced neuroinflammation in BV-2 microglial cells. Int. Immunopharmacol. 2017, 51, 91–98. [Google Scholar] [CrossRef]
- Yang, M.; Lv, Y.; Tian, X.; Lou, J.; An, R.; Zhang, Q.; Li, M.; Xu, L.; Dong, Z. Neuroprotective Effect of β-Caryophyllene on Cerebral Ischemia-Reperfusion Injury via Regulation of Necroptotic Neuronal Death and Inflammation: In Vivo and in Vitro. Front. Neurosci. 2017, 11, 583. [Google Scholar] [CrossRef]
- Galer, B.; Gianas, A.; Jensen, M. Painful diabetic polyneuropathy: Epidemiology, pain description, and quality of life. Diabet. Res. Clin. Pract. 2000, 47, 123–128. [Google Scholar] [CrossRef]
- Niemi, J.P.; Filous, A.R.; DeFrancesco, A.; Lindborg, J.A.; Malhotra, N.A.; Wilson, G.N.; Zhou, B.; Crish, S.D.; Zigmond, R.E. Injury-induced gp130 cytokine signaling in peripheral ganglia is reduced in diabetes mellitus. Exp. Neurol. 2017, 296, 1–15. [Google Scholar] [CrossRef]
- Lees, J.G.; Fivelman, B.; Duffy, S.S.; Makker, P.G.; Perera, C.J.; Moalem-Taylor, G. Cytokines in Neuropathic Pain and Associated Depression. Mod. Trends Pharmacopsychiatr. 2015, 30, 51–66. [Google Scholar] [CrossRef]
- Aguilar-Ávila, D.; Flores Soto, M.; Tapia-Vázquez, C.; Pastor-Zarandona, O.; Rocio Ivette, L.-R.; Viveros Paredes, J.M. β -Caryophyllene, a Natural Sesquiterpene, Attenuates Neuropathic Pain and Depressive-Like Behavior in Experimental Diabetic Mice. J. Med. Food 2019, 22. [Google Scholar] [CrossRef] [PubMed]
- Semprini, R.; Martorana, A.; Ragonese, M.; Motta, C. Observational clinical and nerve conduction study on effects of a nutraceutical combination on painful diabetic distal symmetric sensory-motor neuropathy in patients with diabetes type 1 and type 2. Minerva Med. 2018, 109, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, D.; Chen, Y.; Yang, M. β-Caryophyllene inhibits high glucose-induced oxidative stress, inflammation and extracellular matrix accumulation in mesangial cells. Int. Immunopharmacol. 2020, 84, 106556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhan, M.L.; Tang, Y.; Xiao, M.; Li, M.; Li, Q.S.; Yang, L.; Li, X.; Chen, W.W.; Wang, Y.L. Effects of β-caryophyllene on arginine ADP-ribosyltransferase 1-mediated regulation of glycolysis in colorectal cancer under high-glucose conditions. Int. J. Oncol. 2018, 53, 1613–1624. [Google Scholar] [CrossRef] [Green Version]
- Pant, A.; Saikia, S.K.; Shukla, V.; Asthana, J.; Akhoon, B.A.; Pandey, R. Beta-caryophyllene modulates expression of stress response genes and mediates longevity in Caenorhabditis elegans. Exp. Gerontol. 2014, 57, 81–95. [Google Scholar] [CrossRef]
- Lindsey, L.P.; Daphney, C.M.; Oppong-Damoah, A.; Uchakin, P.N.; Abney, S.E.; Uchakina, O.N.; Khusial, R.D.; Akil, A.; Murnane, K.S. The cannabinoid receptor 2 agonist, β-caryophyllene, improves working memory and reduces circulating levels of specific proinflammatory cytokines in aged male mice. Behav. Brain Res. 2019, 372, 112012. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Pitija, K.; Rujjanawate, C.; Pojanagaroon, S.; Kittakoop, P.; Wongpornchai, S. Adaptogenic-active components from Kaempferia parviflora rhizomes. Food Chem. 2012, 132, 1150–1155. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Levy, R.M. β-Caryophyllene promotes osteoblastic mineralization, and suppresses osteoclastogenesis and adipogenesis in mouse bone marrow cultures in vitro. Exp. Ther. Med. 2016, 12, 3602–3606. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Kim, E.J.; Kwon, G.T.; Jung, Y.J.; Park, T.; Kim, Y.; Yu, R.; Choi, M.-S.; Chun, H.S.; Kwon, S.-H.; et al. β-Caryophyllene potently inhibits solid tumor growth and lymph node metastasis of B16F10 melanoma cells in high-fat diet–induced obese C57BL/6N mice. Carcinogenesis 2015, 36, 1028–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangwal, S.; Stratmann, B.; Bang, C.; Lorenzen, J.M.; Kumarswamy, R.; Fiedler, J.; Falk, C.S.; Scholz, C.J.; Thum, T.; Tschoepe, D. Impairment of Wound Healing in Patients With Type 2 Diabetes Mellitus Influences Circulating MicroRNA Patterns via Inflammatory Cytokines. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1480–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, S.; Purk, A.; Kaur, M.; Soini, H.A.; Novotny, M.V.; Davis, K.; Kao, C.C.; Matsunami, H.; Mescher, A. Beta-caryophyllene enhances wound healing through multiple routes. PLoS ONE 2019, 14, e0216104. [Google Scholar] [CrossRef] [Green Version]
- Yoon, M.S.; Won, K.J.; Kim, D.Y.; Hwang, D.I.; Yoon, S.W.; Kim, B.; Lee, H.M. Skin regeneration effect and chemical composition of essential oil from Artemisia Montana. Nat. Prod. Commun. 2014, 9, 1619–1622. [Google Scholar] [CrossRef] [Green Version]
- Parisotto-Peterle, J.; Bidone, J.; Lucca, L.G.; Araújo, G.M.S.; Falkembach, M.C.; da Silva Marques, M.; Horn, A.P.; Dos Santos, M.K.; da Veiga, V.F., Jr.; Limberger, R.P.; et al. Healing activity of hydrogel containing nanoemulsified β-caryophyllene. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2020, 148, 105318. [Google Scholar] [CrossRef]
- Mazutti da Silva, S.M.; Rezende Costa, C.R.; Martins Gelfuso, G.; Silva Guerra, E.N.; de Medeiros Nóbrega, Y.K.; Gomes, S.M.; Pic-Taylor, A.; Fonseca-Bazzo, Y.M.; Silveira, D.; Magalhães, P.O. Wound Healing Effect of Essential Oil Extracted from Eugenia dysenterica DC (Myrtaceae) Leaves. Molecules 2018, 24, 2. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Beaumont, C.; Rodriguez, D.; Bahr, T. Black pepper (Piper nigrum) essential oil demonstrates tissue remodeling and metabolism modulating potential in human cells. Phytother. Res. PTR 2018, 32, 1848–1852. [Google Scholar] [CrossRef]
- Komakech, R.; Matsabisa, M.G.; Kang, Y. The Wound Healing Potential of Aspilia africana (Pers.) C. D. Adams (Asteraceae). Evid.-Based Complement. Altern. Med. eCAM 2019, 2019, 7957860. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic Acid on regulating glucose and lipids metabolism: A review. Evid.-Based Complement. Altern. Med. eCAM 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Roberts, C.K.; Barnard, R.J.; Sindhu, R.K.; Jurczak, M.; Ehdaie, A.; Vaziri, N.D. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metab. Clin. Exp. 2006, 55, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitturi, S.; Abeygunasekera, S.; Farrell, G.C.; Holmes-Walker, J.; Hui, J.M.; Fung, C.; Karim, R.; Lin, R.; Samarasinghe, D.; Liddle, C.; et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 2002, 35, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Forlani, G. NASH: From liver diseases to metabolic disorders and back to clinical hepatology. Hepatology 2002, 35, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Sirichaiwetchakoon, K.; Lowe, G.M.; Kupittayanant, S.; Churproong, S.; Eumkeb, G. Pluchea indica (L.) Less. Tea Ameliorates Hyperglycemia, Dyslipidemia, and Obesity in High Fat Diet-Fed Mice. Evid.-Based Complement. Altern. Med. eCAM 2020, 2020, 8746137. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Doleski, P.H.; Boligon, A.A.; Stefani, L.M.; Monteiro, S.G. Hypolipidemic effect of beta-caryophyllene to treat hyperlipidemic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 215–223. [Google Scholar] [CrossRef]
- Harb, A.A.; Bustanji, Y.K.; Abdalla, S.S. Hypocholesterolemic effect of beta-caryophyllene in rats fed cholesterol and fat enriched diet. J. Clin. Biochem. Nutr. 2018, 62, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.J.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.; Park, S.; Kim, M.J.; Yang, W.K.; Im, D.U.; Yang, K.R.; Hong, J.; Choe, W.; Kang, I.; Kim, S.S.; et al. AMP-activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. FEBS J. 2014, 281, 4421–4438. [Google Scholar] [CrossRef] [PubMed]
- Kamikubo, R.; Kai, K.; Tsuji-Naito, K.; Akagawa, M. beta-Caryophyllene attenuates palmitate-induced lipid accumulation through AMPK signaling by activating CB2 receptor in human HepG2 hepatocytes. Mol. Nutr. Food Res. 2016, 60, 2228–2242. [Google Scholar] [CrossRef] [PubMed]
- Arizuka, N.; Murakami, T.; Suzuki, K. The effect of β-caryophyllene on nonalcoholic steatohepatitis. J. Toxicol. Pathol. 2017, 30, 263–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chicca, A.; Caprioglio, D.; Minassi, A.; Petrucci, V.; Appendino, G.; Taglialatela-Scafati, O.; Gertsch, J. Functionalization of β-caryophyllene generates novel polypharmacology in the endocannabinoid system. ACS Chem. Biol. 2014, 9, 1499–1507. [Google Scholar] [CrossRef]
- Tambe, Y.; Tsujiuchi, H.; Honda, G.; Ikeshiro, Y.; Tanaka, S. Gastric cytoprotection of the non-steroidal anti-inflammatory sesquiterpene, beta-caryophyllene. Planta Med. 1996, 62, 469–470. [Google Scholar] [CrossRef]
- Ding, Y.; Gu, Z.; Wang, Y.; Wang, S.; Chen, H.; Zhang, H.; Chen, W.; Chen, Y.Q. Clove extract functions as a natural fatty acid synthesis inhibitor and prevents obesity in a mouse model. Food Funct. 2017, 8, 2847–2856. [Google Scholar] [CrossRef]
- EFSA. Flavouring Group Evaluation 78 (FGE.78Rev2) Consideration of Aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic and aromati hydrocarbons evaluated by EFSA in FGE.25Rev3. EFSA J. 2009, 13, 931. [Google Scholar]
- Oliveira, G.; Machado, K.; Machado, K.; Silva, A.; Feitosa, C.; Almeida, F. Non-clinical toxicity of β -caryophyllene, a dietary cannabinoid: Absence of adverse effects in female Swiss mice. Regul. Toxicol. Pharmacol. 2017, 92. [Google Scholar] [CrossRef]
- Schmitt, D.; Levy, R.; Carroll, B. Toxicological Evaluation of β-Caryophyllene Oil: Subchronic Toxicity in Rats. Int. J. Toxicol. 2016, 35, 558–567. [Google Scholar] [CrossRef]
- Bastaki, M.; Api, A.M.; Aubanel, M.; Bauter, M.; Cachet, T.; Demyttenaere, J.C.R.; Diop, M.M.; Harman, C.L.; Hayashi, S.M.; Krammer, G.; et al. Dietary administration of β-caryophyllene and its epoxide to Sprague-Dawley rats for 90 days. Food Chem. Toxicol. 2020, 135, 110876. [Google Scholar] [CrossRef] [PubMed]
- Al-Taee, H.; Azimullah, S.; Meeran, M.F.N.; Alaraj Almheiri, M.K.; Al Jasmi, R.A.; Tariq, S.; Ab Khan, M.; Adeghate, E.; Ojha, S. β-caryophyllene, a dietary phytocannabinoid attenuates oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in rats: An in vitro and in vivo study. Eur. J. Pharm. 2019, 858, 172467. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.F.N.; Al Taee, H.; Azimullah, S.; Tariq, S.; Adeghate, E.; Ojha, S. β-Caryophyllene, a natural bicyclic sesquiterpene attenuates doxorubicin-induced chronic cardiotoxicity via activation of myocardial cannabinoid type-2 (CB(2)) receptors in rats. Chem.-Biol. Interact. 2019, 304, 158–167. [Google Scholar] [CrossRef]
- Kelany, M.E.; Abdallah, M.A. Protective effects of combined β-caryophyllene and silymarin against ketoprofen-induced hepatotoxicity in rats. Can. J. Physiol. Pharmacol. 2016, 94, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Hammad, F.T.; Ojha, S.; Azimullah, S.; Lubbad, L. Does β-caryophyllene protect against renal dysfunction following ischemia-reperfusion injury in the rat? Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 163–171. [Google Scholar] [PubMed]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.; Calixto, J.B. β-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARγ pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol. Cell. Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef]
- Viveros-Paredes, J.M.; González-Castañeda, R.E.; Gertsch, J.; Chaparro-Huerta, V.; López-Roa, R.I.; Vázquez-Valls, E.; Beas-Zarate, C.; Camins-Espuny, A.; Flores-Soto, M.E. Neuroprotective Effects of β-Caryophyllene against Dopaminergic Neuron Injury in a Murine Model of Parkinson’s Disease Induced by MPTP. Pharmaceuticals 2017, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Bahi, A.; Al Mansouri, S.; Al Memari, E.; Al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef]
- Alvarez-González, I.; Madrigal-Bujaidar, E.; Castro-García, S. Antigenotoxic capacity of beta-caryophyllene in mouse, and evaluation of its antioxidant and GST induction activities. J. Toxicol. Sci. 2014, 39, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Lim, L.Y. Effects of spice constituents on P-glycoprotein-mediated transport and CYP3A4-mediated metabolism in vitro. Drug Metab. Dispos. Biol. Fate Chem. 2008, 36, 1283–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Myslivečková, Z.; Szotáková, B.; Špičáková, A.; Lněničková, K.; Ambrož, M.; Kubíček, V.; Krasulová, K.; Anzenbacher, P.; Skálová, L. The inhibitory effects of β-caryophyllene, β-caryophyllene oxide and α-humulene on the activities of the main drug-metabolizing enzymes in rat and human liver in vitro. Chem.-Biol. Interact. 2017, 278, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Calleja, M.A.; Vieites, J.M.; Montero-Meléndez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.I.; Vieira, W.D.; Campos, C.N.; Aarestrup, F.M.; Aarestrup, B.J. Atorvastatin and trans-caryophyllene for the prevention of leukopenia in an experimental chemotherapy model in Wistar rats. Mol. Clin. Oncol. 2015, 3, 825–828. [Google Scholar] [CrossRef] [Green Version]




| Cells | β-Caryophyllene Concentration/Duration | Effects | References |
|---|---|---|---|
| MIN6 β-cells | 0.1–1 μM, 1 h | ↑Insulin, ↑Arf6, Cdc42, and Rac1 | [32] |
| Human Embryonic Kidney (HEK293) | 20, 50, 100, 200 and 500 μM, 24 h | ↓Oxidative stress, ↓Inflammation ↓α-glucosidase | [38] |
| Rat insulinoma (RIN-5F) cells | 500 μmol, 24 h | ↓Glucose absorption, ↑Glucose uptake, ↑Insulin secretion | [37] |
| C2C12 skeletal myotubes | BCP-enriched PipeNig®-FL extract 1, 10, 100 nM, 30 min | ↑Glucose uptake, ↑GLUT4 translocation | [39] |
| C2C12 skeletal myotubes | 1 μM, 48 h | ↑p-SIRT1, ↑p-CREB, ↑Ac-PGC1α, ↑ERRa and ↑NOR1 (fatty acid oxidation transcriptional regulatory genes), ↑CD36 (fatty acid transport genes) ↑CPT1, MCAD, ACO (mitochondrial β-oxidation genes) | [40] |
| Lipid loaded HepG2 cells | 1, 10 and 100 μM | ↓LXR-SREBP-1c pathway, ↑PPAR-α ↓intracellular triglyceride, ↑FATP4, ACS, CPT1, ACOX | [41] |
| Animal | β-Caryophyllene Concentration/Duration | Blood Measures | Other Measures | Reference |
|---|---|---|---|---|
| Male Albino Wistar rats | 200 mg/kg b.w., 45 days | ↓Glucose, ↑Insulin, ↑Vit. C, ↑Vit. E, ↑GSH, ↑Ceruloplasmin, ↓MDA, ↓TNF-α and ↓IL-6 (in plasma) | ↑Pancreatic SOD, CAT, GR, GPx, GST and GSH, ↓Pancreatic MDA, ↓Plasma TNF-α and IL-6 | [29] |
| Male Albino Wistar rats | 100 mg/kg, 200 mg/kg, 400 mg/kg, 45 days | ↓Glucose levels ↑Insulin levels | ↑liver, kidney and skeletal muscle HK, PK, G-6-PD, ↓liver, kidney and skeletal muscle gluconeogenic enzymes (G6pase, F1, 6Bpase, PEPCK and PC), ↑liver and skeletal muscle glycogen synthase, ↓liver and skeletal muscle glycogen phosphorylase | [42] |
| Male Wistar rats | 200 mg/kg, 42 days | ↓Glucose, ↓TGs, ↓SGPT, ↓SGOT ↓cholesterol | ↑pancreatic GSH, ↑SOD, ↑catalase | [37] |
| Male Albino Wistar rats | 200 mg/kg b.w., 45 days | ↓Glucose, ↑Insulin, ↓hexose, ↓hexosamine, ↓fucose and ↓sialic acid | ↓liver and renal sialic acid, ↑liver and renal protein-bound hexose, hexosamine and fucose | [46] |
| Male Wistar rats | 30 mg/kg, P.O, 4 weeks | ↓Glucose, ↓Insulin, ↓TC, ↓VLDL-c,ye ↓TG, ↓HOMA-IR, ↑HDL-c, ↓LDL-c | ↑CB2-R, ↑PPAR-γ, ↑PPAR-α, ↑PGC1-α, ↓TNF-α, ↓NF-κB and ↓VCAM1, ↓MDA, ↑GSH, ↓iNOS, ↓NO, ↑eNOS | [58] |
| Male Wistar rats | 30 mg/kg, P.O, 4 weeks | ↓Glucose, ↓Insulin, ↓HOMA- IR | ↑TAC, ↑GSH, ↓MDA, ↓NO, ↓TNF-α, ↓NF-κB, ↓iNOS, ↑PPAR-γ, ↑PGC-1α, ↑BDNF | [59] |
| Female Sprague Dawley rats | Ethanol extract of Citrus macroptera fruit (500 mg/kg and 1000 mg/kg, P.O) | ↓Glucose levels at 2 h and 3 h after administration (hypoglycemic effect) ↓α-amylase | - | [60] |
| Male Wistar rats | Copaifera duckei containing BCP 21.25% (250 and 500 mg/kg, P.O, 30 days) | ↓Glucose, ↓TG, ↓TC, ↓AST, ↓ALT, ↓urea and ↓creatinine | restore β-cells, ↑quantity and diameter of the Langerhans islets ↓liver mass | [55] |
| Experimental Models | BCP Dose/Concentration/Period | Diabetic Complications | Effects and Mechanisms of BCP | References |
|---|---|---|---|---|
| Human mesangial cells | 6.25, 12.5 and 25 μM for 1 h and then cells stimulated with high-glucose for 24 h | Diabetic nephropathy |
| [117] |
| STZ 40 mg/kg, i.p. at wk 1 (induction), STZ 120 mg/kg at wk 3, (reinforcement) to BALB/c female mice | 10 mg/kg/60 μL, 45 days | Diabetic neuropathic pain |
| [115] |
| B16F10 melanoma cells-induced tumor and lymph node metastasis in high-fat diet (60 kcal%) C57BL/6N mice | 0, 0.15 or 0.3% for 16 weeks with HFD | Diabetes associated cancer |
| [123] |
| Mouse femoral tissues derived bone marrow cells | 0.1–100 μM | Diabetes associated osteoporosis |
| [122] |
| CT26 colorectal tumor cells exposed to high-glucose, and CT26 cells transplanted in STZ (100 mg/kg)-induced DM in male Balb/c mice | 50 μM for 48 h in vitro and 200 mg/kg, P.O to mice for 10 days | Diabetes associated colorectal cancer |
| [118] |
| Distal symmetric polyneuropathy in patients with DM | Diet supplement containing BCP, myrrh, carnosic acid | Diabetic polyneuropathy |
| [116] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashiesh, H.M.; Meeran, M.F.N.; Sharma, C.; Sadek, B.; Kaabi, J.A.; Ojha, S.K. Therapeutic Potential of β-Caryophyllene: A Dietary Cannabinoid in Diabetes and Associated Complications. Nutrients 2020, 12, 2963. https://doi.org/10.3390/nu12102963
Hashiesh HM, Meeran MFN, Sharma C, Sadek B, Kaabi JA, Ojha SK. Therapeutic Potential of β-Caryophyllene: A Dietary Cannabinoid in Diabetes and Associated Complications. Nutrients. 2020; 12(10):2963. https://doi.org/10.3390/nu12102963
Chicago/Turabian StyleHashiesh, Hebaallah Mamdouh, M.F. Nagoor Meeran, Charu Sharma, Bassem Sadek, Juma Al Kaabi, and Shreesh K. Ojha. 2020. "Therapeutic Potential of β-Caryophyllene: A Dietary Cannabinoid in Diabetes and Associated Complications" Nutrients 12, no. 10: 2963. https://doi.org/10.3390/nu12102963
APA StyleHashiesh, H. M., Meeran, M. F. N., Sharma, C., Sadek, B., Kaabi, J. A., & Ojha, S. K. (2020). Therapeutic Potential of β-Caryophyllene: A Dietary Cannabinoid in Diabetes and Associated Complications. Nutrients, 12(10), 2963. https://doi.org/10.3390/nu12102963