Identification of Inflammatory and Disease-Associated Plasma Proteins that Associate with Intake of Added Sugar and Sugar-Sweetened Beverages and Their Role in Type 2 Diabetes Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Dietary Intake Assessment
2.3. Plasma Protein Assessment
2.4. Covariate Assessment
2.5. Outcome Assessment
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
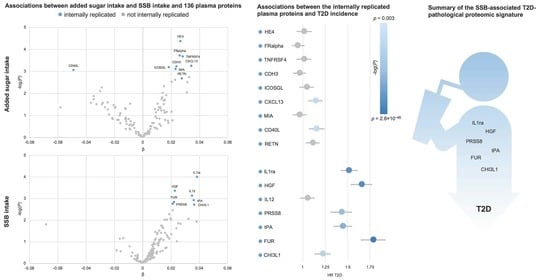
3.2. Plasma Proteins Associated with Added Sugar Intake
3.3. Plasma Proteins Associated with SSB Intake
3.4. Associtions of Plasma Proteins with T2D Incidence
3.5. Associations of Added Sugar and SSB Intake with CRP and T2D Incidence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Minihane, A.-M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [Green Version]
- Lyons, C.L.; Kennedy, E.B.; Roche, H.M. Metabolic Inflammation-Differential Modulation by Dietary Constituents. Nutrients 2016, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Herter-Aeberli, I.; Gerber, P.; Hochuli, M.; Kohler, S.; Haile, S.R.; Gouni-Berthold, I.; Berthold, H.K.; Spinas, G.A.; Berneis, K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, L.B.; Raben, A.; Stender, S.; Astrup, A. Effect of sucrose on inflammatory markers in overweight humans. Am. J. Clin. Nutr. 2005, 82, 421–427. [Google Scholar] [CrossRef]
- Kuzma, J.N.; Cromer, G.; Hagman, D.; Breymeyer, K.L.; Roth, C.L.; Foster-Schubert, K.E.; Holte, S.E.; Weigle, D.S.; Kratz, M. No differential effect of beverages sweetened with fructose, high-fructose corn syrup, or glucose on systemic or adipose tissue inflammation in normal-weight to obese adults: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, C.L.; Stanhope, K.L.; Schwarz, J.M.; Graham, J.L.; Hatcher, B.; Griffen, S.C.; Bremer, A.A.; Berglund, L.; McGahan, J.P.; Keim, N.L.; et al. Circulating Concentrations of Monocyte Chemoattractant Protein-1, Plasminogen Activator Inhibitor-1, and Soluble Leukocyte Adhesion Molecule-1 in Overweight/Obese Men and Women Consuming Fructose- or Glucose-Sweetened Beverages for 10 Weeks. J. Clin. Endocrinol. Metab. 2011, 96, E2034–E2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silbernagel, G.; Machann, J.; Häring, H.-U.; Fritsche, A.; Péter, A. Plasminogen activator inhibitor-1, monocyte chemoattractant protein-1, e-selectin and C-reactive protein levels in response to 4-week very-high-fructose or -glucose diets. Eur. J. Clin. Nutr. 2013, 68, 97–100. [Google Scholar] [CrossRef]
- Buyken, A.E.; Goletzke, J.; Joslowski, G.; Felbick, A.; Cheng, G.; Herder, C.; Brand-Miller, J.C. Association between carbohydrate quality and inflammatory markers: Systematic review of observational and interventional studies. Am. J. Clin. Nutr. 2014, 99, 813–833. [Google Scholar] [CrossRef] [Green Version]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015, 351, h3576. [Google Scholar] [CrossRef] [Green Version]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Willett, W.C.; Hu, F.B. Sugar-Sweetened Beverages and Risk of Metabolic Syndrome and Type 2 Diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colditz, G.A.; Manson, J.E.; Stampfer, M.J.; Rosner, B.; Willett, W.C.; Speizer, F.E. Diet and risk of clinical diabetes in women. Am. J. Clin. Nutr. 1992, 55, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Feskens, E.J.; Virtanen, S.M.; Räsänen, L.; Tuomilehto, J.; Stengård, J.; Pekkanen, J.; Nissinen, A.; Kromhout, D. Dietary Factors Determining Diabetes and Impaired Glucose Tolerance: A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care 1995, 18, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Janket, S.-J.; Manson, J.E.; Sesso, H.; Buring, J.E.; Liu, S. A prospective study of sugar intake and risk of type 2 diabetes in women. Diabetes Care 2003, 26, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Barclay, A.W.; Flood, V.M.; Rochtchina, E.; Mitchell, P.; Brand-Miller, J.C. Mappstat Glycemic Index, Dietary Fiber, and Risk of Type 2 Diabetes in a Cohort of Older Australians. Diabetes Care 2007, 30, 2811–2813. [Google Scholar] [CrossRef] [Green Version]
- Montonen, J.; Järvinen, R.; Knekt, P.; Heliövaara, M.; Reunanen, A. Consumption of Sweetened Beverages and Intakes of Fructose and Glucose Predict Type 2 Diabetes Occurrence. J. Nutr. 2007, 137, 1447–1454. [Google Scholar] [CrossRef] [Green Version]
- Boeing, H.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Carbohydrate intake and incidence of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Br. J. Nutr. 2008, 99, 1107–1116. [Google Scholar] [CrossRef] [Green Version]
- Sluijs, I.; Van Der Schouw, Y.T.; van der Daphne, A.L.; Spijkerman, A.M.; Hu, F.B.; Grobbee, D.E.; Beulens, J.W. Carbohydrate quantity and quality and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition–Netherlands (EPIC-NL) study. Am. J. Clin. Nutr. 2010, 92, 905–911. [Google Scholar] [CrossRef]
- Heikkilä, H.M.; Schwab, U.; Krachler, B.; Männikkö, R.; Rauramaa, R. Dietary associations with prediabetic states—The DR’s EXTRA Study (ISRCTN45977199). Eur. J. Clin. Nutr. 2012, 66, 819–824. [Google Scholar] [CrossRef]
- Ahmadi-Abhari, S.; Luben, R.N.; Powell, N.; Bhaniani, A.; Chowdhury, R.; Wareham, N.J.; Forouhi, N.G.; Khaw, K.-T. Dietary intake of carbohydrates and risk of type 2 diabetes: The European Prospective Investigation into Cancer-Norfolk study. Br. J. Nutr. 2013, 111, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Biggelaar, L.J.C.J.D.; Eussen, S.J.; Sep, S.J.S.; Mari, A.; Ferrannini, E.; Van Dongen, M.C.J.M.; Denissen, K.F.M.; Wijckmans, N.E.G.; Schram, M.T.; Van Der Kallen, C.J.; et al. Associations of Dietary Glucose, Fructose, and Sucrose with β-Cell Function, Insulin Sensitivity, and Type 2 Diabetes in the Maastricht Study. Nutrients 2017, 9, 380. [Google Scholar] [CrossRef] [Green Version]
- Tasevska, N.; Pettinger, M.; Kipnis, V.; Midthune, U.; Tinker, L.F.; Potischman, N.; Neuhouser, M.L.; Beasley, J.M.; Van Horn, L.; Howard, B.V.; et al. Associations of Biomarker-Calibrated Intake of Total Sugars with the Risk of Type 2 Diabetes and Cardiovascular Disease in the Women’s Health Initiative Observational Study. Am. J. Epidemiol. 2018, 187, 2126–2135. [Google Scholar] [CrossRef]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartorelli, D.S.; Franco, L.; Gimeno, S.; Ferreira, S.R.G.; Cardoso, M.A. Dietary fructose, fruits, fruit juices and glucose tolerance status in Japanese–Brazilians. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; English, D.R.; O’Dea, K.; Giles, G.G. Glycemic Index and Dietary Fiber and the Risk of Type 2 Diabetes. Diabetes Care 2004, 27, 2701–2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nat. Cell Biol. 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Wang, X.; Bao, W.; Liu, J.; Ouyang, Y.-Y.; Wang, D.; Rong, S.; Xiao, X.; Shan, Z.-L.; Zhang, Y.; Yao, P.; et al. Inflammatory Markers and Risk of Type 2 Diabetes: A systematic review and meta-analysis. Diabetes Care 2012, 36, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Feng, X.; Li, Q.; Wang, Y.; Li, Q.; Hua, M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 2016, 86, 100–109. [Google Scholar] [CrossRef]
- Ramne, S.; Dias, J.A.; González-Padilla, E.; Olsson, K.; Lindahl, B.; Engström, G.; Ericson, U.; Johansson, I.; Sonestedt, E. Association between added sugar intake and mortality is nonlinear and dependent on sugar source in 2 Swedish population–based prospective cohorts. Am. J. Clin. Nutr. 2018, 109, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Mattisson, I.; Wirfält, E.; Aronsson, C.A.; Wallström, P.; Sonestedt, E.; Gullberg, B.; Berglund, G. Misreporting of energy: Prevalence, characteristics of misreporters and influence on observed risk estimates in the Malmö Diet and Cancer cohort. Br. J. Nutr. 2005, 94, 832–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enhörning, S.; Sjögren, M.; Hedblad, B.; Nilsson, P.M.; Struck, J.; Melander, O. Genetic vasopressin 1b receptor variance in overweight and diabetes mellitus. Eur. J. Endocrinol. 2016, 174, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, G.; Liu, W.; Cheng, C.; Wilson, C.L.; Neale, G.; Yang, J.J.; Ness, K.K.; Robison, L.L.; Hudson, M.M.; Srivastava, D.K. Evaluation of a two-step iterative resampling procedure for internal validation of genome-wide association studies. J. Hum. Genet. 2015, 60, 729–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2018, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olink Proteomics. Complete Human Protein Biomarkers List. Available online: https://www.olink.com/products/complete-protein-biomarkers-list/ (accessed on 16 September 2020).
- Fernandez, C.; Rysä, J.; Almgren, P.; Nilsson, J.; Engström, G.; Orho-Melander, M.; Ruskoaho, H.; Melander, O. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J. Intern. Med. 2018, 284, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.G.; Araújo, T.G.; Carvalho, B.D.M.; Rocha, G.Z.; Dos Santos, A.; Saad, M.J.A. The Role of Hepatocyte Growth Factor (HGF) in Insulin Resistance and Diabetes. Front. Endocrinol. 2018, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Cevik, C.; Nasser, M.; De Caterina, R. Hepatocyte growth factor: Molecular biomarker and player in cardioprotection and cardiovascular regeneration. Thromb. Haemost. 2012, 107, 656–661. [Google Scholar] [CrossRef]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. Dietary Sugars Intake and Cardiovascular Health. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef]
- Wang, J.; Light, K.; Henderson, M.; O’Loughlin, J.; Mathieu, M.-E.; Paradis, G.; Gray-Donald, K. Consumption of Added Sugars from Liquid but Not Solid Sources Predicts Impaired Glucose Homeostasis and Insulin Resistance among Youth at Risk of Obesity. J. Nutr. 2013, 144, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Welsh, J.A.; Wang, Y.; Figueroa, J.; Brumme, C. Sugar intake by type (added vs. naturally occurring) and physical form (liquid vs. solid) and its varying association with children’s body weight, NHANES 2009-2014. Pediatr. Obes. 2018, 13, 213–221. [Google Scholar] [CrossRef]
- De Koning, L.; Malik, V.S.; Kellogg, M.D.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Sweetened Beverage Consumption, Incident Coronary Heart Disease, and Biomarkers of Risk in Men. Circulation 2012, 125, 1735–1741. [Google Scholar] [CrossRef] [Green Version]
- Kosova, E.C.; Auinger, P.; Bremer, A.A. The Relationships between Sugar-Sweetened Beverage Intake and Cardiometabolic Markers in Young Children. J. Acad. Nutr. Diet. 2013, 113, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hert, K.A.; Fisk, P.S.; Rhee, Y.S.; Brunt, A.R. Decreased consumption of sugar-sweetened beverages improved selected biomarkers of chronic disease risk among US adults: 1999 to 2010. Nutr. Res. 2014, 34, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tamez, M.; Monge, A.; Lopez-Ridaura, R.; Fagherazzi, G.; Rinaldi, S.; Ortiz-Panozo, E.; Yunes, E.; Romieu, I.; Lajous, M. Soda Intake Is Directly Associated with Serum C-Reactive Protein Concentration in Mexican Women. J. Nutr. 2018, 148, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Ley, S.H.; Sun, Q.; Hu, F.B.; Malik, V.S. Cross-sectional association between sugar-sweetened beverage intake and cardiometabolic biomarkers in US women. Br. J. Nutr. 2018, 119, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-T.; Kao, Y.-H.; Sothern, M.S.; Seal, D.W.; Lee, C.-H.; Lin, H.-Y.; Chen, T.; Tseng, T.-S. The association between sugar-sweetened beverages intake, body mass index, and inflammation in US adults. Int. J. Public Health 2020, 65, 45–53. [Google Scholar] [CrossRef]
- O’Connor, L.; Imamura, F.; Brage, S.; Griffin, S.J.; Wareham, N.J.; Forouhi, N.G. Intakes and sources of dietary sugars and their association with metabolic and inflammatory markers. Clin. Nutr. 2017, 37, 1313–1322. [Google Scholar] [CrossRef] [Green Version]
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2013, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Ericson, U.; Hindy, G.; Drake, I.; Schulz, C.-A.; Brunkwall, L.; Hellstrand, S.; Almgren, P.; Orho-Melander, M. Dietary and genetic risk scores and incidence of type 2 diabetes. Genes Nutr. 2018, 13, 13. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Borné, Y.; Johnson, L.S.; Muhammad, I.F.; Persson, M.; Niu, K.; Engström, G. Comparing the inflammatory profiles for incidence of diabetes mellitus and cardiovascular diseases: A prospective study exploring the ’common soil’ hypothesis. Cardiovasc. Diabetol. 2018, 17, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, K.; Ramne, S.; Gonzàlez-Padilla, E.; Ericson, U.; Sonestedt, E. Associations of carbohydrates and carbohydrate-rich foods with incidence of type 2 diabetes. Br. J. Nutr. under review.
- Riboli, E.; Elmståhl, S.; Saracci, R.; Gullberg, B.; Lindgärde, F. The Malmo Food Study: Validity of two dietary assessment methods for measuring nutrient intake. Int. J. Epidemiol. 1997, 26, 161S–173S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |


| Added Sugar | ≤5E% | >5–7.5E% | >7.5–10E% | >10–15E% | >15–20E% | >20E% |
|---|---|---|---|---|---|---|
| n = 4382 | 392 | 842 | 1129 | 1544 | 382 | 93 |
| Sex, % women | 60.5 | 62.7 | 61.0 | 62.1 | 61.0 | 54.8 |
| University degree, % | 15.1 | 14.6 | 13.4 | 10.2 | 5.8 | 6.5 |
| Current smoker, % | 32.7 | 26.1 | 24.9 | 24.6 | 28.3 | 41.9 |
| Q5 alcohol, % | 24.0 | 20.2 | 16.9 | 12.8 | 12.0 | 12.9 |
| Incidence T2D, % | 20.4 | 16.3 | 16.5 | 16.9 | 17.3 | 21.5 |
| Age, years | 56.0 (5.8) | 56.5 (5.8) | 57.7 (6.0) | 57.7 (6.0) | 58.1 (5.8) | 57.7 (5.9) |
| BMI, kg/m2 | 26.2 (4.4) | 25.7 (3.9) | 25.4 (3.6) | 25.4 (3.8) | 25.0 (3.8) | 25.2 (4.2) |
| Fasting glucose, mmol/L | 5.66 (0.69) | 5.65 (0.81) | 5.58 (0.65) | 5.60 (0.74) | 5.60 (0.77) | 5.65 (0.72) |
| CRP 1,2, nmol/L | 1.5 (0.7–2.9) | 1.3 (0.7–2.8) | 1.2 (0.6–2.6) | 1.2 (0.6–2.5) | 1.4 (0.6–2.9) | 1.65 (0.7–3.2) |
| Energy intake, kcal | 2095 (718) | 2225 (643) | 2298 (636) | 2381 (660) | 2507 (708) | 2572 (784) |
| SSB intake 1, E% | 0 (0–0) | 0 (0–0.5) | 0 (0–1.1) | 0.65 (0–2.3) | 2.2 (0.4–4.9) | 5.5 (1.4–11.1) |
| SSB:added sugar 1, E% | 0 (0–0) | 0 (0–8.3) | 0 (0–12.3) | 5.5 (0–18.4) | 12.9 (2.4–29.8) | 24.0 (6.2–45.0) |
| SSBs | 0E% | >0–2E% | >2–3E% | >3–5E% | >5E% |
|---|---|---|---|---|---|
| n = 4382 | 2039 | 1471 | 310 | 307 | 255 |
| Sex, % women | 62.7 | 61.5 | 64.2 | 57.0 | 54.5 |
| University degree, % | 13.5 | 11.7 | 8.4 | 8.5 | 7.5 |
| Current smoker, % | 27.1 | 24.3 | 30.0 | 25.7 | 28.2 |
| Q5 alcohol, % | 16.8 | 17.0 | 16.5 | 14.0 | 9.4 |
| Incidence T2D, % | 16.2 | 17.9 | 17.1 | 17.3 | 20.0 |
| Age, years | 57.6 (5.9) | 57.0 (6.1) | 56.9 (6.0) | 57.2 (5.9) | 57.4 (5.9) |
| BMI, kg/m2 | 25.4 (3.8) | 25.3 (3.7) | 25.6 (3.9) | 25.8 (3.8) | 26.2 (4.3) |
| Fasting glucose, mmol/L | 5.60 (0.68) | 5.59 (0.72) | 5.63 (0.74) | 5.64 (0.87) | 5.75 (0.93) |
| CRP 1,2, nmol/L | 1.3 (0.7–2.7) | 1.2 (0.6–2.5) | 1.35 (0.7–2.9) | 1.4 (0.7–2.6) | 1.5 (0.7–3.0) |
| Energy intake, kcal | 2211 (649) | 2422 (677) | 2341 (636) | 2456 (696) | 2391 (711) |
| Added sugar intake, E% | 8.6 (3.6) | 9.99 (3.49) | 11.5 (3.5) | 13.0 (3.5) | 16.4 (4.4) |
| SSB:added sugar 1, E% | 0 (0–0) | 8.22 (4.3–13.1) | 22.2 (17.7–27.8) | 29.6 (24.5–36.2) | 47.3 (37.8–58.4) |
| Added Sugar, E% | Replicated at α1 < 0.05 | Replicated at α1 < 0.01 | Replicated at α1 < FDR0.05 |
|---|---|---|---|
| Epididymial secretory protein E4 (HE4) | 69 | 56 | 20 |
| Folate receptor alpha (FRalpha) | 60 | 37 | 5 |
| Tumor necrosis factor receptor superfamily member 4 (TNFRSF4) | 51 | 27 | 3 |
| Cadherin 3 (CDH3) | 46 | 17 | 1 |
| Inducible T Cell Costimulator Ligand (ICOSLG) | 46 | 14 | 1 |
| C-X-C motif chemokine 13 (CXCL13) | 41 | 14 | 0 |
| Melanoma-derived growth regulatory protein (MIA) | 40 | 11 | 0 |
| CD40 ligand (CD40L) | 37 | 4 | 0 |
| Resistin (RETN) | 23 | 0 | 0 |
| Immunoglobulin-like transcript 3 (ILT3) | 19 | 0 | 0 |
| Interleukin 12 (IL12) | 17 | 0 | 0 |
| Prostasin (PRSS8) | 16 | 0 | 0 |
| Matrix metalloproteinase-10 (MMP10) | 12 | 1 | 0 |
| C-X-C motif chemokine 1 (CXCL1) | 3 | 0 | 0 |
| Transforming growth factor alpha (TGFalpha) | 2 | 0 | 0 |
| Interleukin-1 receptor antagonist (IL1ra) | 2 | 0 | 0 |
| Adrenomedullin (AM) | 1 | 0 | 0 |
| Renin (REN) | 1 | 0 | 0 |
| Agouti-related protein (AGRP) | 1 | 0 | 0 |
| Cathepsin L1 (CTSL1) | 1 | 0 | 0 |
| Furin (FUR) | 1 | 0 | 0 |
| SSB, E% | |||
| Interleukin-1 receptor antagonist (IL1ra) | 60 | 46 | 5 |
| Hepatocyte growth factor (HGF) | 44 | 25 | 0 |
| Interleukin 12 (IL12) | 46 | 12 | 0 |
| Prostasin (PRSS8) | 31 | 1 | 0 |
| Tissue-type plasminogen activator (tPA) | 21 | 5 | 0 |
| Furin (FUR) | 24 | 0 | 0 |
| Chitinase-3-like protein 1 (CHI3L1) | 22 | 0 | 0 |
| Cathepsin D (CTSD) | 8 | 0 | 0 |
| Tartrate-resistant acid phosphatase type 5 (TRAP) | 1 | 0 | 0 |
| Parkinson disease protein 7 (PARK7) | 1 | 0 | 0 |
| Proteinase-activated receptor 1 (PAR1) | 1 | 0 | 0 |
| Prolactin (PRL) | 1 | 0 | 0 |
| Lectin-like oxidized LDL receptor 1 (LOX1) | 1 | 0 | 0 |
| Myoglobin (MB) | 1 | 0 | 0 |
| C-X-C motif chemokine 1 (CXCL1) | 1 | 0 | 0 |
| Added Sugar | n | Lifestyle Adjustments | Lifestyle Adjustments + BMI | Lifestyle Adjustments + BMI + Fasting Glucose | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| HE4 | 4253 | 1.01 (0.93–1.10) | 0.77 | 1.06 (0.97–1.16) | 0.16 | 1.02 (0.93–1.11) | 0.69 |
| FRalpha | 4253 | 0.94 (0.88–1.02) | 0.15 | 1.00 (0.93–1.08) | 0.98 | 0.92 (0.85–0.99) | 0.029 |
| TNFRSF4 | 4175 | 1.01 (0.93–1.09) | 0.84 | 0.97 (0.90–1.05) | 0.52 | 0.87 (0.81–0.95) | 0.0012 * |
| CDH3 | 4241 | 0.96 (0.89–1.03) | 0.23 | 0.98 (0.91–1.05) | 0.52 | 0.94 (0.87–1.01) | 0.11 |
| ICOSLG | 4253 | 1.04 (0.96–1.12) | 0.35 | 1.06 (0.98–1.14) | 0.15 | 0.95 (0.88–1.03) | 0.25 |
| CXCL13 | 4175 | 1.14 (1.06–1.22) | 0.00045 * | 1.12 (1.04–1.21) | 0.0033 | 1.07 (0.98–1.16) | 0.12 |
| MIA | 4252 | 0.96 (0.89–1.04) | 0.34 | 1.01 (0.94–1.09) | 0.79 | 0.99 (0.91–1.07) | 0.74 |
| CD40L | 4382 | 1.15 (1.07–1.24) | 0.00023 * | 1.13 (1.05–1.22) | 0.0012 * | 1.08 (1.00–1.16) | 0.047 |
| RETN | 4382 | 1.11 (1.03–1.19) | 0.0057 | 1.08 (1.01–1.17) | 0.031 | 1.12 (1.04–1.21) | 0.0021 * |
| SSBs | |||||||
| IL1ra | 3761 | 1.51 (1.42–1.61) | 4.6 × 10−37 * | 1.35 (1.26–1.45) | 2.1 × 10−16 * | 1.27 (1.18–1.37) | 6.6 × 10−10 * |
| HGF | 4382 | 1.65 (1.53–1.77) | 2.6 × 10−38 * | 1.48 (1.37–1.60) | 1.0 × 10−22 * | 1.37 (1.27–1.48) | 5.2 × 10−15 * |
| IL12 | 4252 | 1.05 (0.97–1.13) | 0.24 | 0.98 (0.90–1.06) | 0.55 | 0.87 (0.81–0.95) | 0.0014 * |
| PRSS8 | 4252 | 1.43 (1.31–1.55) | 7.7 × 10−17 * | 1.34 (1.23–1.46) | 8.2× 10−12 * | 1.14 (1.05–1.24) | 0.0030 * |
| tPA | 4382 | 1.44 (1.34–1.55) | 8.1 × 10−22 * | 1.33 (1.23–1.43) | 5.6 × 10−13 * | 1.18 (1.09–1.28) | 4.6 × 10−5 * |
| FUR | 4253 | 1.78 (1.64–1.92) | 2.8 × 10−46 * | 1.54 (1.42–1.68) | 4.9 × 10−24 * | 1.30 (1.20–1.42) | 2.2 × 10−9 * |
| CHI3L1 | 4370 | 1.22 (1.13–1.31) | 6.9 × 10−8 * | 1.17 (1.09–1.26) | 2.1 × 10−5 * | 1.17 (1.09–1.26) | 1.5 × 10−5 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramne, S.; Drake, I.; Ericson, U.; Nilsson, J.; Orho-Melander, M.; Engström, G.; Sonestedt, E. Identification of Inflammatory and Disease-Associated Plasma Proteins that Associate with Intake of Added Sugar and Sugar-Sweetened Beverages and Their Role in Type 2 Diabetes Risk. Nutrients 2020, 12, 3129. https://doi.org/10.3390/nu12103129
Ramne S, Drake I, Ericson U, Nilsson J, Orho-Melander M, Engström G, Sonestedt E. Identification of Inflammatory and Disease-Associated Plasma Proteins that Associate with Intake of Added Sugar and Sugar-Sweetened Beverages and Their Role in Type 2 Diabetes Risk. Nutrients. 2020; 12(10):3129. https://doi.org/10.3390/nu12103129
Chicago/Turabian StyleRamne, Stina, Isabel Drake, Ulrika Ericson, Jan Nilsson, Marju Orho-Melander, Gunnar Engström, and Emily Sonestedt. 2020. "Identification of Inflammatory and Disease-Associated Plasma Proteins that Associate with Intake of Added Sugar and Sugar-Sweetened Beverages and Their Role in Type 2 Diabetes Risk" Nutrients 12, no. 10: 3129. https://doi.org/10.3390/nu12103129
APA StyleRamne, S., Drake, I., Ericson, U., Nilsson, J., Orho-Melander, M., Engström, G., & Sonestedt, E. (2020). Identification of Inflammatory and Disease-Associated Plasma Proteins that Associate with Intake of Added Sugar and Sugar-Sweetened Beverages and Their Role in Type 2 Diabetes Risk. Nutrients, 12(10), 3129. https://doi.org/10.3390/nu12103129