The Beneficial Effect of Boswellic Acid on Bone Metabolism and Possible Mechanisms of Action in Experimental Osteoporosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Kits
2.2. Animals
2.3. Experiment Design
2.3.1. Osteoporosis Induction
2.3.2. Treatment Protocol
2.4. Estimation of Calcium Content in Femoral Bone and Lumbar Vertebral Ash
2.5. Determination of BMD
2.6. Femur and Lumbar Vertebra Compression Test
2.7. Histopathological Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of AKBA on Physical Parameters
3.1.1. Effect of AKBA on the Body Weight and Uterus Weight
3.1.2. Effect of AKBA on the Weight, Length, and Thickness of Femur Bone and Lumbar Vertebra
3.1.3. Femur and Lumbar Vertebra Compression Test
3.1.4. Effect of AKBA on Calcium Content of Femur Bone and Lumbar Vertebra
3.1.5. Effect of AKBA on BMD of Femur Bone and Lumbar Vertebra
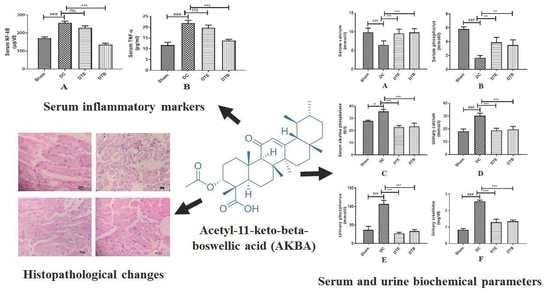
3.2. Effect of AKBA on Serum Biochemical Parameters in Ovariectomized Rats
3.3. Effect of AKBA on Urine Biochemical Parameters in Ovariectomized Rats
3.4. Effect of AKBA on NF-κB and TNF-α Expressions
3.5. Effect of AKBA on Histopathological Changes of Ovariectomized Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwang, Y.H.; Kim, K.J.; Kim, S.J.; Mun, S.K.; Hong, S.G.; Son, Y.J.; Yee, S.T. Suppression Effect of Astaxanthin on Osteoclast Formation In Vitro and Bone Loss In Vivo. Int. J. Mol. Sci. 2018, 19, 912. [Google Scholar] [CrossRef] [Green Version]
- Ström, O.; Borgström, F.; Kanis, J.A.; Compston, J.; Cooper, C.; McCloskey, E.V.; Jönsson, B. Osteoporosis: Burden, health care provision and opportunities in the EU: A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2011, 6, 59–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hua, F.; Ding, K.; Chen, H.; Xu, C.; Ding, W. Angiogenesis Changes in Ovariectomized Rats with Osteoporosis Treated with Estrogen Replacement Therapy. BioMed Res. Int. 2019, 2019, 1283717. [Google Scholar] [CrossRef] [PubMed]
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gonzalez, M.C.; Gallarate, M. Bone Diseases: Current Approach and Future Perspectives in Drug Delivery Systems for Bone Targeted Therapeutics. Nanomaterials 2020, 10, 875. [Google Scholar] [CrossRef] [PubMed]
- D’Oronzo, S.; Coleman, R.; Brown, J.; Silvestris, F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J. Bone Oncol. 2019, 15, 100205. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Al-Dhubiab, B.E.; Chattopadhyaya, I.; Nair, A.; Kumria, R.; Gupta, S. Assessment of pharmacokinetic interaction of spirulina with glitazone in a type 2 diabetes rat model. J. Med. Food 2013, 16, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [Green Version]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef] [Green Version]
- Infante, M.; Fabi, A.; Cognetti, F.; Gorini, S.; Caprio, M.; Fabbri, A. RANKL/RANK/OPG system beyond bone remodeling: Involvement in breast cancer and clinical perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 12. [Google Scholar] [CrossRef] [Green Version]
- Iram, F.; Khan, S.A.; Husain, A. Phytochemistry and potential therapeutic actions of Boswellic acids: A mini-review. Asian Pac. J. Trop. Biomed. 2017, 7, 513–523. [Google Scholar] [CrossRef]
- Siddiqui, M.Z. Boswellia serrata, a potential antiinflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Al-Harrasi, A.; Csuk, R.; Shamraiz, U.; Green, I.R.; Ahmed, I.; Khan, I.A.; Ali, Z. Therapeutic potential of boswellic acids: A patent review (1990–2015). Expert Opin. Ther. Pat. 2017, 27, 81–90. [Google Scholar] [CrossRef]
- Du, Z.; Liu, Z.; Ning, Z.; Liu, Y.; Song, Z.; Wang, C.; Lu, A. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015, 81, 259–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 4101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solanki, N.; Dureja, H. Impact of Surfactants on Formulation Parameters and in vitro Cytotoxicity of Boswellic Acids Loaded Nanoparticles on Human Colon Cancer Cell Lines. Indian J. Pharm. Educ. Res. 2018, 52, S229–S236. [Google Scholar] [CrossRef] [Green Version]
- Pherwani, P.U.; Sathaye, S. Treatment of Osteoporosis: Current Scenario from a Research Perspective. Indian J. Pharm. Educ. Res. 2020, 54, 8–16. [Google Scholar] [CrossRef]
- Cao, X. RANKL-RANK signaling regulates osteoblast differentiation and bone formation. Bone Res. 2018, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wang, X.; Li, G.; Chong, Y.; Zhang, J.; Guo, X.; Li, B.; Bi, Z. Osteoclast regulation of osteoblasts via RANK-RANKL reverse signal transduction in vitro. Mol. Med. Rep. 2017, 16, 3994–4000. [Google Scholar] [CrossRef]
- Bai, F.; Chen, X.; Yang, H.; Xu, H.G. Acetyl-11-Keto-β-Boswellic Acid Promotes Osteoblast Differentiation by Inhibiting Tumor Necrosis Factor-α and Nuclear Factor-κB Activity. J. Craniofacial Surg. 2018, 29, 1996–2002. [Google Scholar] [CrossRef]
- Morsy, M.A.; Patel, S.S.; El-Sheikh, A.A.K.; Savjani, J.K.; Nair, A.B.; Shah, J.N.; Venugopala, K.N. Computational and Biological Comparisons of Plant Steroids as Modulators of Inflammation through Interacting with Glucocorticoid Receptor. Mediat. Inflamm. 2019, 2019, 3041438. [Google Scholar] [CrossRef] [Green Version]
- Ammon, H.P. Boswellic Acids and Their Role in Chronic Inflammatory Diseases. Adv. Exp. Med. Biol. 2016, 928, 291–327. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Ichikawa, H.; Badmaev, V.; Aggarwal, B.B. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-kappa B and NF-kappa B-regulated gene expression. J. Immunol. 2006, 176, 3127–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, L.; Liu, Y.; Zhu, F.; Lin, J.; Wen, D.; Wang, Z.; Bai, J.; Ge, G.; Xu, C.; Gu, Y.; et al. Acetyl-11-keto-β-boswellic acid attenuates titanium particle-induced osteogenic inhibition via activation of the GSK-3β/β-catenin signaling pathway. Theranostics 2019, 9, 7140–7155. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018. [Google Scholar] [CrossRef]
- Taylor, G.T.; Farr, S.; Klinga, K.; Weiss, J. Chronic fluoxetine suppresses circulating estrogen and the enhanced spatial learning of estrogen-treated ovariectomized rats. Psychoneuroendocrinology 2004, 29, 1241–1249. [Google Scholar] [CrossRef]
- Ahmed, M.A.E.; Ahmed, A.A.E.; El Morsy, E.M. Acetyl-11-keto-β-boswellic acid prevents testicular torsion/detorsion injury in rats by modulating 5-LOX/LTB4 and p38-MAPK/JNK/Bax/Caspase-3 pathways. Life Sci. 2020, 260, 118472. [Google Scholar] [CrossRef]
- Nair, A.B.; Kaushik, A.; Attimarad, M.; Al-Dhubiab, B.E. Enhanced oral bioavailability of calcium using bovine serum albumin microspheres. Drug Deliv. 2012, 19, 277–285. [Google Scholar] [CrossRef]
- Reddy, N.P.; Lakshmana, M.; Udupa, U.V. Antiosteoporotic activity of OST-6(Osteocare), a herbomineral preparation in calcium deficient ovariectomized rats. Phytother. Res. PTR 2004, 18, 25–29. [Google Scholar] [CrossRef]
- Washburn, M.L.; Shear, M.J. Composition of bone XIII. Direct gravimetric determination of Ca, Mg, and PO4. J. Biol. Chem. 1932, 99, 21. [Google Scholar] [CrossRef]
- Huang, J.; Hu, Y.; Tong, X.; Zhang, L.; Yu, Z.; Zhou, Z. Untargeted metabolomics revealed therapeutic mechanisms of icariin on low bone mineral density in older caged laying hens. Food Funct. 2020, 11, 3201–3212. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Khan, S.; Malini, S. Antiosteoporotic effect of ethanol extract of Cissus quadrangularis Linn. on ovariectomized rat. J. Ethnopharmacol. 2003, 89, 245–250. [Google Scholar] [CrossRef]
- Morsy, M.A.; Nair, A.B. Prevention of rat liver fibrosis by selective targeting of hepatic stellate cells using hesperidin carriers. Int. J. Pharm. 2018, 552, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016, 23, 2154–2162. [Google Scholar] [CrossRef] [Green Version]
- Gambacciani, M.; Levancini, M. Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Prz Menopauzalny 2014, 13, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, Z.; Zhang, W.; Yang, Y.; Han, B.; Liu, W.; Peng, Y. Dietary Natural N-Acetyl-d-Glucosamine Prevents Bone Loss in Ovariectomized Rat Model of Postmenopausal Osteoporosis. Molecules 2018, 23, 2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alonzo, M.; Bounous, V.E.; Villa, M.; Biglia, N. Current Evidence of the Oncological Benefit-Risk Profile of Hormone Replacement Therapy. Medicina 2019, 55, 573. [Google Scholar] [CrossRef] [Green Version]
- Oršolić, N.; Nemrava, J.; Jeleč, Ž.; Kukolj, M.; Odeh, D.; Terzić, S.; Fureš, R.; Bagatin, T.; Bagatin, D. The Beneficial Effect of Proanthocyanidins and Icariin on Biochemical Markers of Bone Turnover in Rats. Int. J. Mol. Sci. 2018, 19, 2746. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Chacko, K.M.; Aggarwal, M.L.; Bhat, B.; Khandal, R.K.; Sultana, S.; Kuruvilla, B.T. A-90 Day Gavage Safety Assessment of Boswellia serrata in Rats. Toxicol. Int. 2012, 19, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.B.; Bani, S.; Singh, S. Toxicity and safety evaluation of boswellic acids. Phytomed. Int. J. Phytother. Phytopharm. 1996, 3, 87–90. [Google Scholar] [CrossRef]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J. 2020, 19, 89–107. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, N.K.; Thungapathra, M. Resveratrol regulates body weight in healthy and ovariectomized rats. Nutr. Metab. 2017, 14, 30. [Google Scholar] [CrossRef] [Green Version]
- Saleh, N.; Nassef, N.A.; Shawky, M.K.; Elshishiny, M.I.; Saleh, H.A. Novel approach for pathogenesis of osteoporosis in ovariectomized rats as a model of postmenopausal osteoporosis. Exp. Gerontol. 2020, 137, 110935. [Google Scholar] [CrossRef]
- López-Belmonte, J.; Nieto, C.; Estevez, J.; Delgado, J.L.; del Prado, J.M. Comparative uterine effects on ovariectomized rats after repeated treatment with different vaginal estrogen formulations. Maturitas 2012, 72, 353–358. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.T.; Kang, W.B.; Zhao, J.N.; Liu, G.; Zhao, M.G. Osteoprotective Effect of Echinocystic Acid, a Triterpone Component from Eclipta prostrata, in Ovariectomy-Induced Osteoporotic Rats. PLoS ONE 2015, 10, e0136572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, X.; Jin, H.; Wen, G.; Xu, J.; An, J.; Liu, X.; Xie, R.; Tuo, B. Estrogen Regulates Duodenal Calcium Absorption Through Differential Role of Estrogen Receptor on Calcium Transport Proteins. Dig. Dis. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, S.; Shioyama, M.; Kitade, M.; Nagata, M.; Yamaji, T. Involvement of estrogen in phosphorus-induced nephrocalcinosis through fibroblast growth factor 23. Sci. Rep. 2020, 10, 4864. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Suzuki, T.; Kato, H. Serum bone alkaline phosphatase is a useful marker to evaluate lumbar bone mineral density in Japanese postmenopausal osteoporotic women during denosumab treatment. Ther. Clin. Risk Manag. 2017, 13, 1343–1348. [Google Scholar] [CrossRef] [Green Version]
- Elliot, S.J.; Karl, M.; Berho, M.; Potier, M.; Zheng, F.; Leclercq, B.; Striker, G.E.; Striker, L.J. Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. Am. J. Pathol. 2003, 162, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Vorland, C.J.; Lachcik, P.J.; Swallow, E.A.; Metzger, C.E.; Allen, M.R.; Chen, N.X.; Moe, S.M.; Hill Gallant, K.M. Effect of ovariectomy on the progression of chronic kidney disease-mineral bone disorder (CKD-MBD) in female Cy/+ rats. Sci. Rep. 2019, 9, 7936. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.; Chauhan, S.; Nair, A.; Sharma, P. Astilbin: A promising unexplored compound with multidimensional medicinal and health benefits. Pharmacol. Res. 2020, 158. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Takakura, N.; Hiura, F.; Nakamura, I.; Hirata-Tsuchiya, S. The Role of NF-κB in Physiological Bone Development and Inflammatory Bone Diseases: Is NF-κB Inhibition “Killing Two Birds with One Stone”? Cells 2019, 8, 1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Mehla, K.; Chauhan, D.; Nair, A. Anti-inflammatory activity of leaves of Michelia champaca investigated on acute inflammation induced rats. Lat. Am. J. Pharm. 2011, 30, 819. [Google Scholar]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB-Mediated Regulation of Osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siemoneit, U.; Koeberle, A.; Rossi, A.; Dehm, F.; Verhoff, M.; Reckel, S.; Maier, T.J.; Jauch, J.; Northoff, H.; Bernhard, F.; et al. Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense. Br. J. Pharmacol. 2011, 162, 147–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Yasiry, A.R.; Kiczorowska, B. Frankincense—Therapeutic properties. Postepy Hig. Med. Dosw. (Online) 2016, 70, 380–391. [Google Scholar] [CrossRef]
- Lerner, U.H.; Kindstedt, E.; Lundberg, P. The critical interplay between bone resorbing and bone forming cells. J. Clin. Periodontol. 2019, 46, 33–51. [Google Scholar] [CrossRef]




| Parameter | Sham | DC | DTE | DTB |
|---|---|---|---|---|
| Body weight gain (g) | 24.7 ± 1.88 | 45.8 ± 2.95 ### | 40.6 ± 1.43 ### | 39.5 ± 1.97 ### |
| Weight of uterus (g) | 0.64 ± 0.07 | 0.45 ± 0.01 # | 0.68 ± 0.05 ** | 0.74 ± 0.03 ** |
| Weight of the femur bone (g) | 0.59 ± 0.03 | 0.33 ± 0.01 ### | 0.49 ± 0.03 ** | 0.49 ± 0.03 ** |
| Length of femur bone (mm) | 23.1 ± 0.51 | 20.3 ± 0.15 ## | 22.5 ± 0.51 ** | 22.9 ± 0.46 ** |
| Thickness of femur bone (mm) | 5.18 ± 0.15 | 4.27 ± 0.05 ### | 5.19 ± 0.06 ##*** | 5.29 ± 0.18 #*** |
| Hardness of femur bone (kilopond) | 4.98 ± 0.18 | 2.69 ± 0.12 ### | 5.14 ± 0.06 *** | 5.31 ± 0.25 *** |
| % Calcium in femur bone | 1.33 ± 0.07 | 0.41 ± 0.14 ### | 1.02 ± 0.06 ** | 1.05 ± 0.14 ** |
| BMD of femur bone (mg/mm2) | 178 ± 8.36 | 118 ± 9.13 ### | 150 ± 8.67 * | 162 ± 5.55 ** |
| Weight of the lumbar vertebra (g) | 0.33 ± 0.03 | 0.18 ± 0.01 ## | 0.30 ± 0.03 * | 0.32 ± 0.04 * |
| Length of lumbar vertebra (mm) | 8.04 ± 0.44 | 6.20 ± 0.40 ## | 7.55 ± 0.23 * | 7.96 ± 0.19 ** |
| Thickness of lumbar vertebra (mm) | 3.89 ± 0.13 | 2.25 ± 0.12 ### | 2.93 ± 0.16 ##* | 3.20 ± 0.20 #** |
| Hardness of lumbar vertebra (kilopond) | 12.9 ± 0.56 | 9.47 ± 0.73 ## | 11.9 ± 0.44 * | 12.4 ± 0.58 * |
| % Calcium in lumbar vertebra | 1.13 ± 0.09 | 0.49 ± 0.11 ### | 0.91 ± 0.06 * | 1.03 ± 0.10 ** |
| BMD of lumbar vertebra (mg/mm2) | 106 ± 5.28 | 71.2 ± 7.56 ## | 96.0 ± 4.54 * | 105 ± 5.44 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dhubiab, B.E.; Patel, S.S.; Morsy, M.A.; Duvva, H.; Nair, A.B.; Deb, P.K.; Shah, J. The Beneficial Effect of Boswellic Acid on Bone Metabolism and Possible Mechanisms of Action in Experimental Osteoporosis. Nutrients 2020, 12, 3186. https://doi.org/10.3390/nu12103186
Al-Dhubiab BE, Patel SS, Morsy MA, Duvva H, Nair AB, Deb PK, Shah J. The Beneficial Effect of Boswellic Acid on Bone Metabolism and Possible Mechanisms of Action in Experimental Osteoporosis. Nutrients. 2020; 12(10):3186. https://doi.org/10.3390/nu12103186
Chicago/Turabian StyleAl-Dhubiab, Bandar E., Snehal S. Patel, Mohamed A. Morsy, Harika Duvva, Anroop B. Nair, Pran Kishore Deb, and Jigar Shah. 2020. "The Beneficial Effect of Boswellic Acid on Bone Metabolism and Possible Mechanisms of Action in Experimental Osteoporosis" Nutrients 12, no. 10: 3186. https://doi.org/10.3390/nu12103186
APA StyleAl-Dhubiab, B. E., Patel, S. S., Morsy, M. A., Duvva, H., Nair, A. B., Deb, P. K., & Shah, J. (2020). The Beneficial Effect of Boswellic Acid on Bone Metabolism and Possible Mechanisms of Action in Experimental Osteoporosis. Nutrients, 12(10), 3186. https://doi.org/10.3390/nu12103186