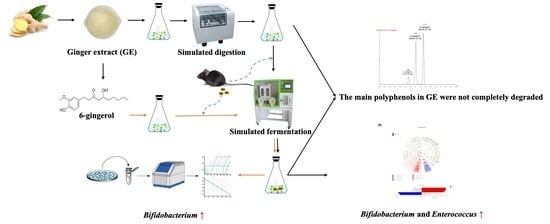
Assessing the Effects of Ginger Extract on Polyphenol Profiles and the Subsequent Impact on the Fecal Microbiota by Simulating Digestion and Fermentation In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.2. Preparation of Ginger Extract
2.3. Simulated Digestion Model In Vitro
2.4. Simulated Fecal Fermentation In Vitro
2.5. Identification and Quantification of 6-, 8-, and 10-Gingerols and 6-Shogaol at Different Time Points of Digestion and Fermentation In Vitro
2.6. Estimation of the Total Polyphenol Content (TPC)
2.7. DNA Extraction and MiSeq Sequencing
2.8. Processing and Bioinformatics Analysis of Sequencing Data
2.9. Analysis of pH Values and Short-Chain Fatty Acid (SCFA) Production
2.10. DNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.11. Statistical Analysis
3. Results
3.1. Degradation of Polyphenols during Simulated Digestion and Fermentation
3.2. Changes in Bacterial Populations with In Vitro Batch Culture Fermentation
3.3. Effects of Ginger Extract on the pH Value and SCFA Production during In Vitro Fermentation
3.4. Effect of 6G on the Specific Microbial Population during In Vitro Fermentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kou, X.; Wang, X.; Ji, R.; Liu, L.; Qiao, Y.; Lou, Z.; Ma, C.; Li, S.; Wang, H.; Ho, C. Occurrence, biological activity and metabolism of 6-shogaol. Food Funct. 2018, 9, 1310–1327. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Rida, P.C.G.; Kucuk, O.; Aneja, R. Ginger augmented chemotherapy: A novel multitarget nontoxic approach for cancer management. Mol. Nutr. Food Res. 2016, 60, 1364–1373. [Google Scholar] [CrossRef]
- Palatty, P.L.; Haniadka, R.; Valder, B.; Arora, R.; Baliga, M.S. Ginger in the prevention of nausea and vomiting: A review. Crit. Rev. Food Sci. 2013, 53, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.; Posadzki, P.; Watson, L.K.; Ernst, E. The use of ginger (Zingiber officinale) for the treatment of pain: A systematic review of clinical trials. Pain Med. 2011, 12, 1808–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haniadka, R.; Saldanha, E.; Sunita, V.; Palatty, P.L.; Fayad, R.; Baliga, M.S. A review of the gastroprotective effects of ginger (Zingiber officinale Roscoe). Food Funct. 2013, 4, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.M.; Folmer, V.N.; Bliddal, H.; Altman, R.D.; Juhl, C.; Tarp, S.; Zhang, W.; Christensen, R. Efficacy and safety of ginger in osteoarthritis patients: A meta-analysis of randomized placebo-controlled trials. Osteoarthr. Cartil. 2015, 23, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ke, W.; Bao, R.; Hu, X.; Chen, F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: A review. Ann. N. Y. Acad. Sci. 2017, 1398, 83–98. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidem. Biomar. 2008, 17, 1930–1936. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Li, Y.; Wang, Y.; Gao, J.; Xia, L.; Hong, Y. Comparison of fresh, dried and stir-frying gingers in decoction with blood stasis syndrome in rats based on a GC-TOF/MS metabolomics approach. J. Pharm. Biomed. 2016, 129, 339–349. [Google Scholar] [CrossRef]
- Mukkavilli, R.; Yang, C.; Tanwar, R.S.; Ghareeb, A.; Luthra, L.; Aneja, R. Absorption, metabolic stability, and pharmacokinetics of ginger phytochemicals. Molecules 2017, 22, 553. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Tran, V.H.; Duke, C.C. Stability of [6]-gingerol and [6]-shogaol in simulated gastric and intestinal fluids. J. Pharm. Biomed. 2007, 45, 648–653. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.; Li, D.; Hu, X.; Chen, F. Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. Eur. J. Nutr. 2020, 59, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosele, J.I.; Macia, A.; Romero, M.P.; Motilua, M.J.; Rubio, L. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds. J. Funct. Foods 2015, 14, 529–540. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Chen, F.; Xie, Y.Q.; Han, M.D.; Luo, C.X.; Zhao, Y.Y.; Gao, Y.Q. Nutraceutical potential and antioxidant benefits of selected fruit seeds subjected to an in vitro digestion. J. Funct. Foods 2016, 20, 317–331. [Google Scholar] [CrossRef]
- Alqurashi, R.M.; Alarifi, S.N.; Walton, G.E.; Costabile, A.F.; Rowland, I.R.; Commane, D.M. In vitro approaches to assess the effects of acai (Euterpe oleracea) digestion on polyphenol availability and the subsequent impact on the faecal microbiota. Food Chem. 2017, 234, 190–198. [Google Scholar] [CrossRef]
- Condezo-Hoyos, L.; Mohanty, I.P.; Noratto, G.D. Assessing non-digestible compounds in apple cultivars and their potential as modulators of obese faecal microbiota in vitro. Food Chem. 2014, 161, 208–215. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.-S.; Isabel Calvo, M.; Yolanda Cavero, R.; Astiasaran, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Valdes-Varela, L.; Ruas-Madiedo, P.; Gueimonde, M. In vitro fermentation of different fructo-oligosaccharides by Bifidobacterium strains for the selection of synbiotic combinations. Int. J. Food Microbiol. 2017, 242, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimlou, M.; Yari, Z.; Hekmatdoost, A.; Alavian, S.M.; Keshavarz, S.A. Ginger supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Hepat. Mon. 2016, 16, e34897. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gao, H.; Ke, D.; Zuo, G.; Yang, Y.; Yamahara, J.; Li, Y. Improvement of liquid fructose-induced adipose tissue insulin resistance by ginger treatment in rats is associated with suppression of adipose macrophage-related proinflammatory cytokines. Evid.-Based Compl. Alt. 2013, 2013, 590376. [Google Scholar] [CrossRef] [Green Version]
- Kazeem, M.I.; Akanji, M.A.; Yakubu, M.T.; Ashafa, A.O.T. Protective effect of free and bound polyphenol extracts from ginger (Zingiber officinale Roscoe) on the hepatic antioxidant and some carbohydrate metabolizing enzymes of streptozotocin-induced diabetic rats. Evid.-Based Compl. Alt. 2013, 2013, 935486. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.L.; Yu, X.; Dong, L.E.; Shen, R.L. In vitro fermentation of oat β-glucan and hydrolysates by fecal microbiota and selected probiotic strains. J. Sci. Food Agric. 2017, 97, 4198–4203. [Google Scholar] [CrossRef]
- Attri, S.; Sharma, K.; Raigond, P.; Goel, G. Colonic fermentation of polyphenolics from Sea buckthorn (Hippophae rhamnoides) berries: Assessment of effects on microbial diversity by Principal Component Analysis. Food Res. Int. 2018, 105, 324–332. [Google Scholar] [CrossRef]
- Wu, T.; Grootaert, C.; Pitart, J.; Vidovic, N.K.; Kamiloglu, S.; Possemiers, S.; Glibetic, M.; Smagghe, G.; Raes, K.; Van de Wiele, T.; et al. Aronia (Aronia melanocarpa) Polyphenols Modulate the Microbial Community in a Simulator of the Human Intestinal Microbial Ecosystem (SHIME) and Decrease Secretion of Proinflammatory Markers in a Caco-2/endothelial Cell Coculture Model. Mol. Nutr. Food Res. 2018, 62, 1800607. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Ulamek-Koziol, M. Mutual Two-Way Interactions of Curcumin and Gut Microbiota. Int. J. Mol. Sci. 2020, 21, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.-Z.; Li, X.-Y.; Wang, S.; Shen, L.; Ji, H.-F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Patan, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, M.G.R.; Quintanilla-Lopez, J.E.; Lebron-Aguilar, R.; Martin-Alvarez, P.J.; Victoria Moreno-Arribas, M.; Bartolome, B. In vitro fermentation of a red wine extract by human gut microbiota: Changes in microbial groups and formation of phenolic metabolites. J. Agric. Food Chem. 2012, 60, 2136–2147. [Google Scholar] [CrossRef]
- Brown, E.M.; McDougall, G.J.; Stewart, D.; Pereira-Caro, G.; Gonzalez-Barrio, R.; Allsopp, P.; Magee, P.; Crozier, A.; Rowland, I.; Gill, C.I.R. Persistence of anticancer activity in berry extracts after simulated gastrointestinal digestion and colonic fermentation. PLoS ONE 2012, 7, e49740. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.E.; Gibson, G.R.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Hoffmann, M.; Messlik, A.; Kim, S.C.; Sartor, R.B.; Haller, D. Impact of a probiotic Enterococcus faecalis in a gnotobiotic mouse model of experimental colitis. Mol. Nutr. Food Res. 2011, 55, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, W.; Zhu, X.; Sun, X.; Xiao, J.; Li, D.; Cui, Y.; Wang, C.; Shi, Y. Response of gut microbiota to dietary fiber and metabolic interaction with SCFAs in piglets. Front. Microbiol. 2018, 9, 2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]




| Treatments | TPC (GAE mg/g) | Polyphenolic Compounds (μg/mg of Digesta) | |||
|---|---|---|---|---|---|
| 6G | 8G | 10G | 6S | ||
| Blank | 884.89 ± 21.41 a | 13.63 ± 0.49 a | 2.85 ± 0.30 a | 1.33 ± 0.05 a | 1.85 ± 0.11 a |
| Gastric | 610.58 ± 12.45 c | 9.02 ± 0.51 b | 2.91 ± 0.35 a | 1.26 ± 0.14 a | 1.75 ± 0.20 a |
| Small intestine | 751.03 ± 31.39 b | 7.09 ± 0.53 c | 2.80 ± 0.19 a | 1.05 ± 0.00 a | 1.68 ± 0.10 a |
| Incubation Period (h) | TPC (GAE mg/g) | Polyphenolic Compounds (μg/mg of Fermented Mixture) | |||
|---|---|---|---|---|---|
| 6G | 8G | 10G | 6S | ||
| 0 | 523.86 ± 13.57 b | 4.81 ± 0.65 a | 1.36 ± 0.20 a | 1.10 ± 0.02 b | 0.86 ± 0.22 a |
| 6 | 542.25 ± 25.41 b | 1.43 ± 0.1 bc | 0.17 ± 0.01 b | 0.86 ± 0.00 c | 0.30 ± 0.00 b |
| 12 | 691.81 ± 21.35 a | 2.45 ± 0.68 b | 1.42 ± 0.36 a | 1.27 ± 0.09 a | 0.54 ± 0.10 ab |
| 24 | 432.64 ± 21.89 c | 0.50 ± 0.02 c | 0.17 ± 0.00 b | 0.86 ± 0.00 c | 0.30 ± 0.00 b |
| Bacteria | Group | Fermentation Time (h) | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | Treatment | Fermentation Time | Treatment × Fermentation Time | ||
| Total bacteria | Control | 9.83 ± 0.63 | 9.70 ± 0.30 | 9.95 ± 0.20 | 10.10 ± 0.50 | 0.741 | 0.409 | 0.559 |
| 6G | 9.81 ± 0.49 | 9.78 ± 0.49 | 10.25 ± 0.16 | 9.87 ± 0.60 | ||||
| Bifidobacterium | Control | 4.55 ± 0.45 | 3.90 ± 0.16 | 3.75 ± 0.99 | 3.31 ± 0.22 | <0.001 | 0.015 | 0.606 |
| 6G | 5.42 ± 0.33 | 5.15 ± 0.43 | 4.99 ± 0.55 | 4.79 ± 0.66 | ||||
| Enterococcus | Control | 7.33 ± 0.55 | 7.20 ± 0.27 | 6.43 ± 0.37 | 6.37 ± 0.56 | 0.425 | 0.003 | 0.71 |
| 6G | 7.47 ± 0.47 | 7.38 ± 0.69 | 6.96 ± 0.63 | 6.18 ± 0.70 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, Y.; Hu, X.; Feng, F.; Cai, L.; Chen, F. Assessing the Effects of Ginger Extract on Polyphenol Profiles and the Subsequent Impact on the Fecal Microbiota by Simulating Digestion and Fermentation In Vitro. Nutrients 2020, 12, 3194. https://doi.org/10.3390/nu12103194
Wang J, Chen Y, Hu X, Feng F, Cai L, Chen F. Assessing the Effects of Ginger Extract on Polyphenol Profiles and the Subsequent Impact on the Fecal Microbiota by Simulating Digestion and Fermentation In Vitro. Nutrients. 2020; 12(10):3194. https://doi.org/10.3390/nu12103194
Chicago/Turabian StyleWang, Jing, Yong Chen, Xiaosong Hu, Fengqin Feng, Luyun Cai, and Fang Chen. 2020. "Assessing the Effects of Ginger Extract on Polyphenol Profiles and the Subsequent Impact on the Fecal Microbiota by Simulating Digestion and Fermentation In Vitro" Nutrients 12, no. 10: 3194. https://doi.org/10.3390/nu12103194
APA StyleWang, J., Chen, Y., Hu, X., Feng, F., Cai, L., & Chen, F. (2020). Assessing the Effects of Ginger Extract on Polyphenol Profiles and the Subsequent Impact on the Fecal Microbiota by Simulating Digestion and Fermentation In Vitro. Nutrients, 12(10), 3194. https://doi.org/10.3390/nu12103194